e3bec840e997a40f68bc4404417aadab.ppt
- Количество слайдов: 25
 Current Sequence Listing Process for Nucleic Acids and Polypeptides Dave Nguyen Technology Center 1600
Current Sequence Listing Process for Nucleic Acids and Polypeptides Dave Nguyen Technology Center 1600
 Current Sequence Listing Process Technology Center 1600 (TC 1600) is a primary stakeholder in the current sequence listing process and is taking a major role in – Conducting Automation Workgroup Meetings (AWG) on a biweekly basis to sustain the USPTO business function of performing quality searching of molecular sequences by TC 1600 – Sustaining the PTO's best business practices for processing genetic sequences claimed in patent applications – Continuing to improve our business process for maintaining the infrastructure for processing molecular sequence listings and searching genetic sequences from applicant submissions and commercial databases of published sequence listings. 2
Current Sequence Listing Process Technology Center 1600 (TC 1600) is a primary stakeholder in the current sequence listing process and is taking a major role in – Conducting Automation Workgroup Meetings (AWG) on a biweekly basis to sustain the USPTO business function of performing quality searching of molecular sequences by TC 1600 – Sustaining the PTO's best business practices for processing genetic sequences claimed in patent applications – Continuing to improve our business process for maintaining the infrastructure for processing molecular sequence listings and searching genetic sequences from applicant submissions and commercial databases of published sequence listings. 2
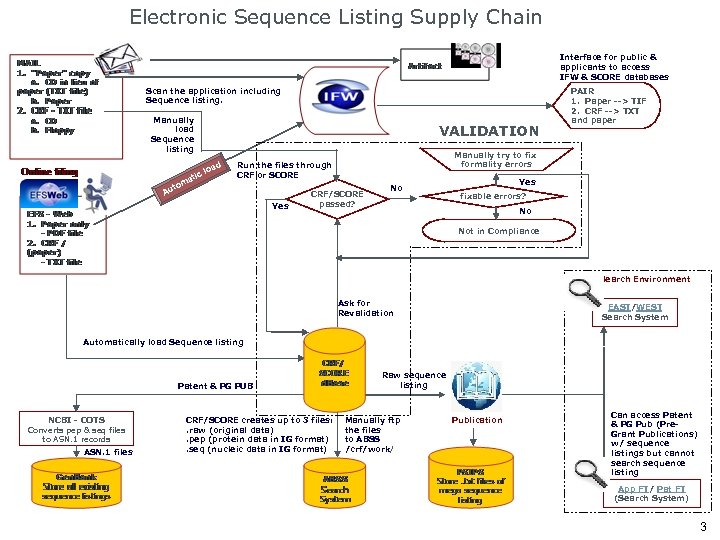 Electronic Sequence Listing Supply Chain Interface for public & applicants to access IFW & SCORE databases Scan the application including Sequence listing. Manually load Sequence listing c ati m uto VALIDATION d loa Manually try to fix formality errors Run the files through CRF or SCORE A Yes PAIR 1. Paper --> TIF 2. CRF --> TXT and paper CRF/SCORE passed? No Yes fixable errors? No Not in Compliance Search Environment Ask for Revalidation EAST/WEST Search System Automatically load Sequence listing Patent & PG PUB NCBI - COTS Converts pep & seq files to ASN. 1 records ASN. 1 files CRF/SCORE creates up to 3 files: . raw (original data). pep (protein data in IG format). seq (nucleic data in IG format) Raw sequence listing Manually ftp the files to ABSS /crf/work/ Publication Can access Patent & PG Pub (Pre. Grant Publications) w/ sequence listings but cannot search sequence listing App FT/ Pat FT (Search System) 3
Electronic Sequence Listing Supply Chain Interface for public & applicants to access IFW & SCORE databases Scan the application including Sequence listing. Manually load Sequence listing c ati m uto VALIDATION d loa Manually try to fix formality errors Run the files through CRF or SCORE A Yes PAIR 1. Paper --> TIF 2. CRF --> TXT and paper CRF/SCORE passed? No Yes fixable errors? No Not in Compliance Search Environment Ask for Revalidation EAST/WEST Search System Automatically load Sequence listing Patent & PG PUB NCBI - COTS Converts pep & seq files to ASN. 1 records ASN. 1 files CRF/SCORE creates up to 3 files: . raw (original data). pep (protein data in IG format). seq (nucleic data in IG format) Raw sequence listing Manually ftp the files to ABSS /crf/work/ Publication Can access Patent & PG Pub (Pre. Grant Publications) w/ sequence listings but cannot search sequence listing App FT/ Pat FT (Search System) 3
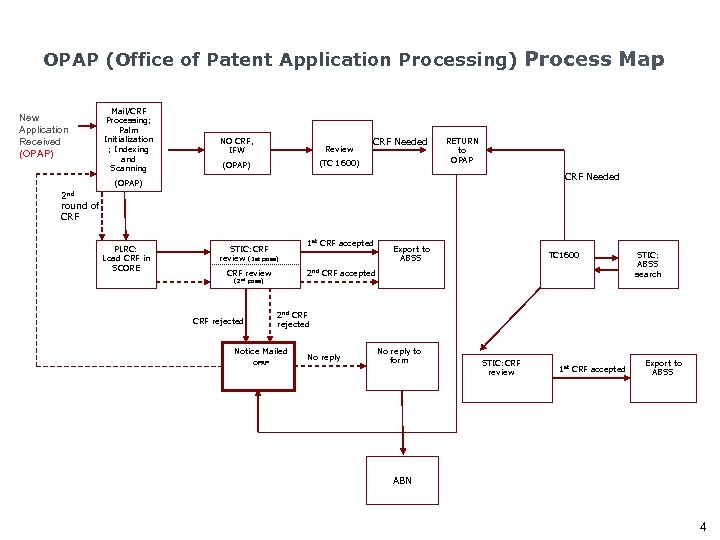 OPAP (Office of Patent Application Processing) Process Map New Application Received (OPAP) Mail/CRF Processing; Palm Initialization ; Indexing and Scanning NO CRF, IFW Review (OPAP) CRF Needed (TC 1600) RETURN to OPAP CRF Needed (OPAP) 2 nd round of CRF PLRC: Load CRF in SCORE STIC: CRF review (1 st pass) 1 st CRF accepted Export to ABSS TC 1600 2 nd CRF accepted CRF review (2 nd pass) STIC: ABSS search 2 nd CRF rejected Notice Mailed OPAP No reply to form STIC: CRF review 1 st CRF accepted Export to ABSS ABN 4
OPAP (Office of Patent Application Processing) Process Map New Application Received (OPAP) Mail/CRF Processing; Palm Initialization ; Indexing and Scanning NO CRF, IFW Review (OPAP) CRF Needed (TC 1600) RETURN to OPAP CRF Needed (OPAP) 2 nd round of CRF PLRC: Load CRF in SCORE STIC: CRF review (1 st pass) 1 st CRF accepted Export to ABSS TC 1600 2 nd CRF accepted CRF review (2 nd pass) STIC: ABSS search 2 nd CRF rejected Notice Mailed OPAP No reply to form STIC: CRF review 1 st CRF accepted Export to ABSS ABN 4
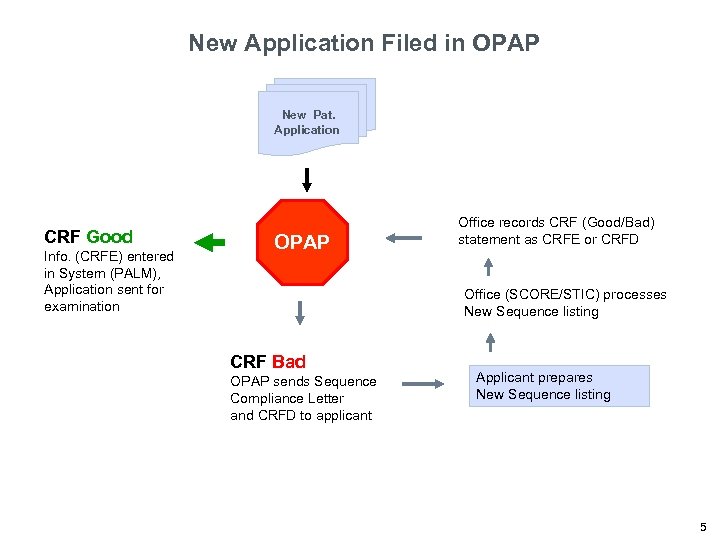 New Application Filed in OPAP New Pat. Application CRF Good Info. (CRFE) entered in System (PALM), Application sent for examination OPAP Office records CRF (Good/Bad) statement as CRFE or CRFD Office (SCORE/STIC) processes New Sequence listing CRF Bad OPAP sends Sequence Compliance Letter and CRFD to applicant Applicant prepares New Sequence listing 5
New Application Filed in OPAP New Pat. Application CRF Good Info. (CRFE) entered in System (PALM), Application sent for examination OPAP Office records CRF (Good/Bad) statement as CRFE or CRFD Office (SCORE/STIC) processes New Sequence listing CRF Bad OPAP sends Sequence Compliance Letter and CRFD to applicant Applicant prepares New Sequence listing 5
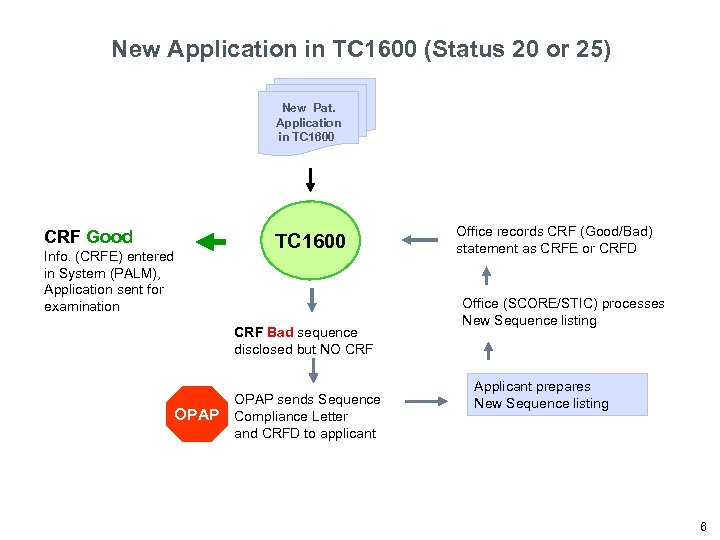 New Application in TC 1600 (Status 20 or 25) New Pat. Application in TC 1600 CRF Good Info. (CRFE) entered in System (PALM), Application sent for examination TC 1600 CRF Bad sequence disclosed but NO CRF OPAP sends Sequence OPAP Compliance Letter Office records CRF (Good/Bad) statement as CRFE or CRFD Office (SCORE/STIC) processes New Sequence listing Applicant prepares New Sequence listing and CRFD to applicant 6
New Application in TC 1600 (Status 20 or 25) New Pat. Application in TC 1600 CRF Good Info. (CRFE) entered in System (PALM), Application sent for examination TC 1600 CRF Bad sequence disclosed but NO CRF OPAP sends Sequence OPAP Compliance Letter Office records CRF (Good/Bad) statement as CRFE or CRFD Office (SCORE/STIC) processes New Sequence listing Applicant prepares New Sequence listing and CRFD to applicant 6
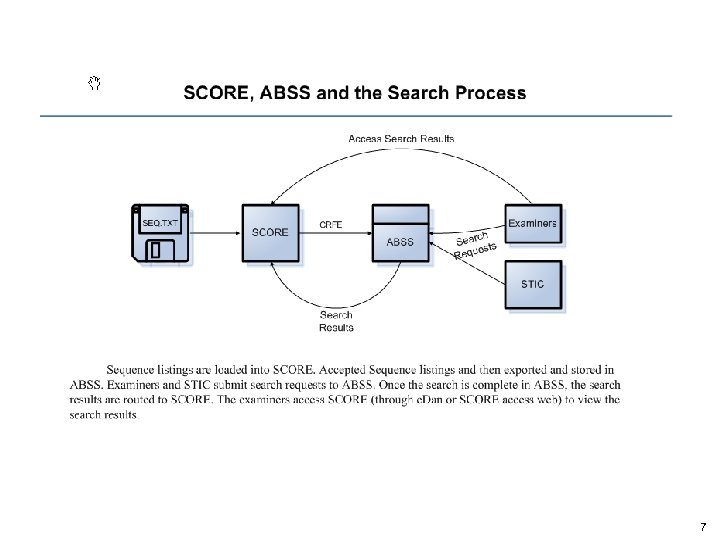 7
7
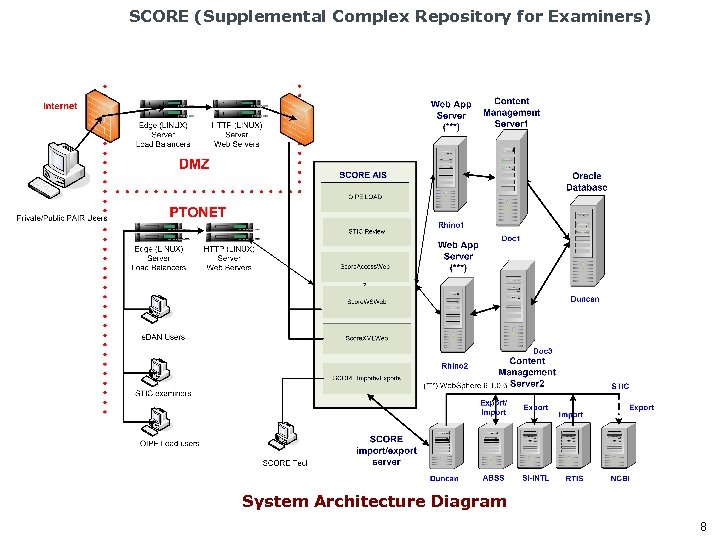 SCORE (Supplemental Complex Repository for Examiners) System Architecture Diagram 8
SCORE (Supplemental Complex Repository for Examiners) System Architecture Diagram 8
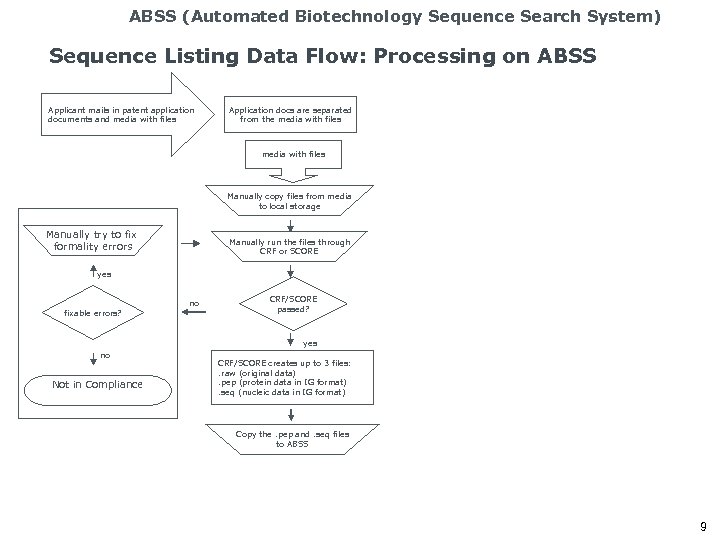 ABSS (Automated Biotechnology Sequence Search System) Sequence Listing Data Flow: Processing on ABSS Applicant mails in patent application documents and media with files Application docs are separated from the media with files Manually copy files from media to local storage Manually try to fix formality errors Manually run the files through CRF or SCORE yes fixable errors? no CRF/SCORE passed? yes no Not in Compliance CRF/SCORE creates up to 3 files: . raw (original data). pep (protein data in IG format). seq (nucleic data in IG format) Copy the. pep and. seq files to ABSS 9
ABSS (Automated Biotechnology Sequence Search System) Sequence Listing Data Flow: Processing on ABSS Applicant mails in patent application documents and media with files Application docs are separated from the media with files Manually copy files from media to local storage Manually try to fix formality errors Manually run the files through CRF or SCORE yes fixable errors? no CRF/SCORE passed? yes no Not in Compliance CRF/SCORE creates up to 3 files: . raw (original data). pep (protein data in IG format). seq (nucleic data in IG format) Copy the. pep and. seq files to ABSS 9
 Guidance on Common Issues Joseph Woitach Technology Center 1600
Guidance on Common Issues Joseph Woitach Technology Center 1600
 What Sequence Rules Apply? • US Rules - 37 CFR 1. 821 -825 – Original rules -- 55 Fed. Reg. 18230 (May 1, 1990) (effective October 1, 1990) – Amended rules -- 63 Fed. Reg. 29620 (June 1 1998) (effective July 1, 1998). • International Rules - WIPO Standard ST. 25, effective July 1, 1998 – http: //www. wipo. int/standards/en/pdf/03 -2501. pdf 11
What Sequence Rules Apply? • US Rules - 37 CFR 1. 821 -825 – Original rules -- 55 Fed. Reg. 18230 (May 1, 1990) (effective October 1, 1990) – Amended rules -- 63 Fed. Reg. 29620 (June 1 1998) (effective July 1, 1998). • International Rules - WIPO Standard ST. 25, effective July 1, 1998 – http: //www. wipo. int/standards/en/pdf/03 -2501. pdf 11
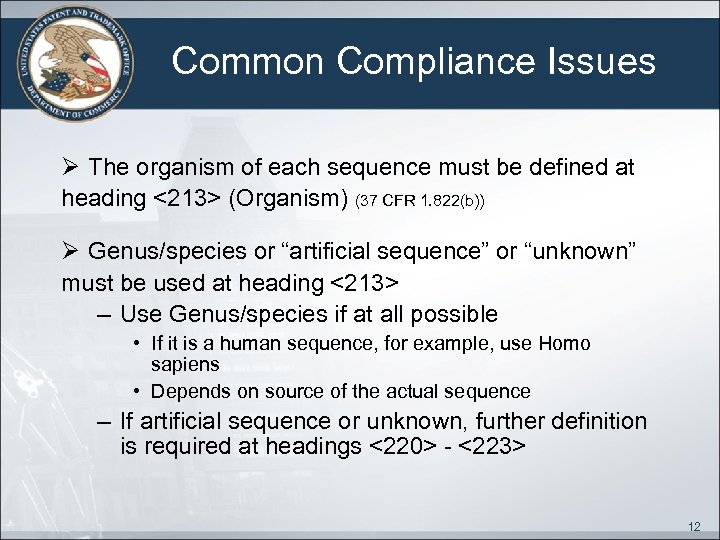 Common Compliance Issues Ø The organism of each sequence must be defined at heading <213> (Organism) (37 CFR 1. 822(b)) Ø Genus/species or “artificial sequence” or “unknown” must be used at heading <213> – Use Genus/species if at all possible • If it is a human sequence, for example, use Homo sapiens • Depends on source of the actual sequence – If artificial sequence or unknown, further definition is required at headings <220> - <223> 12
Common Compliance Issues Ø The organism of each sequence must be defined at heading <213> (Organism) (37 CFR 1. 822(b)) Ø Genus/species or “artificial sequence” or “unknown” must be used at heading <213> – Use Genus/species if at all possible • If it is a human sequence, for example, use Homo sapiens • Depends on source of the actual sequence – If artificial sequence or unknown, further definition is required at headings <220> - <223> 12
 Common Compliance Issues Artificial Sequence at heading <213>? Explain why the sequence is artificial (not naturally-occurring) If a portion of the sequence was derived from a natural source, e. g. , a bacterial gene, then provide the source and explain how the sequence differs from the naturally occurring source material 13
Common Compliance Issues Artificial Sequence at heading <213>? Explain why the sequence is artificial (not naturally-occurring) If a portion of the sequence was derived from a natural source, e. g. , a bacterial gene, then provide the source and explain how the sequence differs from the naturally occurring source material 13
 Common Compliance Issues Unknown – Use if there is no scientific name disclosed or only a partial scientific name, e. g. , Bacillus sp. – Use if only the source of the organism is disclosed, e. g. , “soil sample from Pittsburgh” – Example sequence listing section: • <213> Unknown • <223> Bacillus species 14
Common Compliance Issues Unknown – Use if there is no scientific name disclosed or only a partial scientific name, e. g. , Bacillus sp. – Use if only the source of the organism is disclosed, e. g. , “soil sample from Pittsburgh” – Example sequence listing section: • <213> Unknown • <223> Bacillus species 14
 Sequence Listing Examples Example 1 Example 2 15
Sequence Listing Examples Example 1 Example 2 15
 Common compliance issues Ø Sequences having a gap or gaps must be displayed as separate sequences in the Sequence Listing. For example, if a chemical moiety has several strands of protein attached to it, each protein sequence should appear in the Sequence Listing separately. The chemical moiety should NOT be shown (37 CFR 1. 822(e)) Ø Sequences made of fragments of other sequences must be displayed as separate sequences in the Sequence Listing (37 CFR 1. 822(e)) 16
Common compliance issues Ø Sequences having a gap or gaps must be displayed as separate sequences in the Sequence Listing. For example, if a chemical moiety has several strands of protein attached to it, each protein sequence should appear in the Sequence Listing separately. The chemical moiety should NOT be shown (37 CFR 1. 822(e)) Ø Sequences made of fragments of other sequences must be displayed as separate sequences in the Sequence Listing (37 CFR 1. 822(e)) 16
 Common compliance issues Ø Sequence Listings are often non-compliant because of minor formatting issues- Use of Patent. In minimizes such occurrences Ø Patent. In’s “Copy to Disk” function results in loss of hard returns on the CRF - Regenerate the Sequence Listing and use Windows Explorer to copy the text file to the CRF 17
Common compliance issues Ø Sequence Listings are often non-compliant because of minor formatting issues- Use of Patent. In minimizes such occurrences Ø Patent. In’s “Copy to Disk” function results in loss of hard returns on the CRF - Regenerate the Sequence Listing and use Windows Explorer to copy the text file to the CRF 17
 Filing option • Electronic Filing System (EFS) - Legal Framework: http: //www. uspto. gov/patents/process/file/efs/guidance/New_legal_framework. jsp 18
Filing option • Electronic Filing System (EFS) - Legal Framework: http: //www. uspto. gov/patents/process/file/efs/guidance/New_legal_framework. jsp 18
 Filing option • Electronic Filing System (EFS) – Learn about EFS at this website: http: //www. uspto. gov/ebc/efs_help. html • Add the sequence listing to your EFS-Web submission • No paper copy or statement needed for initial filing – If filing in response to a Notice to Comply a statement that there is no new matter is needed. – Sequence listing is automatically processed by SCORE and immediately placed in ABSS (if compliant) 19
Filing option • Electronic Filing System (EFS) – Learn about EFS at this website: http: //www. uspto. gov/ebc/efs_help. html • Add the sequence listing to your EFS-Web submission • No paper copy or statement needed for initial filing – If filing in response to a Notice to Comply a statement that there is no new matter is needed. – Sequence listing is automatically processed by SCORE and immediately placed in ABSS (if compliant) 19
 FAQs Why does my CRF has an error on Field Code <213>? Numeric identifier <213> is something other than “Scientific name, i. e. , Genus/species, Unknown or Artificial Sequence” 20
FAQs Why does my CRF has an error on Field Code <213>? Numeric identifier <213> is something other than “Scientific name, i. e. , Genus/species, Unknown or Artificial Sequence” 20
 FAQs Why does my CRF has an error on Field Code <223>? A major reason for noncompliance is that the information provided in field <223> to explain an artificial or unknown organism is improper. Solution: Indicating what the artificial sequences are is acceptable, e. g. , primer, aptamer, linker, adapter, cloning vector, expression vector, si. RNA, probe, expressed sequence tag, etc. Chimeric constructs should identify sources of the parts, etc. 21
FAQs Why does my CRF has an error on Field Code <223>? A major reason for noncompliance is that the information provided in field <223> to explain an artificial or unknown organism is improper. Solution: Indicating what the artificial sequences are is acceptable, e. g. , primer, aptamer, linker, adapter, cloning vector, expression vector, si. RNA, probe, expressed sequence tag, etc. Chimeric constructs should identify sources of the parts, etc. 21
 FAQs • Do genes identified by gene accession numbers in the specification need to comply with the sequence rule requirements? 22
FAQs • Do genes identified by gene accession numbers in the specification need to comply with the sequence rule requirements? 22
 FAQs No, they are not considered disclosures of Sequences. When accession numbers appear in claims, they may raise an issue of improper incorporation of essential material by reference. If the sequences need to be brought into the disclosure then they must comply with the sequence rules. 23
FAQs No, they are not considered disclosures of Sequences. When accession numbers appear in claims, they may raise an issue of improper incorporation of essential material by reference. If the sequences need to be brought into the disclosure then they must comply with the sequence rules. 23
 FAQs A sequence listing was prepared via Patent. In. Why did the sequence listing submission get rejected by the Patent Office? The USPTO uses an in-house verification software for validation. In addition, information provided in field <223> for artificial sequence or unknown organism must be manually verified. 24
FAQs A sequence listing was prepared via Patent. In. Why did the sequence listing submission get rejected by the Patent Office? The USPTO uses an in-house verification software for validation. In addition, information provided in field <223> for artificial sequence or unknown organism must be manually verified. 24
 Q and A Thank You! Dave Nguyen TC 1600 Management Dave. Nguyen@uspto. gov 571 -272 -0731 Joseph Woitach TC 1600 Management Joseph. Woitach@uspto. gov 571 -272 -0739 25
Q and A Thank You! Dave Nguyen TC 1600 Management Dave. Nguyen@uspto. gov 571 -272 -0731 Joseph Woitach TC 1600 Management Joseph. Woitach@uspto. gov 571 -272 -0739 25


