fd69e79f8fbf6943d1061bf6362861d3.ppt
- Количество слайдов: 33
 CWD Friends For Life 7 July 2011 Update on the Closed-Loop Artificial Pancreas Project Stuart A Weinzimer, MD Associate Professor of Pediatrics Yale University School of Medicine
CWD Friends For Life 7 July 2011 Update on the Closed-Loop Artificial Pancreas Project Stuart A Weinzimer, MD Associate Professor of Pediatrics Yale University School of Medicine
 Rationale for a Closed-Loop System • Present methods of diabetes treatment improve, but don’t normalize, blood glucose levels, even with CGM • Burden of care extremely high • CL would advance our ability to control BG levels while at the same time REDUCE burden on user
Rationale for a Closed-Loop System • Present methods of diabetes treatment improve, but don’t normalize, blood glucose levels, even with CGM • Burden of care extremely high • CL would advance our ability to control BG levels while at the same time REDUCE burden on user
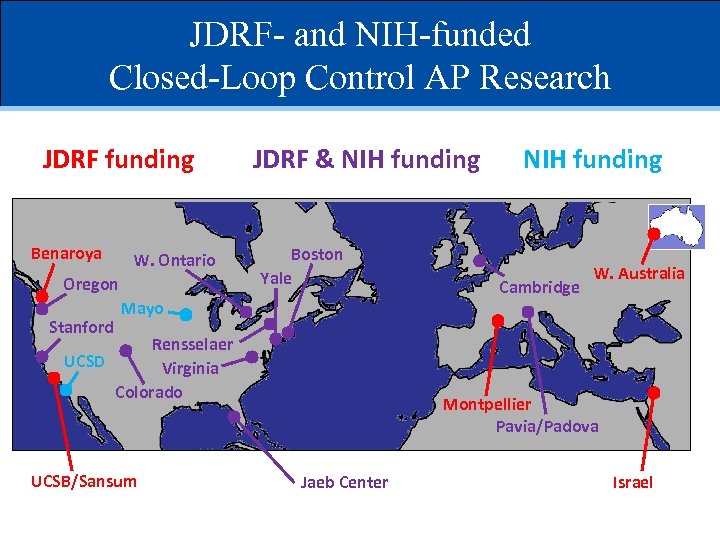 JDRF- and NIH-funded Closed-Loop Control AP Research JDRF funding Benaroya W. Ontario Oregon Stanford JDRF & NIH funding Boston Yale Mayo Rensselaer UCSD Virginia Colorado UCSB/Sansum NIH funding Cambridge W. Australia Montpellier Pavia/Padova Jaeb Center Israel
JDRF- and NIH-funded Closed-Loop Control AP Research JDRF funding Benaroya W. Ontario Oregon Stanford JDRF & NIH funding Boston Yale Mayo Rensselaer UCSD Virginia Colorado UCSB/Sansum NIH funding Cambridge W. Australia Montpellier Pavia/Padova Jaeb Center Israel
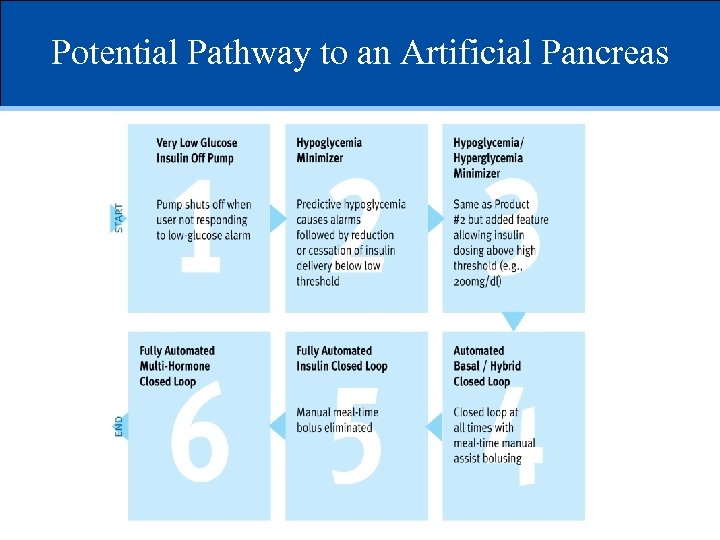 Potential Pathway to an Artificial Pancreas
Potential Pathway to an Artificial Pancreas
 Medtronic e. PID closed-loop system • Paradigm 715 insulin pump • MMT sensor adapted for one-minute transmission • Laptop computer with software program and algorithm
Medtronic e. PID closed-loop system • Paradigm 715 insulin pump • MMT sensor adapted for one-minute transmission • Laptop computer with software program and algorithm
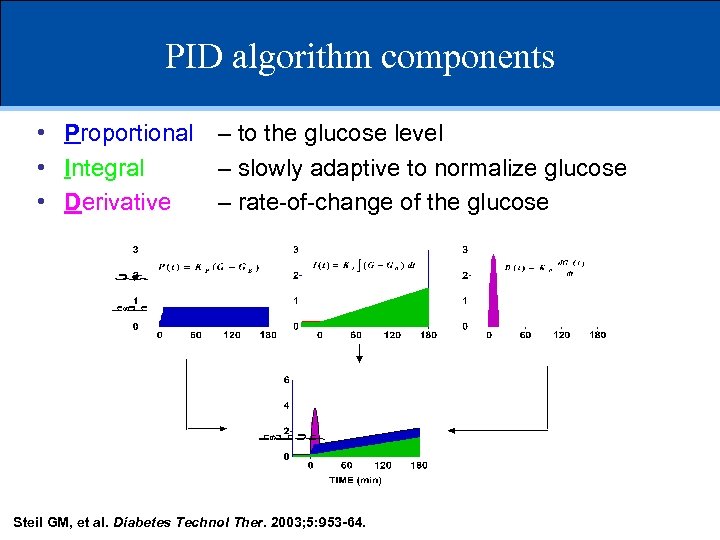 PID algorithm components • Proportional – to the glucose level • Integral – slowly adaptive to normalize glucose • Derivative – rate-of-change of the glucose Steil GM, et al. Diabetes Technol Ther. 2003; 5: 953 -64.
PID algorithm components • Proportional – to the glucose level • Integral – slowly adaptive to normalize glucose • Derivative – rate-of-change of the glucose Steil GM, et al. Diabetes Technol Ther. 2003; 5: 953 -64.
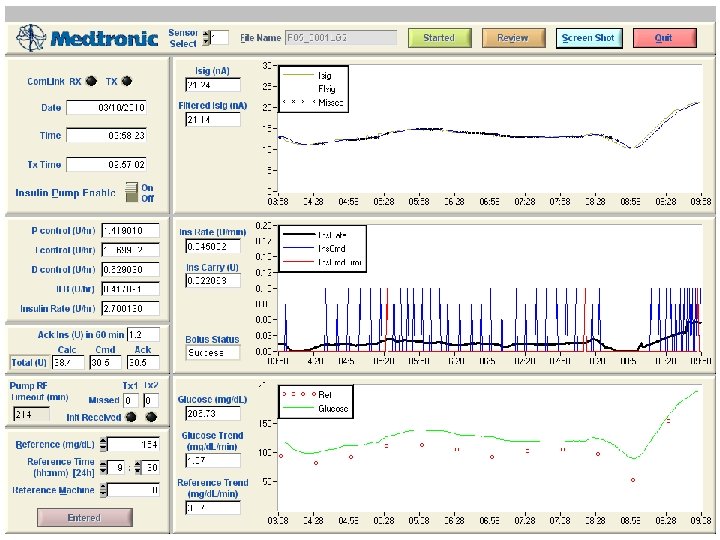
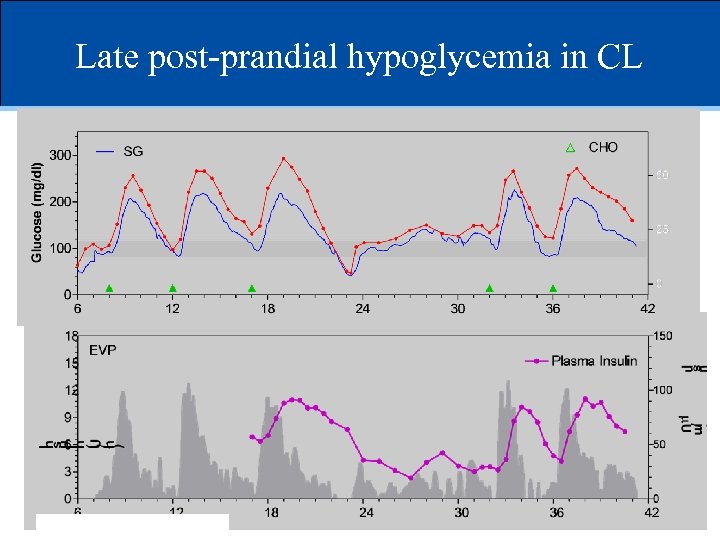 Late post-prandial hypoglycemia in CL
Late post-prandial hypoglycemia in CL
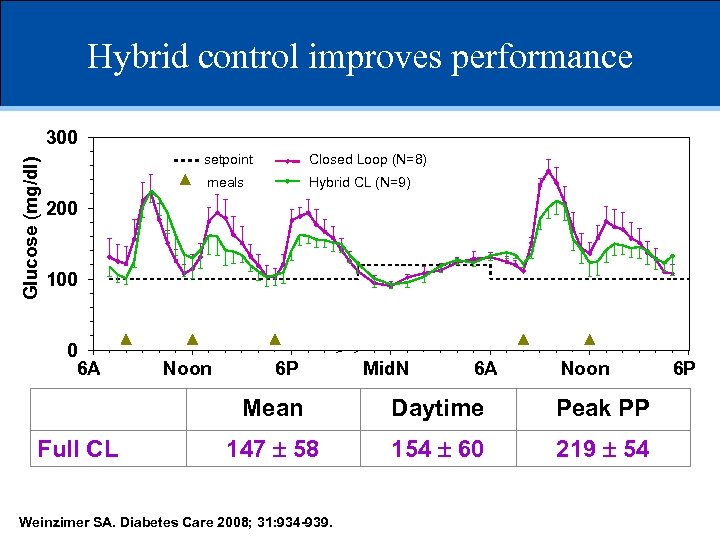 Hybrid control improves performance Glucose (mg/dl) 300 setpoint Closed Loop (N=8) meals Hybrid CL (N=9) 200 100 0 6 A Noon 6 P Mid. N 6 A Noon Mean Full CL Hybrid Daytime Peak PP 147 58 138 49 154 60 143 50 219 54 196 52 Weinzimer SA. Diabetes Care 2008; 31: 934 -939. 6 P
Hybrid control improves performance Glucose (mg/dl) 300 setpoint Closed Loop (N=8) meals Hybrid CL (N=9) 200 100 0 6 A Noon 6 P Mid. N 6 A Noon Mean Full CL Hybrid Daytime Peak PP 147 58 138 49 154 60 143 50 219 54 196 52 Weinzimer SA. Diabetes Care 2008; 31: 934 -939. 6 P
 Conclusions of study • CL control feasible in youth with T 1 D • Manual insulin “priming bolus” improved meal excursions • Tendency to late post-prandial hypoglycemia • Limitations – No OL control – Subjects were sedentary
Conclusions of study • CL control feasible in youth with T 1 D • Manual insulin “priming bolus” improved meal excursions • Tendency to late post-prandial hypoglycemia • Limitations – No OL control – Subjects were sedentary
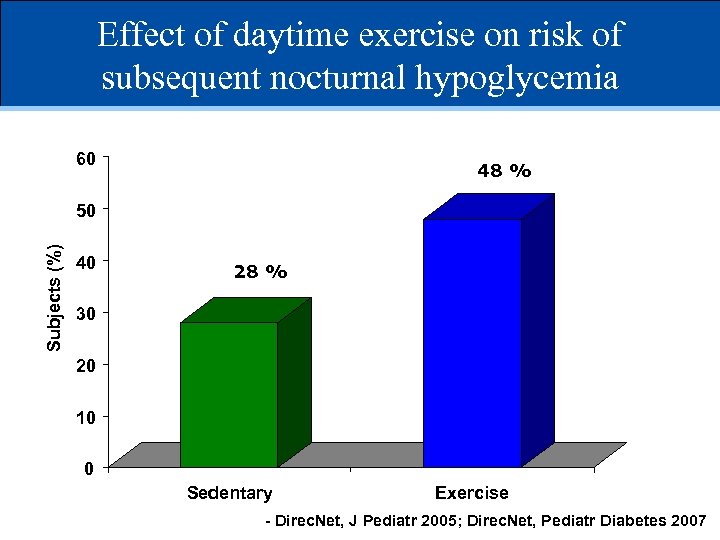 Effect of daytime exercise on risk of subsequent nocturnal hypoglycemia 60 48 % Subjects (%) 50 40 28 % 30 20 10 0 Sedentary Exercise - Direc. Net, J Pediatr 2005; Direc. Net, Pediatr Diabetes 2007
Effect of daytime exercise on risk of subsequent nocturnal hypoglycemia 60 48 % Subjects (%) 50 40 28 % 30 20 10 0 Sedentary Exercise - Direc. Net, J Pediatr 2005; Direc. Net, Pediatr Diabetes 2007
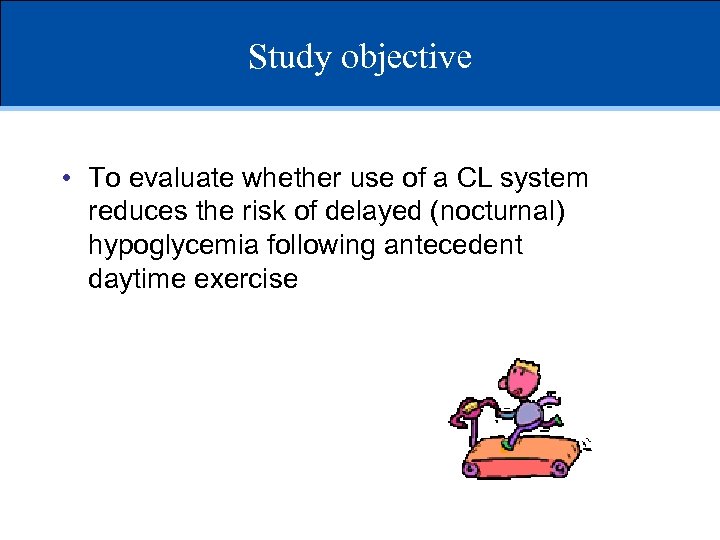 Study objective • To evaluate whether use of a CL system reduces the risk of delayed (nocturnal) hypoglycemia following antecedent daytime exercise
Study objective • To evaluate whether use of a CL system reduces the risk of delayed (nocturnal) hypoglycemia following antecedent daytime exercise
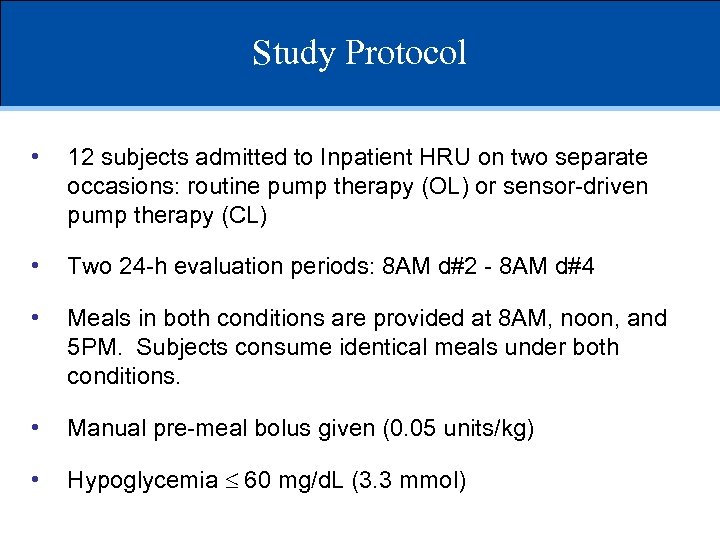 Study Protocol • 12 subjects admitted to Inpatient HRU on two separate occasions: routine pump therapy (OL) or sensor-driven pump therapy (CL) • Two 24 -h evaluation periods: 8 AM d#2 - 8 AM d#4 • Meals in both conditions are provided at 8 AM, noon, and 5 PM. Subjects consume identical meals under both conditions. • Manual pre-meal bolus given (0. 05 units/kg) • Hypoglycemia 60 mg/d. L (3. 3 mmol)
Study Protocol • 12 subjects admitted to Inpatient HRU on two separate occasions: routine pump therapy (OL) or sensor-driven pump therapy (CL) • Two 24 -h evaluation periods: 8 AM d#2 - 8 AM d#4 • Meals in both conditions are provided at 8 AM, noon, and 5 PM. Subjects consume identical meals under both conditions. • Manual pre-meal bolus given (0. 05 units/kg) • Hypoglycemia 60 mg/d. L (3. 3 mmol)
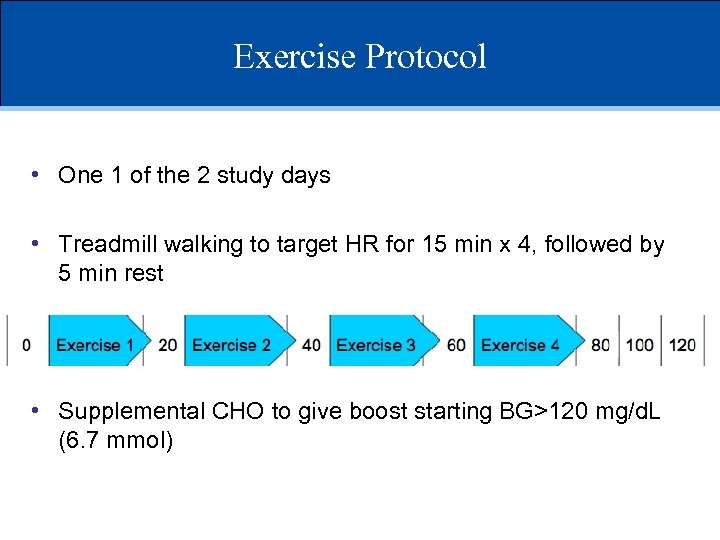 Exercise Protocol • One 1 of the 2 study days • Treadmill walking to target HR for 15 min x 4, followed by 5 min rest • Supplemental CHO to give boost starting BG>120 mg/d. L (6. 7 mmol)
Exercise Protocol • One 1 of the 2 study days • Treadmill walking to target HR for 15 min x 4, followed by 5 min rest • Supplemental CHO to give boost starting BG>120 mg/d. L (6. 7 mmol)
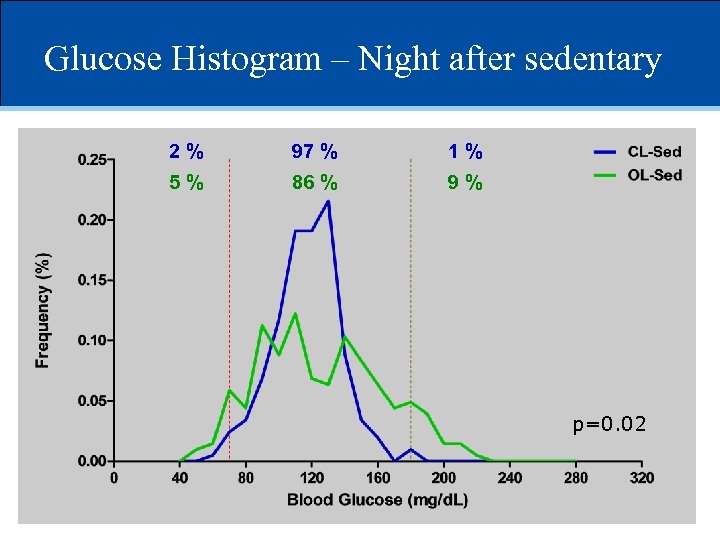 Glucose Histogram – Night after sedentary 2% 97 % 1% 5% 86 % 9% p=0. 02
Glucose Histogram – Night after sedentary 2% 97 % 1% 5% 86 % 9% p=0. 02
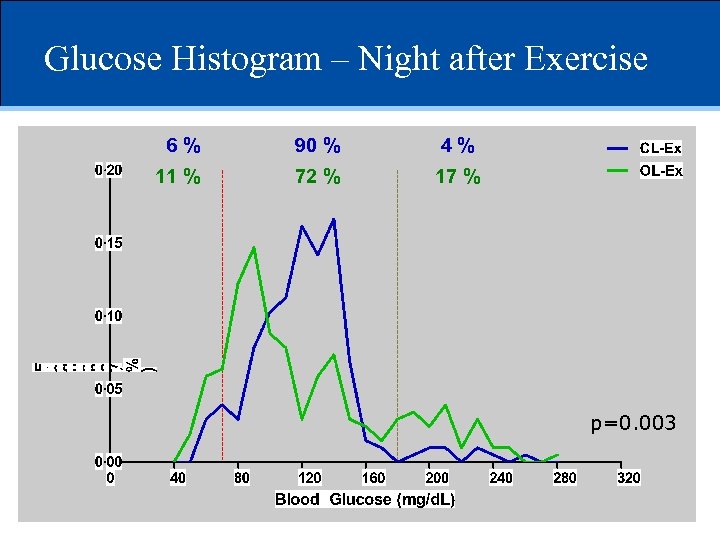 Glucose Histogram – Night after Exercise 6% 90 % 4% 11 % 72 % 17 % p=0. 003
Glucose Histogram – Night after Exercise 6% 90 % 4% 11 % 72 % 17 % p=0. 003
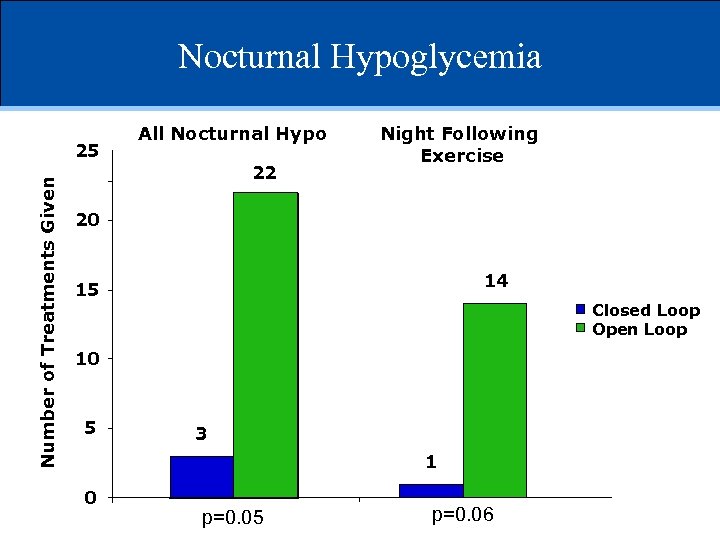 Nocturnal Hypoglycemia Number of Treatments Given 25 All Nocturnal Hypo 22 Night Following Exercise 20 14 15 Closed Loop Open Loop 10 5 3 1 0 p=0. 05 p=0. 06
Nocturnal Hypoglycemia Number of Treatments Given 25 All Nocturnal Hypo 22 Night Following Exercise 20 14 15 Closed Loop Open Loop 10 5 3 1 0 p=0. 05 p=0. 06
 Summary and Conclusions • CL control was associated with: – Greater time within target range at night compared to OL for both sedentary and exercise days – Fewer episodes of frank hypoglycemia • Use of a CL, even if only at night, may be effective in reducing hypoglycemia • Prandial glycemic excursions still undesirable
Summary and Conclusions • CL control was associated with: – Greater time within target range at night compared to OL for both sedentary and exercise days – Fewer episodes of frank hypoglycemia • Use of a CL, even if only at night, may be effective in reducing hypoglycemia • Prandial glycemic excursions still undesirable
 Next study questions • Can the addition of pramlintide improve the performance of a CL system by reducing the peak post-prandial glucose excursions?
Next study questions • Can the addition of pramlintide improve the performance of a CL system by reducing the peak post-prandial glucose excursions?
 Pramlintide • Analog of human amylin • Co-secreted with insulin from -cell • Used as adjunct to insulin in T 1 D to reduce postprandial glycemic excursions – Delay gastric emptying – Suppress endogenous glucagon
Pramlintide • Analog of human amylin • Co-secreted with insulin from -cell • Used as adjunct to insulin in T 1 D to reduce postprandial glycemic excursions – Delay gastric emptying – Suppress endogenous glucagon
 Study Protocol • 8 subjects admitted to Inpatient HRU for CL control • Two 24 -h evaluation periods: 8 AM d#2 - 8 AM d#4 • Meals provided at 8 AM, 1 PM, and 6 PM. Subjects consume identical meals under both conditions. • Pramlintide 30 mcg given prior to each meal on one study day • Hypoglycemia 60 mg/d. L (3. 3 mmol)
Study Protocol • 8 subjects admitted to Inpatient HRU for CL control • Two 24 -h evaluation periods: 8 AM d#2 - 8 AM d#4 • Meals provided at 8 AM, 1 PM, and 6 PM. Subjects consume identical meals under both conditions. • Pramlintide 30 mcg given prior to each meal on one study day • Hypoglycemia 60 mg/d. L (3. 3 mmol)
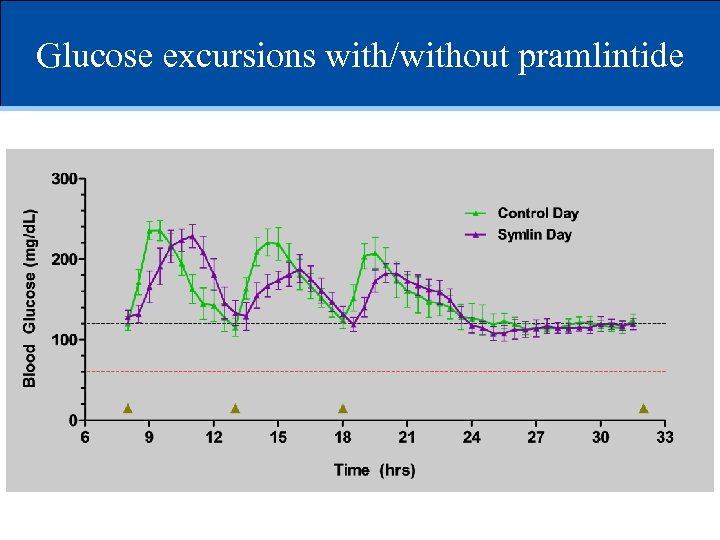 Glucose excursions with/without pramlintide
Glucose excursions with/without pramlintide
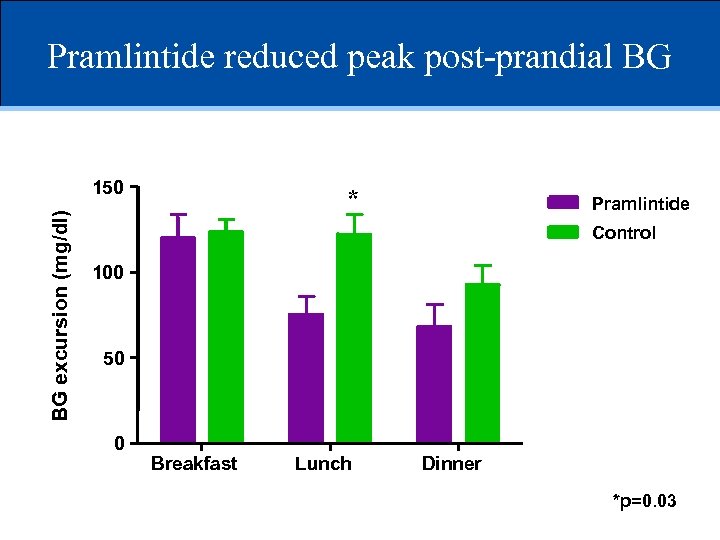 Pramlintide reduced peak post-prandial BG BG excursion (mg/dl) 150 * Pramlintide Control 100 50 0 Breakfast Lunch Dinner *p=0. 03
Pramlintide reduced peak post-prandial BG BG excursion (mg/dl) 150 * Pramlintide Control 100 50 0 Breakfast Lunch Dinner *p=0. 03
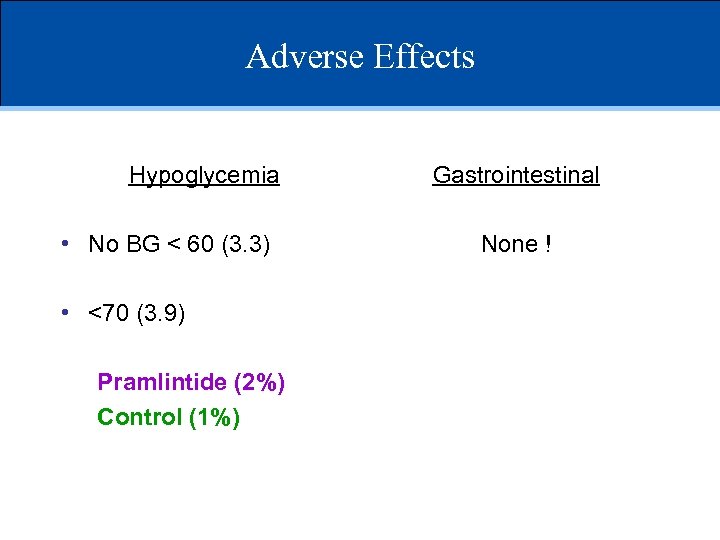 Adverse Effects Hypoglycemia • No BG < 60 (3. 3) • <70 (3. 9) Pramlintide (2%) Control (1%) Gastrointestinal None !
Adverse Effects Hypoglycemia • No BG < 60 (3. 3) • <70 (3. 9) Pramlintide (2%) Control (1%) Gastrointestinal None !
 Summary and conclusions • Pramlintide had modest effect on prandial glucose • Would require manual injection or at best, manual bolus • Faster insulin absorption / action clearly needed
Summary and conclusions • Pramlintide had modest effect on prandial glucose • Would require manual injection or at best, manual bolus • Faster insulin absorption / action clearly needed
 Next Steps • Evaluation of other incretins • Strategies to accelerate insulin absorption / action
Next Steps • Evaluation of other incretins • Strategies to accelerate insulin absorption / action
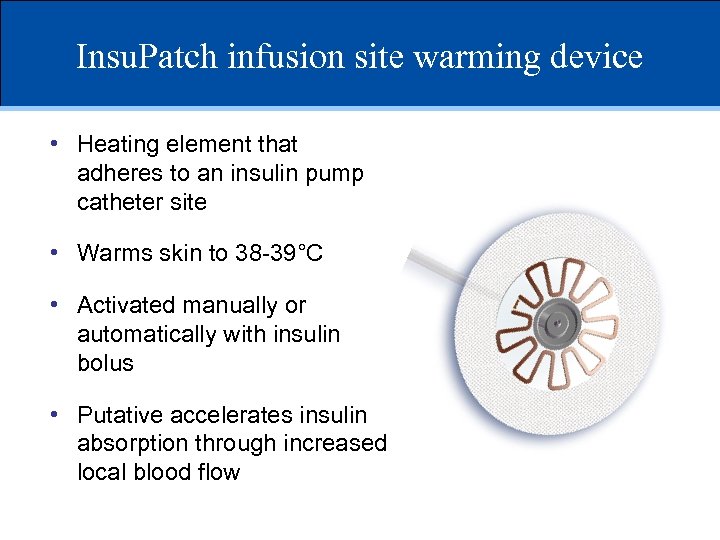 Insu. Patch infusion site warming device • Heating element that adheres to an insulin pump catheter site • Warms skin to 38 -39°C • Activated manually or automatically with insulin bolus • Putative accelerates insulin absorption through increased local blood flow
Insu. Patch infusion site warming device • Heating element that adheres to an insulin pump catheter site • Warms skin to 38 -39°C • Activated manually or automatically with insulin bolus • Putative accelerates insulin absorption through increased local blood flow
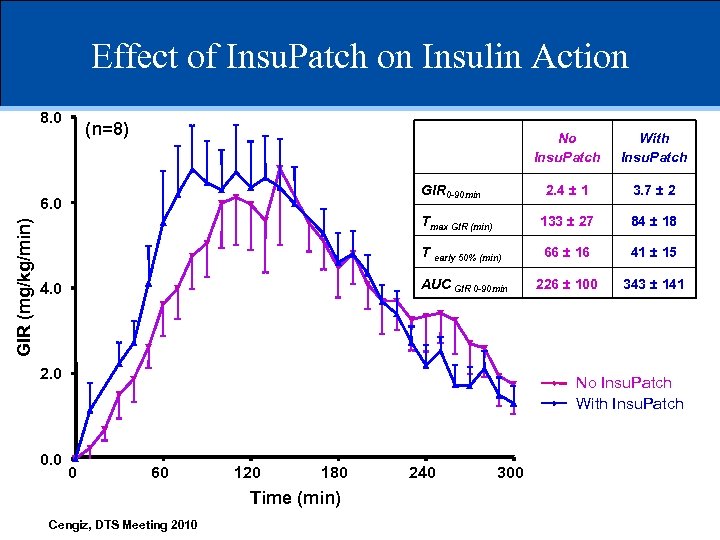 Effect of Insu. Patch on Insulin Action 8. 0 (n=8) No Insu. Patch 2. 4 ± 1 3. 7 ± 2 Tmax GIR (min) 133 ± 27 84 ± 18 T early 50% (min) 66 ± 16 41 ± 15 AUC GIR 0 -90 min 226 ± 100 343 ± 141 GIR 0 -90 min 6. 0 GIR (mg/kg/min) With Insu. Patch 4. 0 2. 0 0. 0 No Insu. Patch With Insu. Patch 0 60 120 180 Time (min) Cengiz, DTS Meeting 2010 240 300
Effect of Insu. Patch on Insulin Action 8. 0 (n=8) No Insu. Patch 2. 4 ± 1 3. 7 ± 2 Tmax GIR (min) 133 ± 27 84 ± 18 T early 50% (min) 66 ± 16 41 ± 15 AUC GIR 0 -90 min 226 ± 100 343 ± 141 GIR 0 -90 min 6. 0 GIR (mg/kg/min) With Insu. Patch 4. 0 2. 0 0. 0 No Insu. Patch With Insu. Patch 0 60 120 180 Time (min) Cengiz, DTS Meeting 2010 240 300
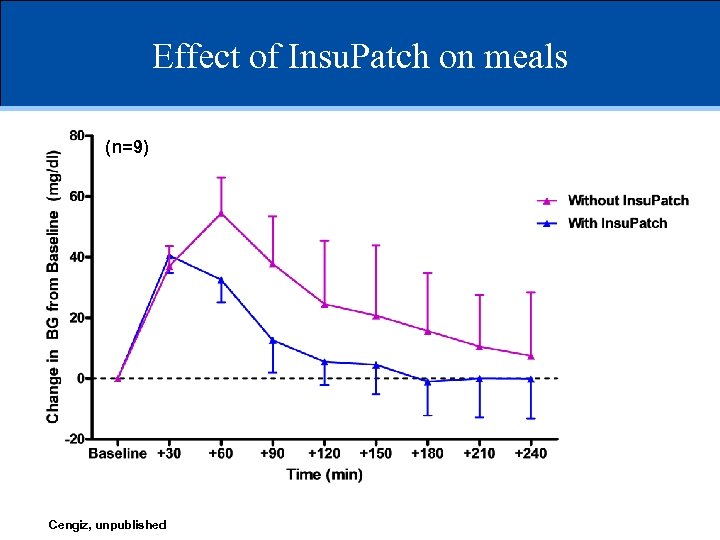 Effect of Insu. Patch on meals (n=9) Cengiz, unpublished
Effect of Insu. Patch on meals (n=9) Cengiz, unpublished
 Other approaches to AP • “Control to Range” – OL when BGs within target – Automatic pump suspension for actual or predicted hypoglycemia – Pump augmentation for hyperglycemia
Other approaches to AP • “Control to Range” – OL when BGs within target – Automatic pump suspension for actual or predicted hypoglycemia – Pump augmentation for hyperglycemia
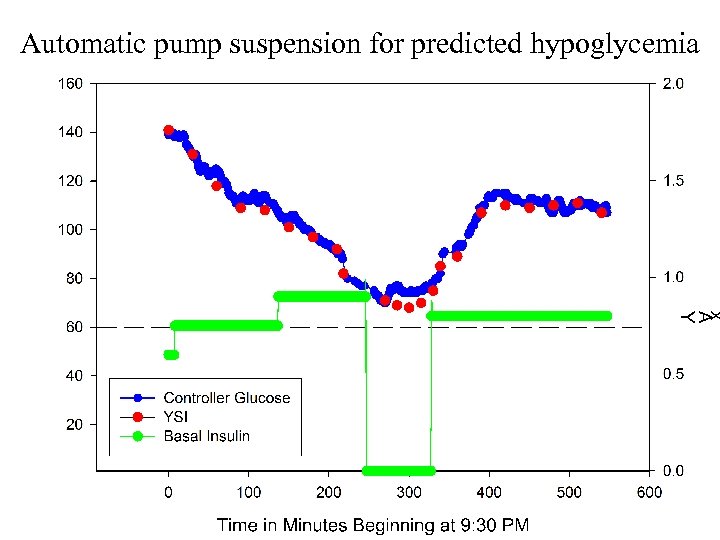 Automatic pump suspension for predicted hypoglycemia
Automatic pump suspension for predicted hypoglycemia
 The take-home message • Pumps and sensors are becoming increasingly integrated and automated, but self-care burden is still high • Full CL delivery is possible with current technologies but will likely require manual interfaces to completely optimize BG control • Dual hormonal control will improve performance of CL systems but will additional regulatory complexity • Path to a true product will be iterative
The take-home message • Pumps and sensors are becoming increasingly integrated and automated, but self-care burden is still high • Full CL delivery is possible with current technologies but will likely require manual interfaces to completely optimize BG control • Dual hormonal control will improve performance of CL systems but will additional regulatory complexity • Path to a true product will be iterative
 Thank you! • Yale Closed Loop Team – – – – – Stu Weinzimer Jennifer Sherr Eda Cengiz William Tamborlane Grace Kim Lori Carria Amy Steffen Kate Weyman Melinda Zgorski
Thank you! • Yale Closed Loop Team – – – – – Stu Weinzimer Jennifer Sherr Eda Cengiz William Tamborlane Grace Kim Lori Carria Amy Steffen Kate Weyman Melinda Zgorski


