52be7d1ca90111fa29f46f44f60d8f12.ppt
- Количество слайдов: 2
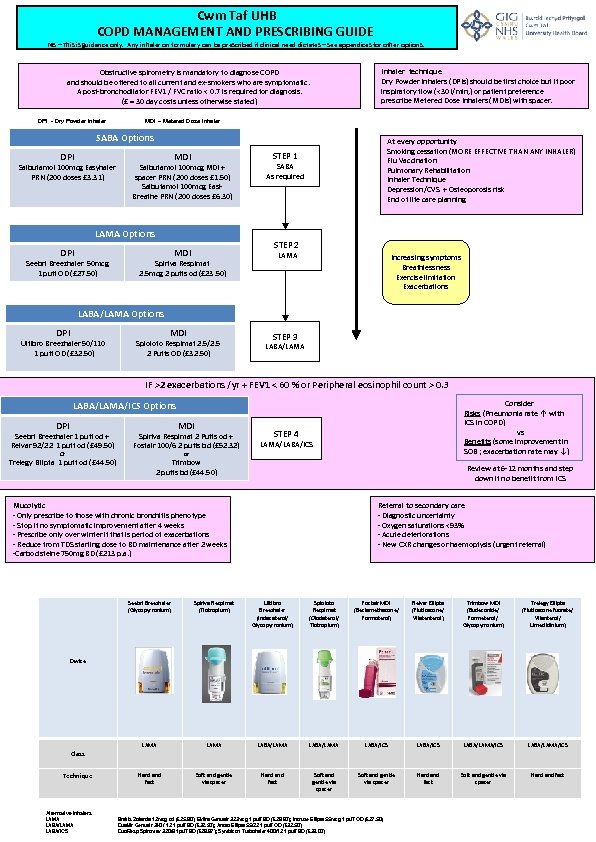
Cwm Taf UHB COPD MANAGEMENT AND PRESCRIBING GUIDE NB – This is guidance only. Any inhaler on formulary can be prescribed if clinical need dictates – see appendices for other options. Inhaler technique Dry Powder Inhalers (DPIs) should be first choice but if poor inspiratory flow (<30 l/min, ) or patient preference prescribe Metered Dose Inhalers (MDIs) with spacer. Obstructive spirometry is mandatory to diagnose COPD and should be offered to all current and ex-smokers who are symptomatic. A post-bronchodilator FEV 1 / FVC ratio < 0. 7 is required for diagnosis. (£ = 30 day costs unless otherwise stated) DPI - Dry Powder Inhaler MDI – Metered Dose Inhaler SABA Options DPI Salbutamol 100 mcg Easyhaler PRN (200 doses £ 3. 31) MDI STEP 1 Salbutamol 100 mcg MDI + spacer PRN (200 doses £ 1. 50) Salbutamol 100 mcg Easi. Breathe PRN (200 doses £ 6. 30) LAMA Options DPI Seebri Breezhaler 50 mcg 1 puff OD (£ 27. 50) At every opportunity Smoking cessation (MORE EFFECTIVE THAN ANY INHALER) Flu Vaccination Pulmonary Rehabilitation Inhaler Technique Depression/CVS + Osteoporosis risk End of life care planning SABA As required STEP 2 MDI Spiriva Respimat 2. 5 mcg 2 puffs od (£ 23. 50) LAMA Increasing symptoms Breathlessness Exercise limitation Exacerbations LABA/LAMA Options DPI Ultibro Breezhaler 50/110 1 puff OD (£ 32. 50) MDI Spioloto Respimat 2. 5/2. 5 2 Puffs OD (£ 32. 50) STEP 3 LABA/LAMA IF >2 exacerbations /yr + FEV 1 < 60 % or Peripheral eosinophil count > 0. 3 Consider Risks (Pneumonia rate ↑ with ICS in COPD) vs Benefits (some improvement in SOB ; exacerbation rate may ↓) LABA/LAMA/ICS Options DPI MDI Seebri Breezhaler 1 puff od + Relvar 92/22 1 puff od (£ 49. 50) Spiriva Respimat 2 Puffs od + Fostair 100/6 2 puffs bd (£ 52. 32) Trelegy Ellipta 1 puff od (£ 44. 50) STEP 4 Trimbow 2 puffs bd (£ 44. 50) Or LAMA/LABA/ICS or Review at 6 -12 months and step down if no benefit from ICS Mucolytic - Only prescribe to those with chronic bronchitis phenotype - Stop if no symptomatic improvement after 4 weeks - Prescribe only over winter if that is period of exacerbations - Reduce from TDS starting dose to BD maintenance after 2 weeks -Carbocisteine 750 mg BD (£ 213 p. a. ) Referral to secondary care - Diagnostic uncertainty - Oxygen saturations <93% - Acute deteriorations - New CXR changes or haemoptysis (urgent referral) Seebri Breezhaler (Glycopyrronium) Spiriva Respimat (Tiotropium) Ultibro Breezhaler (Indacaterol/ Glycopyrronium) Spioloto Respimat (Olodaterol/ Tiotropium) Fostair MDI (Beclamethasone/ Formoterol) Relvar Ellipta (Fluticasone/ Vilatenterol) Trimbow MDI (Budesonide/ Formeterol/ Glycopyrronium) Trelegy Ellipta (Fluticasone fuorate/ Vilanterol/ Umeclidinium) LAMA LABA/LAMA LABA/ICS LABA/LAMA/ICS Hard and fast Soft and gentle via spacer Hard and fast Device Class Technique Alternative Inhalers LAMA LABA/ICS Braltis Zolanda 12 mcg od (£ 25. 80); Eklira Genuair 322 mcg 1 puff BD (£ 28. 60); Incruse Ellipta 55 mcg 1 puff OD (£ 27. 50) Duaklir Genuair 340 / 12 1 puff BD (£ 32. 50); Anoro Ellipta 55/22 1 puff OD (£ 32. 50) Duo. Resp Spiromax 320/9 1 puff BD (£ 29. 97); Symbicort Turbohaler 400/12 1 puff BD (£ 38. 00)

References 1. Prof C. Jensen et al. Pneumonia and pneumonia related mortality in patients with COPD treated with fixed combinations of inhaled corticosteroid and long acting β 2 agonist: observational matched cohort study (PATHOS). British medical journal. 2013; 346: f 3306 available online [http: //www. bmj. com/content/346/bmj. f 3306 ] 2. National Institute for Health and Clinical Excellence (2014). Chronic obstructive pulmonary disease: beclometasone/formoterol (Fostair). National Institute for Health and Clincal Excellence, 2014. 3. National Institute for Health and Clinical Excellence (2013). ESNM 21. Chronic obstructive pulmonary disease: fluticasone furorate and vilanterol. National Institute for Health and Clincal Excellence, 2013 4. Calverley PMA, Kuna P, Monsó E, et al. (2010) Beclomethasone/formoterol in the management of COPD: a randomised controlled trial. Respiratory Medicine 104: 1858− 68 5. Nannini LJ, Lasserson TJ, Poole P. (2012). Combined corticosteroid and long-acting beta 2 -agonist in one inhaler versus long-acting beta 2 -agonists for chronic obstructive pulmonary disease (Review). The Cochrane Collaboration. 2012 6. Nannini LJ, Poole P, Milan SJ, Kesterton A. (2013). Combined corticosteroid and long-acting beta 2 -agonist in oneinhaler versus inhaled corticosteroids alone for chronic obstructive pulmonary disease (Review) The Cochrane Collaboration. 2013 7. Bateman E. D, Ferguson G. T, Barnes N, et al. (2013). Dual bronchodilation with QVA 149 versus single bronchodilator therapy: the SHINE study. European Respiratory Journal. 2013; 42(6): 1484 -94. 8. Beeh K. M, Korn S, Beier J, et al. (2014). Effect of QVA 149 on lung volumes and exercise tolerance in COPD patients: the BRIGHT study. Respiratory Medicine. 2014 Apr; 108(4): 584 -92. 9. Dahl R, Jadayel D, Alagappan V. K. T, et al. (2013). Efficacy and safety of QVA 149 compared to the concurrent administration of its monocomponents indacaterol and glycopyrronium: the BEACON study [Corrigendum]. International Journal of Chronic Obstructive Pulmonary Disease. 2013; 8: 501– 508. 10. Scottish Medicines Consortium. (2015). Aclidinium/formoterol fumarate dihydrate 340/12 micrograms inhalation powder (Duaklir Genuair) SMC No. (1034/15). 2015. 11. Zhong, C. Wang, X. Zhou. (2015). LATEBREAKING ABSTRACT: Efficacy and safety of once daily QVA 149 compared with twice daily salmeterol/fluticasone combination (SFC) in patients with COPD: the LANTERN study. European Respiratory Society. 2015 12. National Institute for Health and Clinical Excellence (2014). Chronic obstructive pulmonary disease: umeclidinium/vilanterol combination inhaler (Anoro Ellipta). National Institute for Health and Clinical Excellence, 2014 13. National Institute for Health and Clinical Excellence (2014). Chronic obstructive pulmonary disease: indacaterol/glycopyrronium (Ultibro Breezhaler). National Institute for Health and Clinical Excellence, 2014 14. Wedzicha J. A, Decramer M, Ficker H. J, . (2013). Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA 149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. The Lancet. 2013 20. Vogelmeier, C. F, Bateman, E. D. , Pallante, J. (2012). Efficacy and safety daily QVA 149 compared with twice daily salmeterol-fluticasone in patients with chronic obstructive pulmonary disease (ILLUMINATE): a randomised, double-blind, parallel group study. The Lancet Respiratory Medicine. 2012; 1: 151 -60 21. Appleton S, Poole P, Smith B. J, et al (2006). Long-acting beta 2 -agonists for poorly reversible chronic obstructive pulmonary disease (Review). The Cochrane Collaboration. 2006 22. Geake J. B, Dabscheck E. J, Wood-Baker R, . (2015). Indacaterol, a once-daily beta 2 -agonist, versus twice-daily beta 2 -agonists or placebo for chronic obstructive pulmonary disease (Review). The Cochrane Collaboration. 2015 23. Donohue, J. F. , Fogarty, C. , Lo¨tvall, J. et al. (2010) Once-Daily Bronchodilators for Chronic Obstructive Pulmonary Disease Indacaterol Versus Tiotropium. The American Journal Respiratory and Critical Care Medicine. 2010; 182: 155 -162 24. Dahl, R. Chung, K. F. Buhl, F. et al (2010). Efficacy of a new once-daily long-acting inhaled b 2 -agonist indacaterol versus twice-daily formoterol in COPD. Thorax 2010; 65: 473 -479. 25. Kornmann, O. Dahl R, Centann, R. et al (2011). Once-daily indacaterol versus twice-daily salmeterol for COPD: a placebo-controlled comparison. European Respiratory Jouranal 2011; 37: 273– 279 26. Spencer S, Karner C, Cates C. J, et al. Inhaled corticosteroids versus long-acting beta 2 -agonists for chronic obstructive pulmonary disease (Review). The Cochrane Collaboration. 2011 27. National Institute for Health and Clinical Excellence (2015). Chronic obstructive pulmonary disease: Olodaterol. National Institute for Health and Clinical Excellence, 2015 28. Rossi, A. Kristufek, P. Levine, B. E. et al (2002). Comparison of the Efficacy, Tolerability, and Safety of Formoterol Dry Powder and Oral, Slow-Release Theophylline inthe Treatment of COPD. Chest 2002; 121: 1058 -1069 29. Wadbo, M. Lo C. G. , Larsson, K. et al. (2002). Effects of formoterol and ipratropium bromide in COPD: a 3 -month placebo-controlled study. European Respiratory Journal 2002; 20: 1138– 1146 30. Beier, J. , Kirsten, A-M, Mróz, R. (2012). Effi cacy and Safety of Aclidinium Bromide Compared with Placebo and Tiotropium in Patients with Moderate-to-Severe Chronic Obstructive Pulmonary Disease: Results from a 6 -week, Randomized, Controlled Phase IIIb Study. COPD, 10: 511– 522 31. Ni, H. , Soe, Z. , Moe S. (2014). Aclidinium bromide for stable chronic obstructive pulmonary disease (Review). The Cochrane Collaboration. 2015 32. Kerwin, E. He´ber, J. Gallagher, N. et al. (2012). Efficacy and safety of NVA 237 versus placebo and tiotropium in patients with COPD: the GLOW 2 study. European Respiratory Journal. 2012; 40: 1106– 1114 33. D’Urzo, A. Ferguson, G. T. Van Noord, J. A et al. (2011). Efficacy and safety of once-daily NVA 237 in patients with moderate-to-severe COPD: the GLOW 1 trial. Respiratory Research. 2012; 12: 156 34. Beeh. , M. K. , Singh. D, . Scala, L. D, Drollmann, A. (2012). Once-daily NVA 237 improves exercise tolerance from the first dose in patients with COPD: the GLOW 3 trial. International journal of COPD. 2012; 4: 503 -515 35. National Institute for Health and Clinical Excellence (2015). Chronic obstructive pulmonary disease: umeclidinium (incruse)l. National institute for Health and Clinical Excellence, 2015 36. National Institute for Health and Clinical Excellence (2013). Chronic obstructive pulmonary disease: glycopyrronium (seebri). National Institute for Health and Clinical Excellence, 2013 37. National Institute for Health and Clinical Excellence (2013). Chronic obstructive pulmonary disease: aclidinium (Eklira). National Institute for Health and Clinical Excellence, 2013 38. Jones, P. W. , Singh, D. , Bateman, E. D. et al (2012). Efficacy and safety of twice-daily aclidinium bromide in COPD patients: the ATTAIN study. European Respiratory Journal. 2012; 40: 830– 836 39. Kerwin, E. M. , D’Urzo, A. D. , Gelb, A. F. , et al. (2012). Efficacy and Safety of a 12 -week Treatment with Twice-daily Aclidinium Bromide in COPD Patients (ACCORD COPD I). Journal of COPD. 2012; 9: 1 -12 40. Karner, C. , Chong, J. , & Poole, P. (2012) Tiotropium versus placebo for chronic obstructive pulmonary disease (Review). The Cochrane Collaboration. 2012 41. Chrystyn, H et al. Device errors in asthma and COPD: systematic literature review and meta-analysis. Primary Care Respiratory Medicine (2017) 27: 22 42. Rogliani, P et al. Optimizing drug delivery in COPD: The role of inhaler devices. Respiratory Medicine 124 (2017) 6 -14 43. Vestbo J et al. Effectiveness of Fluticasone Furoate–Vilanterol for COPD in Clinical Practice. N Engl J Med 2016; 375: 1253 -60. Preparation date: June 2015, updated May 2016 and August 2017. Prices from MIMS and Drug Tariff online (accessed 5 th Aug 2017)
52be7d1ca90111fa29f46f44f60d8f12.ppt