0cb261587594747d2868cdbec9089048.ppt
- Количество слайдов: 37

CUBICIN® (daptomycin for injection) for S. aureus Bacteremia Including Those With Known or Suspected Endocarditis Anti-Infective Drugs Advisory Committee Meeting March 6, 2006 C-1

Staphylococcus aureus Bacteremia Henry F. Chambers, M. D. Professor of Medicine, UCSF Chief of Infectious Diseases San Francisco General Hospital C-2

Case 1 • 38 y/o man, new CHF, alcoholic cardiomyopathy, Hct = 13 (normal 40 -45) • Given PRBCs, diuretics, afterload reducers • HD 6: upper + lower endoscopy • Post-procedure T = 39 o. C, blood cultures taken • HD 7: afebrile but BC x 2 = GPC in clusters; R forearm former IV site red, tender, indurated • Vancomycin administered • HD 8: BC isolate = MSSA; f/u BC sterile C-3

Management Issues • What is the risk of a poor outcome? • What antibiotic should be used? • What is the duration of therapy? C-4
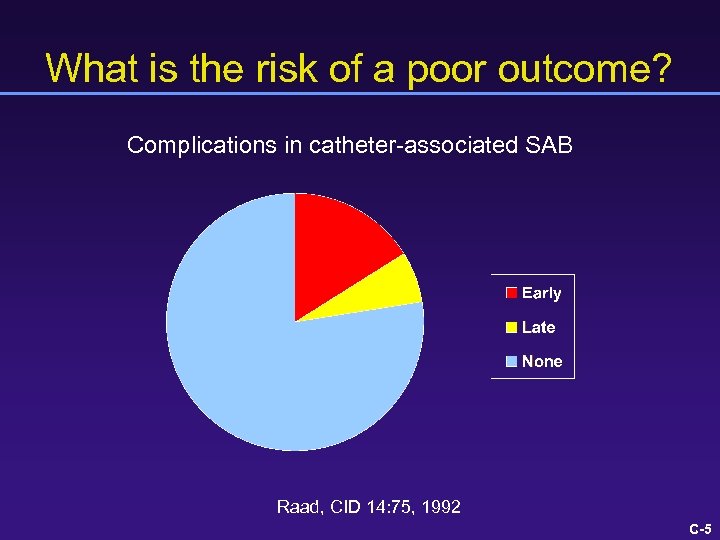
What is the risk of a poor outcome? Complications in catheter-associated SAB Raad, CID 14: 75, 1992 C-5
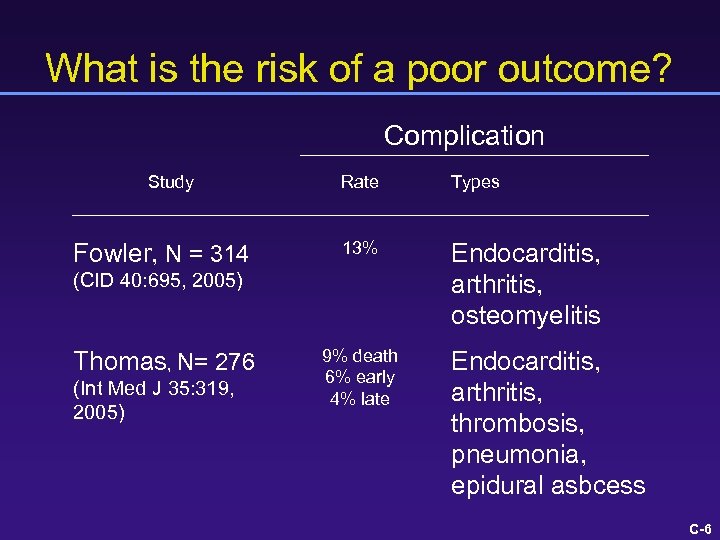
What is the risk of a poor outcome? Complication Study Fowler, N = 314 Rate Types 13% Endocarditis, arthritis, osteomyelitis (CID 40: 695, 2005) Thomas, N= 276 (Int Med J 35: 319, 2005) 9% death 6% early 4% late Endocarditis, arthritis, thrombosis, pneumonia, epidural asbcess C-6
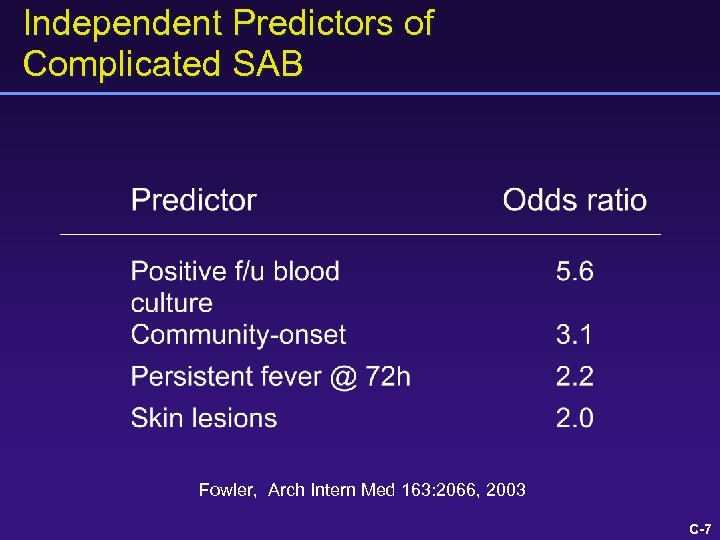
Independent Predictors of Complicated SAB Fowler, Arch Intern Med 163: 2066, 2003 C-7
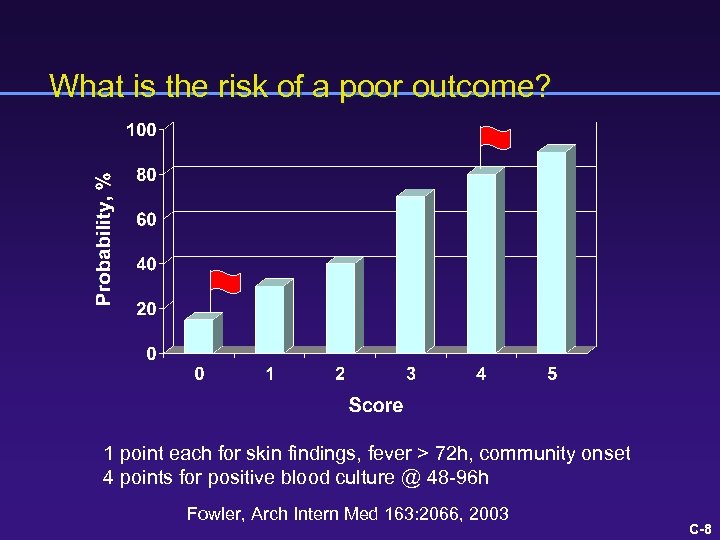
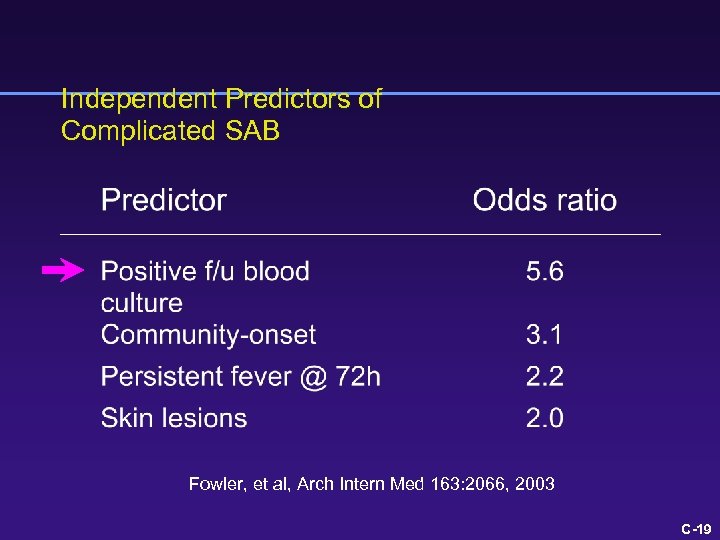
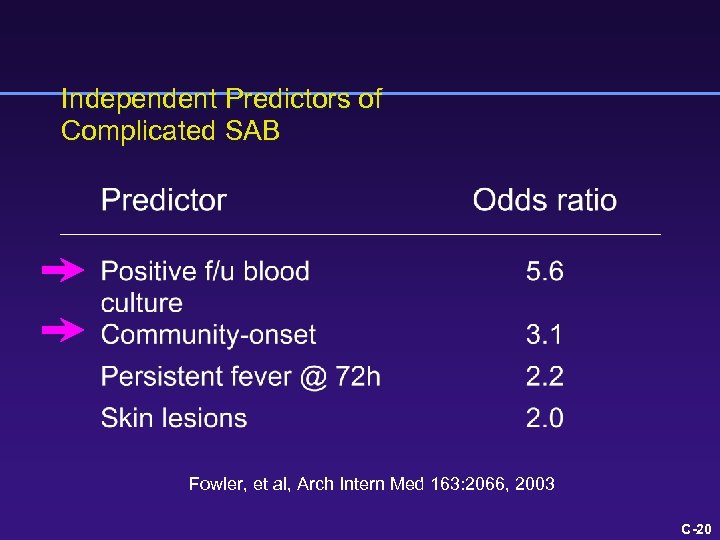
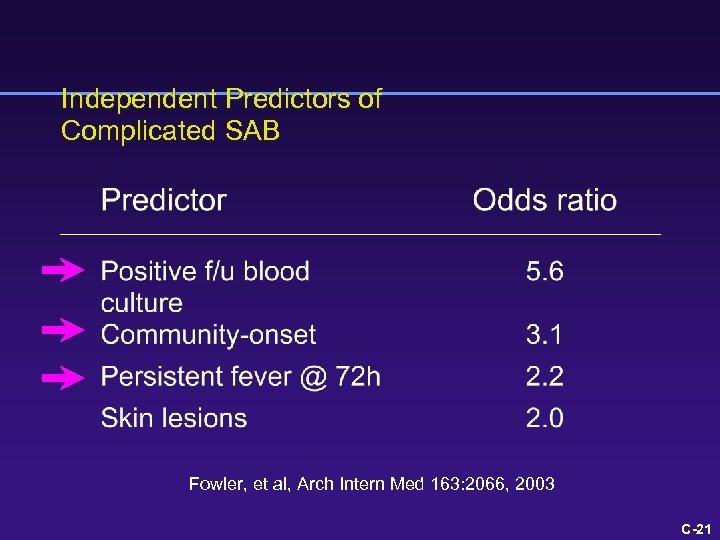
What is the risk of a poor outcome? 1 point each for skin findings, fever > 72 h, community onset 4 points for positive blood culture @ 48 -96 h Fowler, Arch Intern Med 163: 2066, 2003 C-8
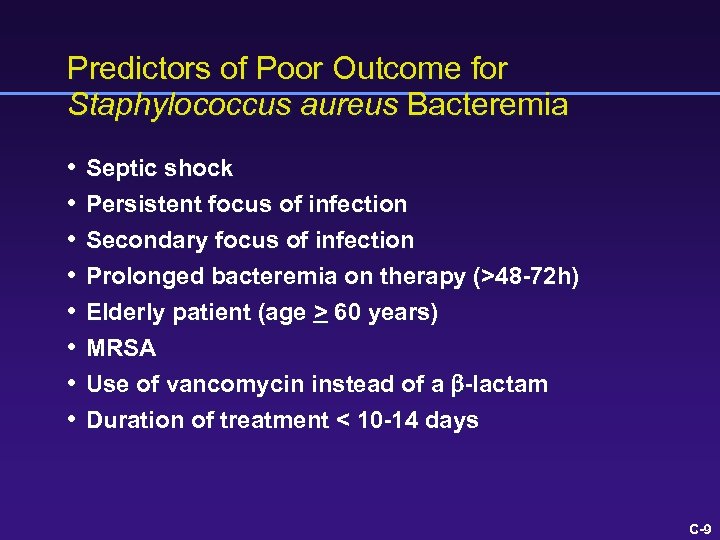
Predictors of Poor Outcome for Staphylococcus aureus Bacteremia • Septic shock • Persistent focus of infection • Secondary focus of infection • Prolonged bacteremia on therapy (>48 -72 h) • Elderly patient (age > 60 years) • MRSA • Use of vancomycin instead of a b-lactam • Duration of treatment < 10 -14 days C-9

Criteria for Antimicrobial Therapy of Staphylococcus aureus Bacteremia 1. 2. 3. 4. Bactericidal activity Non-toxic, well-tolerated Parenteral administration, at least initially Convenient dosing C-10

What antibiotic should be used? “If the focus of infection has been promptly removed with rapid documented resolution of the bacteremia (< 3 days), 2 weeks of antibiotic therapy with a penicillinase-resistant penicillin, first-generation cephalosporin, or glycopeptide is likely to be enough…. . Under no circumstances should patients simply have the catheter removed without antibiotic treatment. ” Antimicrobial Therapy and Vaccines, 2 nd Ed. , 2002, page 641 C-11

What antibiotic should be used? Fowler, CID 27: 478 -86, 1998 C-12
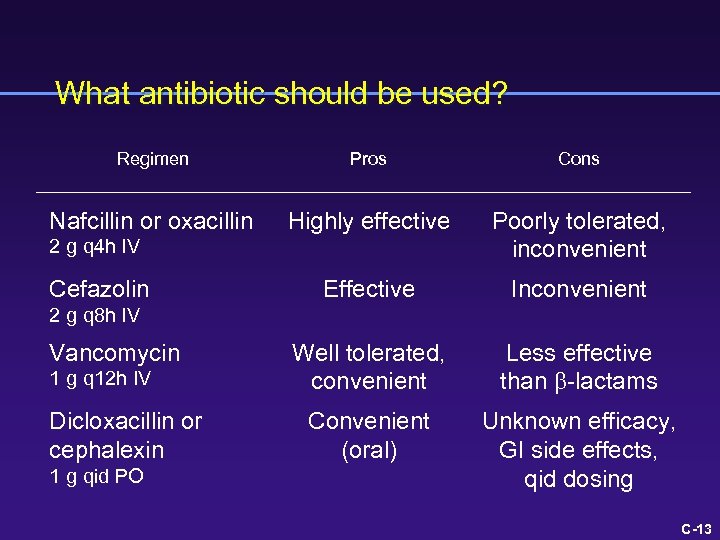
What antibiotic should be used? Regimen Pros Cons Nafcillin or oxacillin Highly effective Poorly tolerated, inconvenient Effective Inconvenient Well tolerated, convenient Less effective than b-lactams Convenient (oral) Unknown efficacy, GI side effects, qid dosing 2 g q 4 h IV Cefazolin 2 g q 8 h IV Vancomycin 1 g q 12 h IV Dicloxacillin or cephalexin 1 g qid PO C-13

What is the duration of therapy? 7 -10 or fewer days? – Associated with high relapse, complication rates 10 -14 days? – Standard recommended duration 4 -6 weeks? – For endocarditis, osteomyelitis, complicated SAB C-14

What was done? • PICC placed • Ceftriaxone 2 g IV q 24 h for 14 days • Home infusion therapy arranged C-15

Case 2 • 44 y/o man, homeless, IVDU with fever and back pain, non-localizing exam • Vancomycin administered • 3/3 BC positive MRSA • TTE negative, MRI spine negative • Fever persists during first week • 1/3 BC + MRSA 72 h after admission C-16
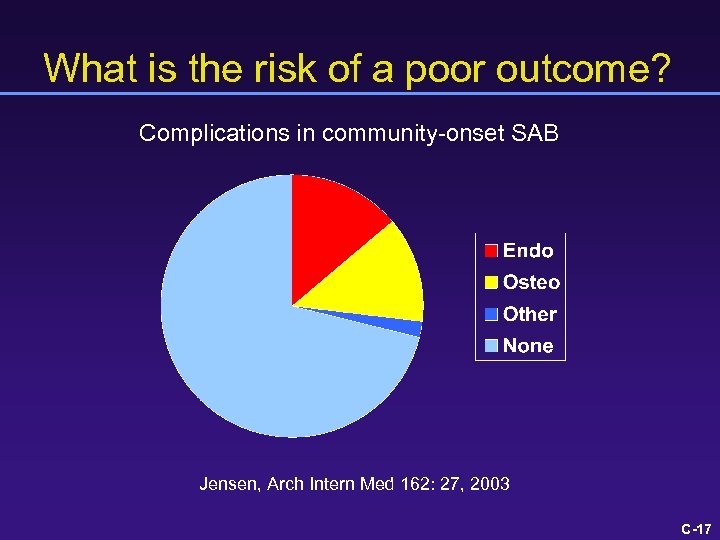
What is the risk of a poor outcome? Complications in community-onset SAB Jensen, Arch Intern Med 162: 27, 2003 C-17
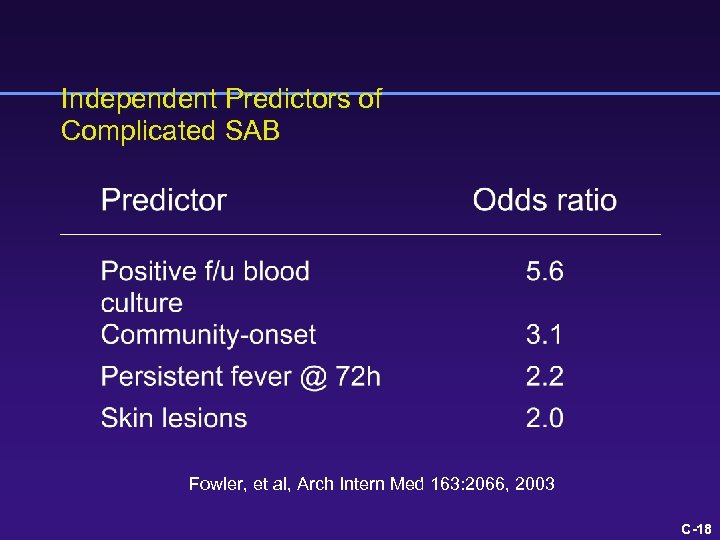
Independent Predictors of Complicated SAB Fowler, et al, Arch Intern Med 163: 2066, 2003 C-18
Independent Predictors of Complicated SAB Fowler, et al, Arch Intern Med 163: 2066, 2003 C-19
Independent Predictors of Complicated SAB Fowler, et al, Arch Intern Med 163: 2066, 2003 C-20
Independent Predictors of Complicated SAB Fowler, et al, Arch Intern Med 163: 2066, 2003 C-21

What antibiotic should be used? Regimen Pros Cons Nafcillin or oxacillin Highly effective Poorly tolerated, inconvenient Effective Inconvenient Well tolerated, convenient Less effective than b-lactams Convenient (oral) Unknown efficacy, GI side effects, qid dosing 2 g q 4 h IV Cefazolin 2 g q 8 h IV Vancomycin 1 g q 12 h IV Dicloxacillin or cephalexin 1 g qid PO C-22

What was done? • PICC placed • Methadone maintenance • Vancomycin ~1 g q 12 h IV for 6 weeks • Trough serum concentrations of ~15 mg/ml C-23

What happened? • Patient returned 3 mo later complaining of back pain • Afebrile, normal exam • Blood cultures negative • MRI: T 10 -T 11 osteomyelitis, discitis • Bone biopsy culture: MRSA C-24

What was done? • PICC placed • Methadone maintenance • Vancomycin ~1 g q 12 h IV for 6 weeks • Trough serum concentrations or ~15 mg/ml C-25

Management Issues • Is this a vancomcyin failure? • Is so, why did it fail? • What is the risk of a poor outcome now? • What antibiotic(s) should be used now? • What is the duration of therapy? C-26
“State of the Art” Treatment of Staphylococcus aureus Bacteremia • Current armamentarium is inadequate for – Out patient treatment – MRSA – Patients who fail or cannot tolerate therapy • Physicians often must rely on – Drugs not approved for treatment of complicated staphylococcal infections – Drugs of unknown or poorly documented efficacy – Second-line agents – Combinations of agents of uncertain benefit C-27

CUBICIN® (daptomycin for injection) for S. aureus Bacteremia Including Those With Known or Suspected Endocarditis David Mantus, Ph. D. Vice President, Regulatory Affairs Cubist Pharmaceuticals Adjunct Assistant Professor Massachusetts College of Pharmacy C-28

Adjudication Committee Member and Affiliation Elias Abrutyn, M. D. Associate Provost and Associate Dean for Faculty Affairs, and Interim Chief, Infectious Disease Drexel University G. Ralph Corey, M. D. Professor of Internal Medicine & Infectious Disease Duke University Medical Center Sara Cosgrove, M. D. Assistant Professor, Dept of Medicine, Div of Infectious Disease Johns Hopkins University School of Medicine and Johns Hopkins Bloomberg School of Public Health Vance G. Fowler, M. D. Associate Professor of Medicine Duke University Medical Center Adolf W. Karchmer, M. D. Professor of Medicine, Chief, Division of Infectious Diseases Beth Israel Deaconess Medical Center C-29

Experts Available for Questions and Answers Christopher H. Cabell, M. D. , M. H. S. , F. A. C. C. Assistant Professor of Medicine Division of Cardiology Duke University School of Medicine Henry F. “Chip” Chambers, M. D. Professor of Medicine University of California - San Francisco Chief, Division of Infectious Diseases San Francisco General Hospital George Drusano, M. D. Professor of Medicine and Pharmacology Albany Medical College Co-director Ordway Research Institute Donald Levine, M. D. Professor of Medicine Chief, General/Internal Medicine Wayne State University Albert Sheldon, Jr. , Ph. D. President C-30
What is Daptomycin? • Cyclic lipopeptide natural product • Approved (IV, 4 mg/kg q 24 h) for complicated skin and skin structure infections, including MRSA – – US 2003 Israel 2004 Argentina 2005 EU 2006 C-31
Post-licensure Experience • 150, 000+ patients treated – No new toxicities – ~1/3 of doses delivered in outpatient setting • Potency vs. S. aureus maintained – Microbiologic surveillance studies demonstrate > 99. 9% of isolates are daptomycin susceptible • ~25% of use is for bacteremia (off-label) – ~50% of this use at the 4 mg/kg dose approved for skin, NOT the 6 mg/kg dose studied in S. aureus bacteremia C-32

Rationale for Daptomycin in S. aureus Bacteremia and Endocarditis • Rapidly bactericidal in vitro and in vivo • Potency against MRSA and MSSA • Proven clinical efficacy in skin (MRSA and MSSA) • Proven efficacy in animal models of S. aureus endocarditis at 6 mg/kg human equivalent dose • Potential for outpatient treatment – Monotherapy – Once-daily C-33

Continuous Dialogue with FDA on Development • Study design (2001 -2002) – – Open-label Comparators Enrollment of all patients with S. aureus Data Safety Monitoring Board • Study assessments and analyses (2004 -2005) – Adjudication Committee – Primary endpoints – Statistical Analysis Plan agreed upon prior to unblinding • Study results (2005) – s. NDA filed – Priority review granted C-34
S. aureus Bacteremia and Endocarditis Supplemental Indication and Dose • Proposed Indication – Staphylococcus aureus bacteremia (SAB) including those with known or suspected endocarditis (SAIE) caused by methicillin-susceptible and methicillin-resistant strains • Proposed Dose – 6 mg/kg monotherapy administered as a 30 -minute intravenous (IV) infusion once per day for a minimum duration of 2 to 6 weeks, depending on the clinical condition C-35

Agenda Introduction David Mantus, Ph. D. Efficacy Helen Boucher, M. D. Microbiology Jeff Alder, Ph. D. Safety Gloria Vigliani, M. D. Conclusions G. Ralph Corey, M. D. V. P. Regulatory Affairs Cubist Pharmaceuticals Assistant Professor of Medicine Dir. Infectious Diseases Fellowship Program Div. of Infectious Diseases and Geographic Medicine Tufts University-NEMC V. P. Drug Discovery & Evaluation Cubist Pharmaceuticals V. P. Medical Strategy Cubist Pharmaceuticals Professor of Internal Medicine & Infectious Disease Duke University Medical Center C-36
Overall Findings Daptomycin 6 mg/kg once daily: • Effective in the treatment of S. aureus bacteremia and endocarditis – Response higher in MRSA • Well tolerated for extended treatment durations • Less nephrotoxic than standard-of-care agents • Provides a much needed option for treatment of patients with S. aureus bacteremia including those with known or suspected endocarditis C-37
0cb261587594747d2868cdbec9089048.ppt