2bf075b4453594674e8bf99700380660.ppt
- Количество слайдов: 32

CTOS-Seattle, November 2007 TERAPEUTIC CONSEQUENCES FROM MOLECOLAR BIOLOGY FOR GIST PATIENTS AFFECTED BY NEUROFIBROMATOSIS TYPE 1 Mussi C, Schildhaus HU, Gronchi A, Wardelmann E, Hohenberger P Mannheim University Hospital, Germany Bonn University Hospital, Germany Istituto Nazionale Tumori, Italy supported by Conticanet
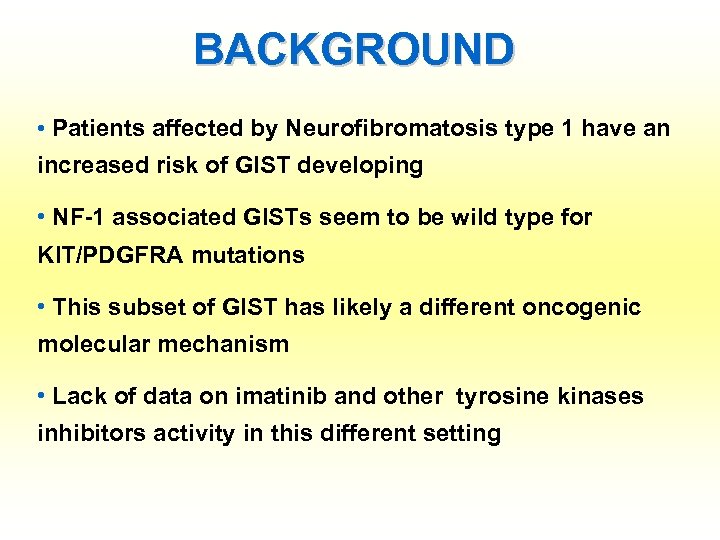
BACKGROUND • Patients affected by Neurofibromatosis type 1 have an increased risk of GIST developing • NF-1 associated GISTs seem to be wild type for KIT/PDGFRA mutations • This subset of GIST has likely a different oncogenic molecular mechanism • Lack of data on imatinib and other tyrosine kinases inhibitors activity in this different setting
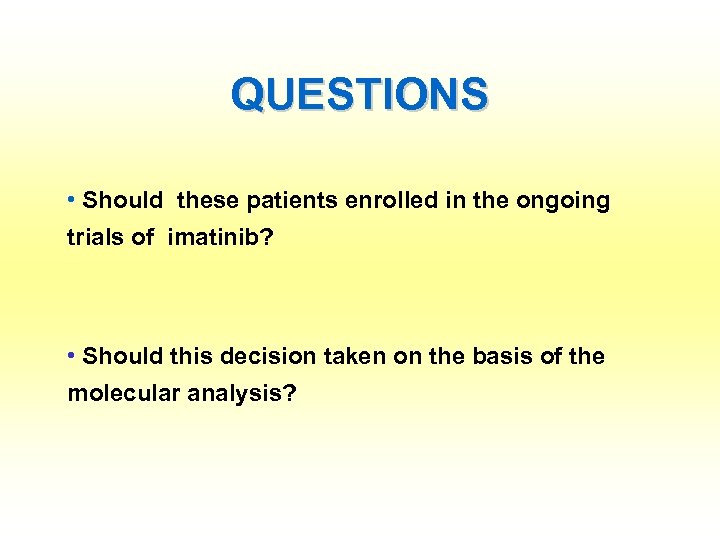
QUESTIONS • Should these patients enrolled in the ongoing trials of imatinib? • Should this decision taken on the basis of the molecular analysis?
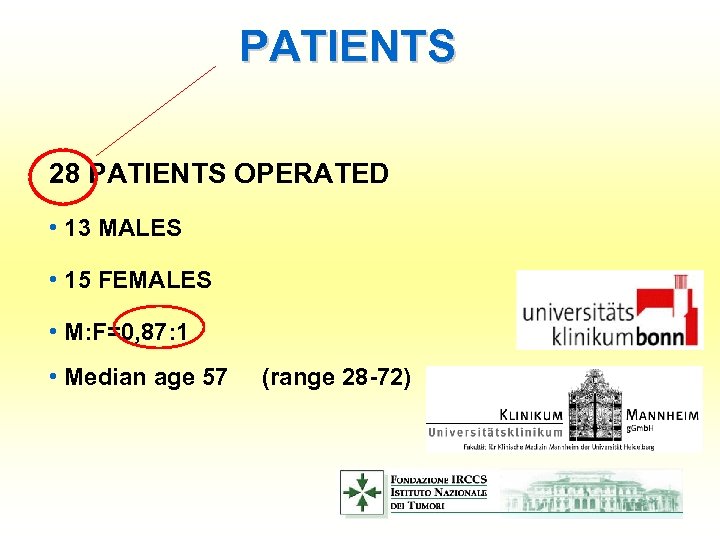
PATIENTS 28 PATIENTS OPERATED • 13 MALES • 15 FEMALES • M: F=0, 87: 1 • Median age 57 (range 28 -72)
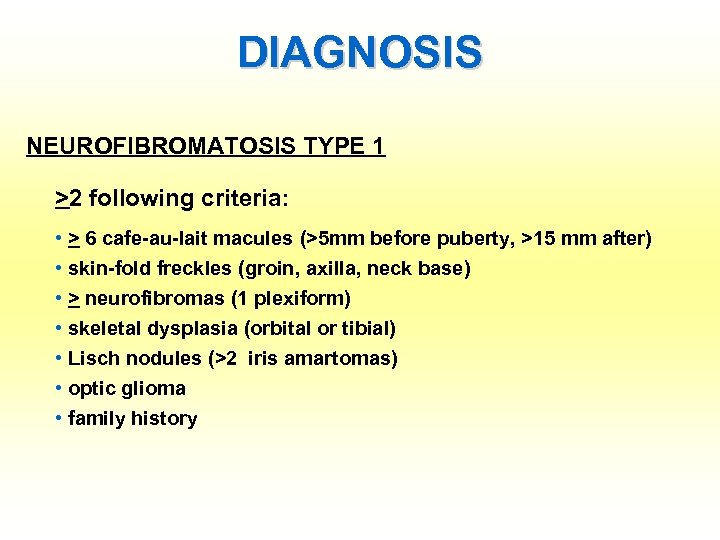
DIAGNOSIS NEUROFIBROMATOSIS TYPE 1 >2 following criteria: • > 6 cafe-au-lait macules (>5 mm before puberty, >15 mm after) • skin-fold freckles (groin, axilla, neck base) • > neurofibromas (1 plexiform) • skeletal dysplasia (orbital or tibial) • Lisch nodules (>2 iris amartomas) • optic glioma • family history

DIAGNOSIS All tumors were sympthomatic except one GIST • The diagnosis was confirmed histologically in terms of morphology and immunophenotyping • Seven tumors were reclassified as GIST after pathologic review • 2 MPNST • 1 Schwannoma, 1 Neurofibroma • 2 Leiomyosarcoma • 1 Leiomyoma
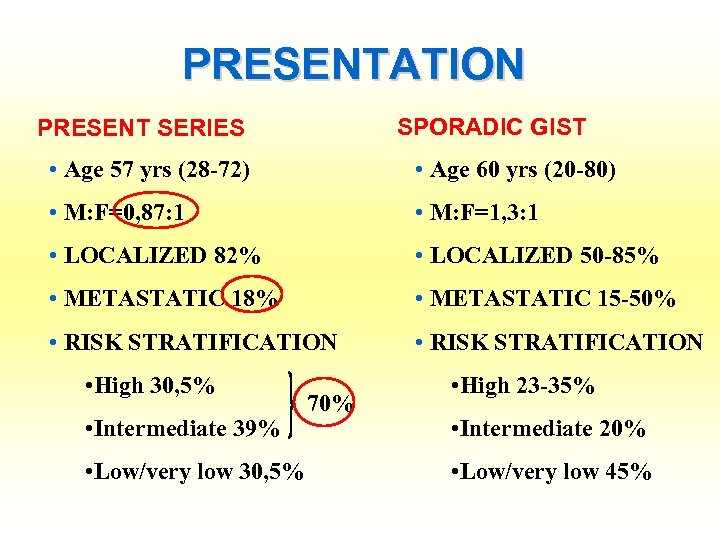
PRESENTATION SPORADIC GIST PRESENT SERIES • Age 57 yrs (28 -72) • Age 60 yrs (20 -80) • M: F=0, 87: 1 • M: F=1, 3: 1 • LOCALIZED 82% • LOCALIZED 50 -85% • METASTATIC 18% • METASTATIC 15 -50% • RISK STRATIFICATION • High 30, 5% • Intermediate 39% • Low/very low 30, 5% 70% • High 23 -35% • Intermediate 20% • Low/very low 45%
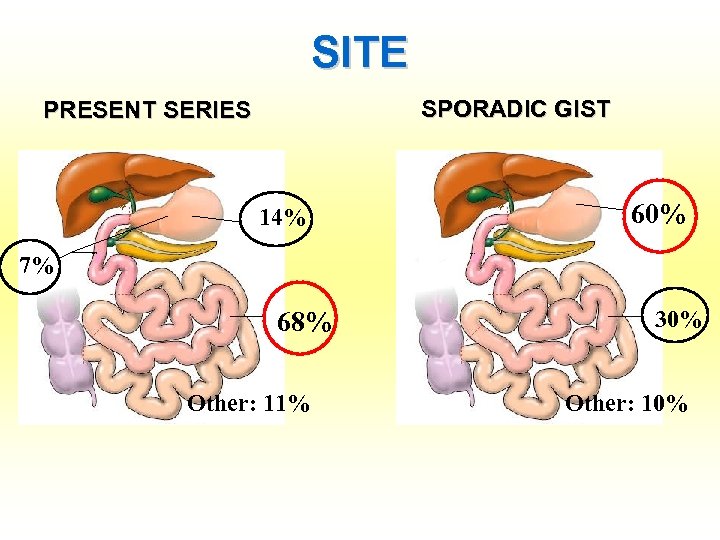
SITE SPORADIC GIST PRESENT SERIES 14% 25% 60% 7% 68% Other: 11% 30% Other: 10%
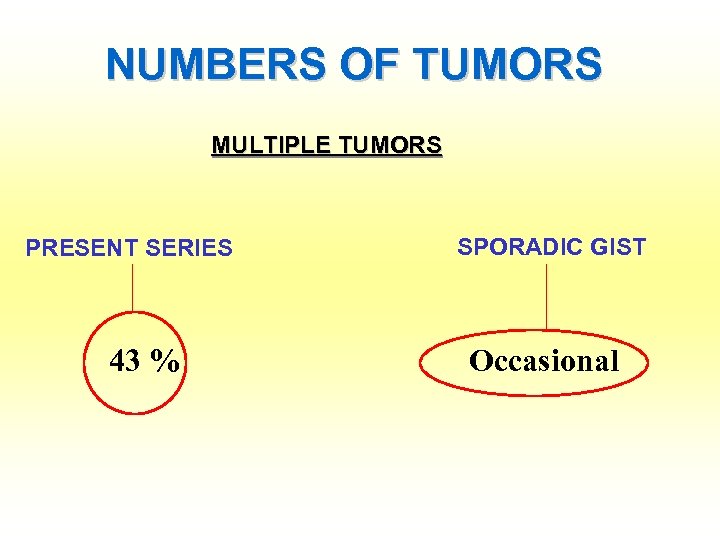
NUMBERS OF TUMORS MULTIPLE TUMORS PRESENT SERIES 43 % SPORADIC GIST Occasional
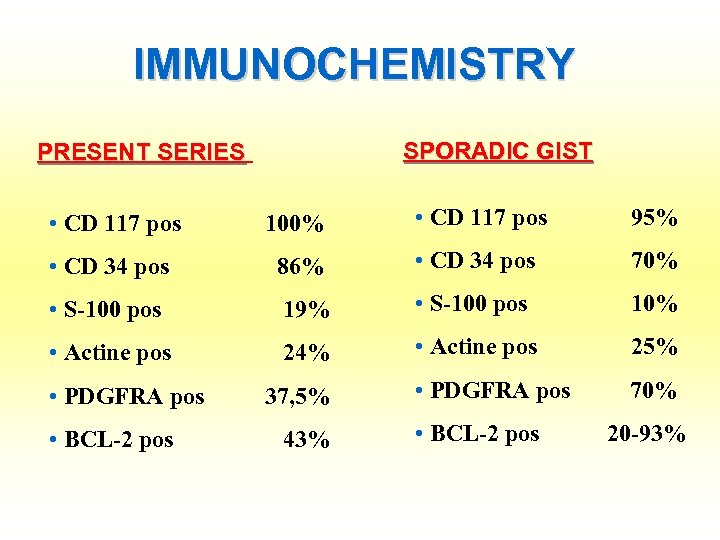
IMMUNOCHEMISTRY SPORADIC GIST PRESENT SERIES • CD 117 pos 100% • CD 117 pos 95% • CD 34 pos 86% • CD 34 pos 70% 19% • S-100 pos 10% • Actine pos 24% • Actine pos 25% • PDGFRA pos 37, 5% • PDGFRA pos 70% • BCL-2 pos 43% • BCL-2 pos 20 -93% • S-100 pos
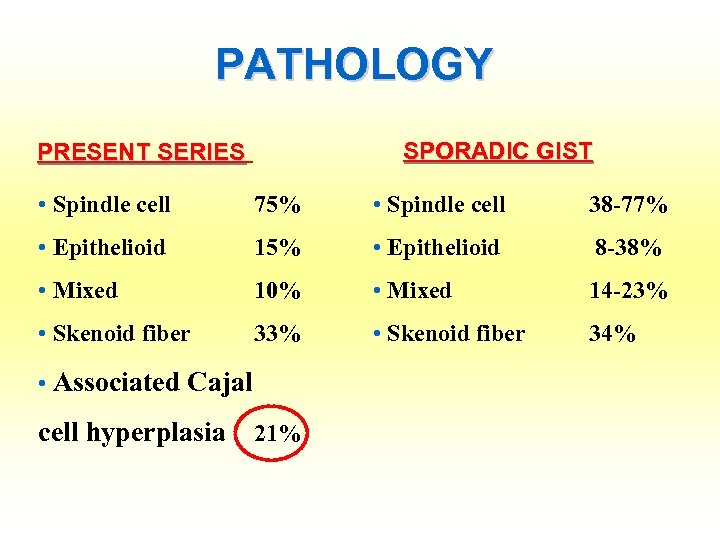
PATHOLOGY SPORADIC GIST PRESENT SERIES • Spindle cell 75% • Spindle cell 38 -77% • Epithelioid 15% • Epithelioid 8 -38% 10% • Mixed 33% • Skenoid fiber • Mixed • Skenoid fiber • Associated Cajal cell hyperplasia 21% 14 -23% 34%
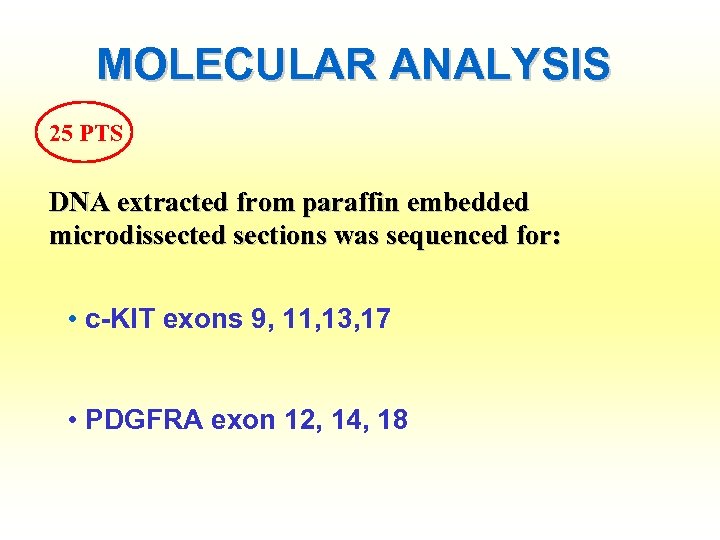
MOLECULAR ANALYSIS 25 PTS DNA extracted from paraffin embedded microdissected sections was sequenced for: • c-KIT exons 9, 11, 13, 17 • PDGFRA exon 12, 14, 18
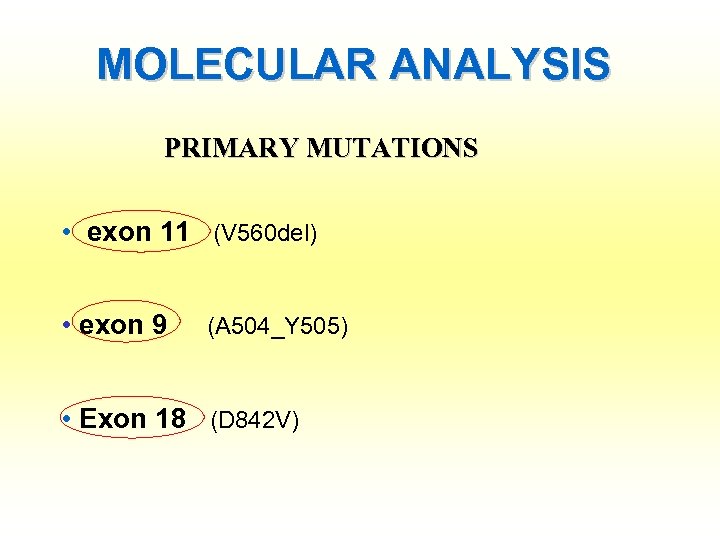
MOLECULAR ANALYSIS PRIMARY MUTATIONS • exon 11 (V 560 del) • exon 9 (A 504_Y 505) • Exon 18 (D 842 V)
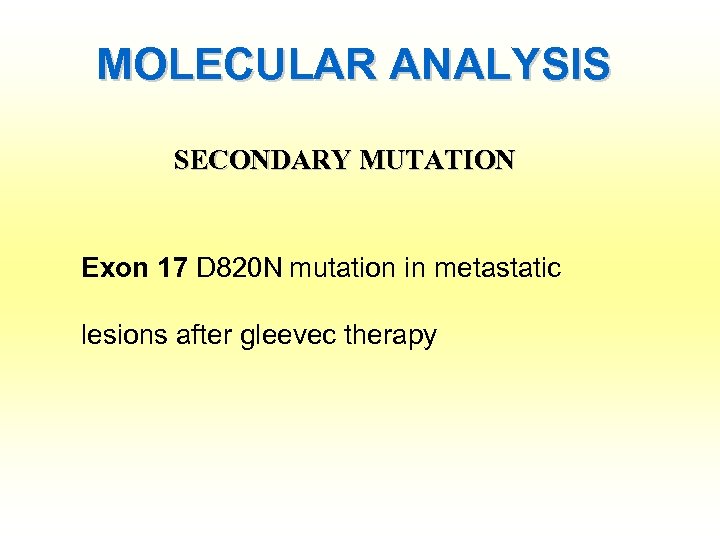
MOLECULAR ANALYSIS SECONDARY MUTATION Exon 17 D 820 N mutation in metastatic lesions after gleevec therapy
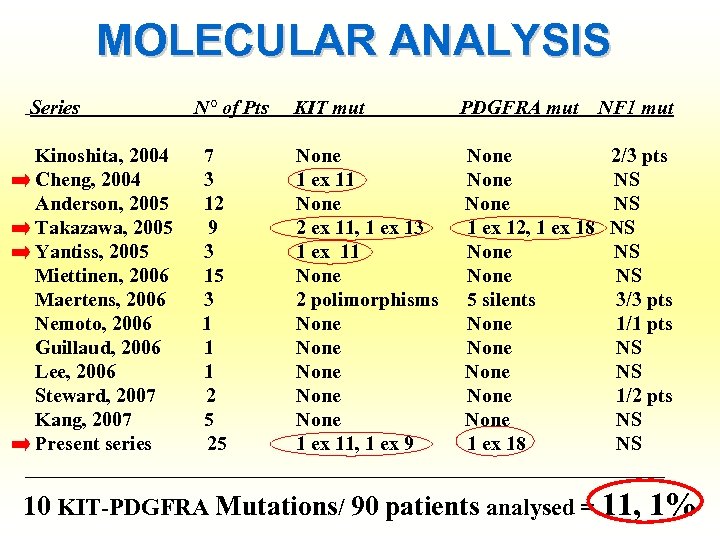
MOLECULAR ANALYSIS Series N° of Pts KIT mut PDGFRA mut NF 1 mut Kinoshita, 2004 7 None 2/3 pts Cheng, 2004 3 1 ex 11 None NS Anderson, 2005 12 None NS Takazawa, 2005 9 2 ex 11, 1 ex 13 1 ex 12, 1 ex 18 NS Yantiss, 2005 3 1 ex 11 None NS Miettinen, 2006 15 None NS Maertens, 2006 3 2 polimorphisms 5 silents 3/3 pts Nemoto, 2006 1 None 1/1 pts Guillaud, 2006 1 None NS Lee, 2006 1 None NS Steward, 2007 2 None 1/2 pts Kang, 2007 5 None NS Present series 25 1 ex 11, 1 ex 9 1 ex 18 NS ________________________________ 10 KIT-PDGFRA Mutations/ 90 patients analysed = 11, 1%
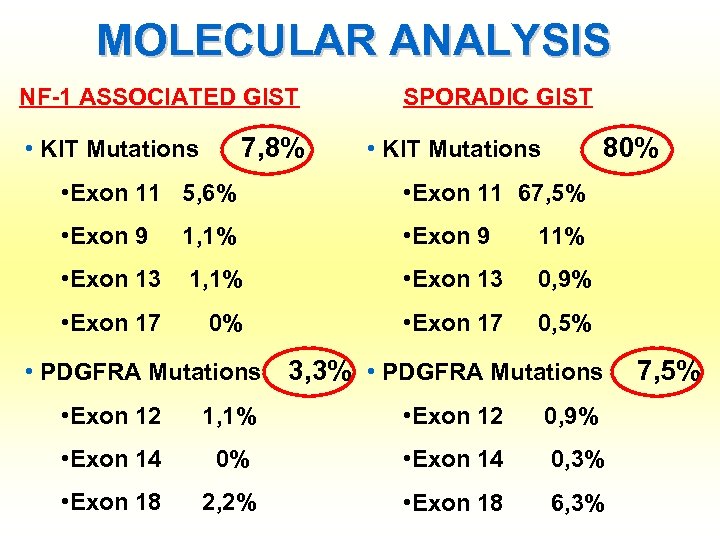
MOLECULAR ANALYSIS NF-1 ASSOCIATED GIST 7, 8% • KIT Mutations SPORADIC GIST 80% • KIT Mutations • Exon 11 5, 6% • Exon 11 67, 5% • Exon 9 1, 1% • Exon 9 11% • Exon 13 1, 1% • Exon 13 0, 9% • Exon 17 0, 5% • PDGFRA Mutations 3, 3% • PDGFRA Mutations • Exon 12 1, 1% • Exon 12 0, 9% • Exon 14 0, 3% • Exon 18 2, 2% • Exon 18 6, 3% 7, 5%
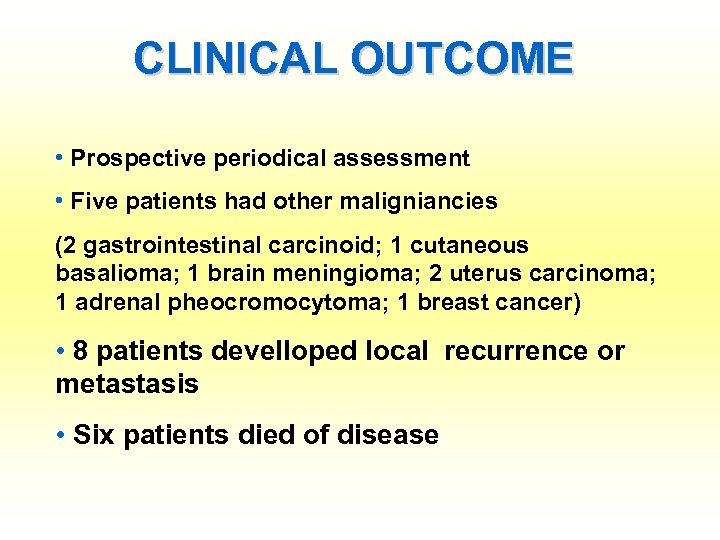
CLINICAL OUTCOME • Prospective periodical assessment • Five patients had other maligniancies (2 gastrointestinal carcinoid; 1 cutaneous basalioma; 1 brain meningioma; 2 uterus carcinoma; 1 adrenal pheocromocytoma; 1 breast cancer) • 8 patients develloped local recurrence or metastasis • Six patients died of disease
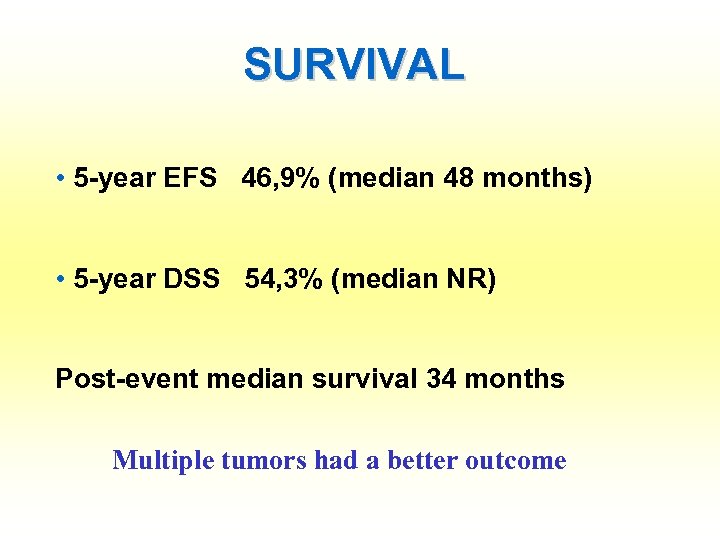
SURVIVAL • 5 -year EFS 46, 9% (median 48 months) • 5 -year DSS 54, 3% (median NR) Post-event median survival 34 months Multiple tumors had a better outcome
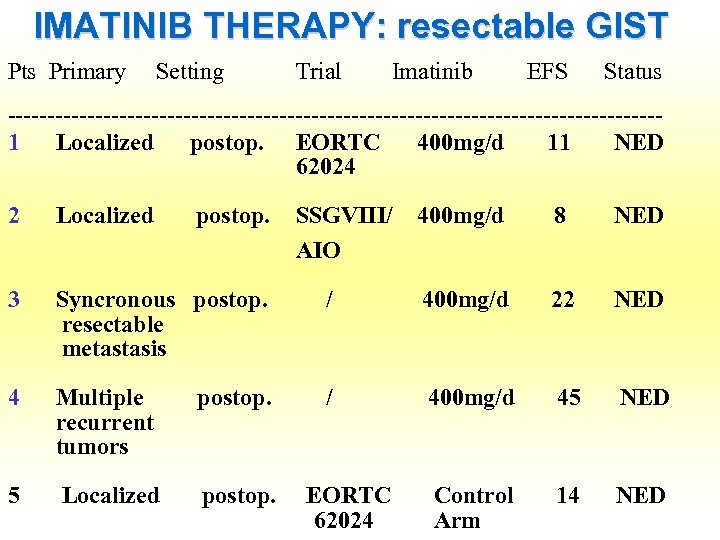
IMATINIB THERAPY: resectable GIST Pts Primary Setting Trial Imatinib EFS Status -----------------------------------------1 Localized postop. EORTC 400 mg/d 11 NED 62024 2 Localized postop. SSGVIII/ 400 mg/d 8 NED AIO 3 Syncronous postop. resectable metastasis / 400 mg/d 22 NED 4 Multiple postop. recurrent tumors / 400 mg/d 45 NED 5 Localized postop. EORTC Control 14 NED 62024 Arm
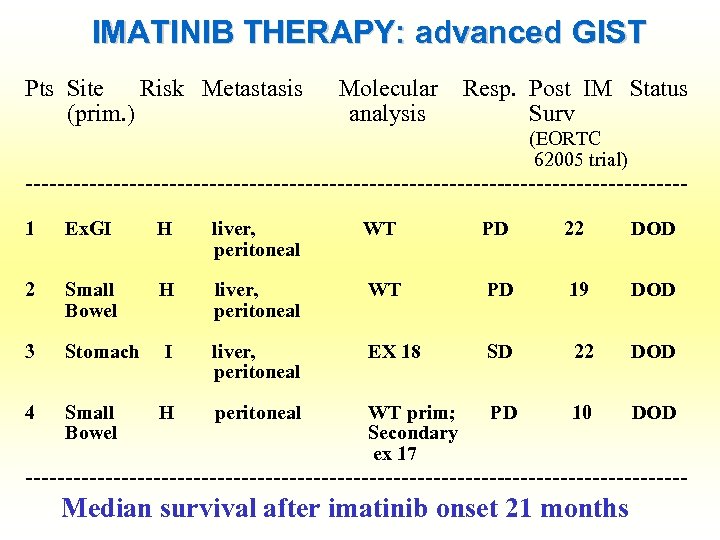
IMATINIB THERAPY: advanced GIST Pts Site Risk Metastasis (prim. ) Molecular analysis Resp. Post IM Status Surv (EORTC 62005 trial) -----------------------------------------1 Ex. GI H liver, WT peritoneal PD 22 DOD 2 Small H liver, WT Bowel peritoneal PD 19 DOD 3 Stomach I liver, EX 18 peritoneal SD 22 DOD 4 Small H peritoneal WT prim; PD 10 DOD Bowel Secondary ex 17 ------------------------------------------ Median survival after imatinib onset 21 months
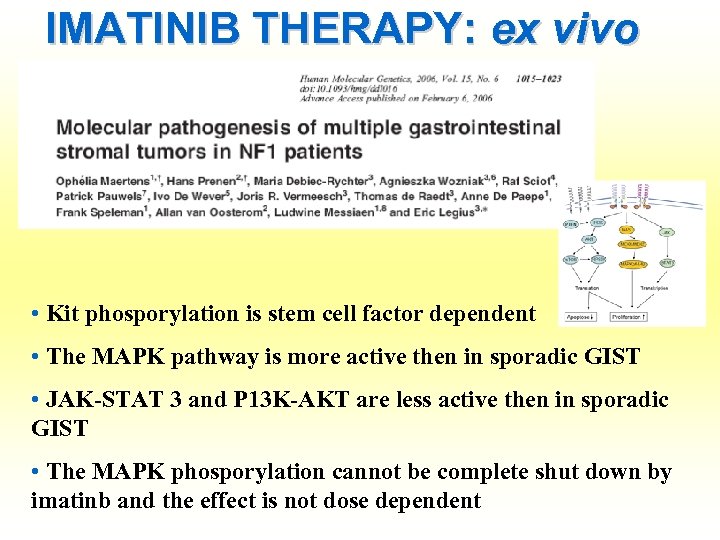
IMATINIB THERAPY: ex vivo • Kit phosporylation is stem cell factor dependent • The MAPK pathway is more active then in sporadic GIST • JAK-STAT 3 and P 13 K-AKT are less active then in sporadic GIST • The MAPK phosporylation cannot be complete shut down by imatinb and the effect is not dose dependent

IMATINIB THERAPY: neurofibrin deficit is associated with high levels of Kit expression

IMATINIB THERAPY: in vivo

IMATINIB THERAPY: in vivo
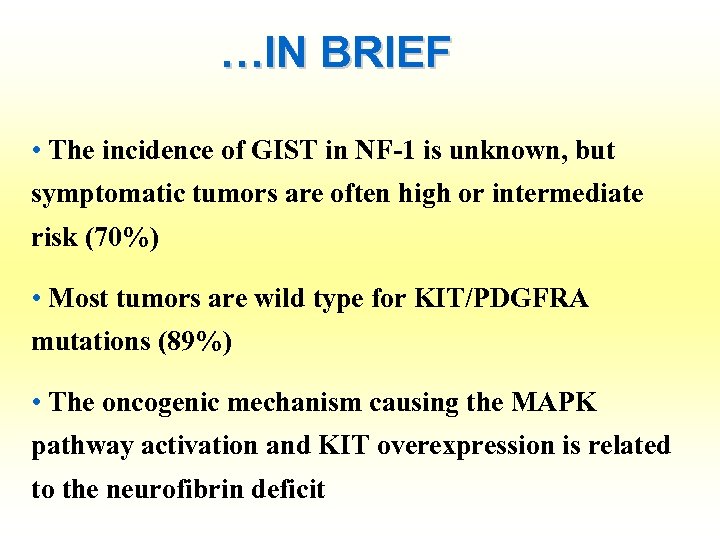
…IN BRIEF • The incidence of GIST in NF-1 is unknown, but symptomatic tumors are often high or intermediate risk (70%) • Most tumors are wild type for KIT/PDGFRA mutations (89%) • The oncogenic mechanism causing the MAPK pathway activation and KIT overexpression is related to the neurofibrin deficit
CONCLUSIONS The molecular analysis is always raccomended • to individuate sporadic mutation • to clarify the prognostic meaning of mutations in this subset of GIST
CONCLUSIONS Further studies are necessary to clarify the effecacy of IM and other inhibitors of tyrosine kinases in this setting
CONCLUSIONS • Localized wild type GIST should not be elegible for adjuvant trials • The molecular analysis should be done before the enrollement

CONCLUSIONS • Local advanced GIST enrolled in trials of preoperative imatinib should be carefully surveilled
CONCLUSIONS • Metastatic GIST could benefit from imatinib treatment • Sunitinib could be the first alternative in non responder tumors

CONCLUSIONS The future treatent of this subset of GIST is likely dependent from further investigations of the molecular pathways activated by neurofibrin as new molecular targets

THANKS! …. chiara. mussi@chir. ma. uni-heidelberg. de
2bf075b4453594674e8bf99700380660.ppt