 Crystal Structures & X-ray Diffraction Chemistry 123 Spring 2008 Dr. Woodward
Crystal Structures & X-ray Diffraction Chemistry 123 Spring 2008 Dr. Woodward
 Crystals
Crystals
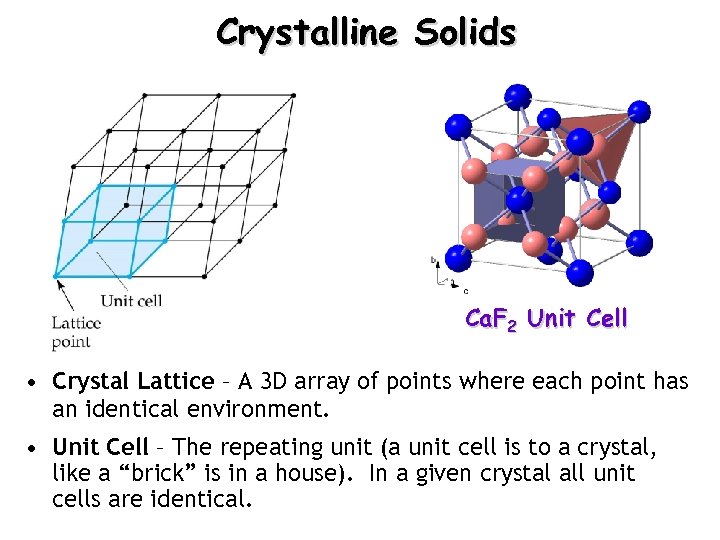 Crystalline Solids Ca. F 2 Unit Cell • Crystal Lattice – A 3 D array of points where each point has an identical environment. • Unit Cell – The repeating unit (a unit cell is to a crystal, like a “brick” is in a house). In a given crystal all unit cells are identical.
Crystalline Solids Ca. F 2 Unit Cell • Crystal Lattice – A 3 D array of points where each point has an identical environment. • Unit Cell – The repeating unit (a unit cell is to a crystal, like a “brick” is in a house). In a given crystal all unit cells are identical.
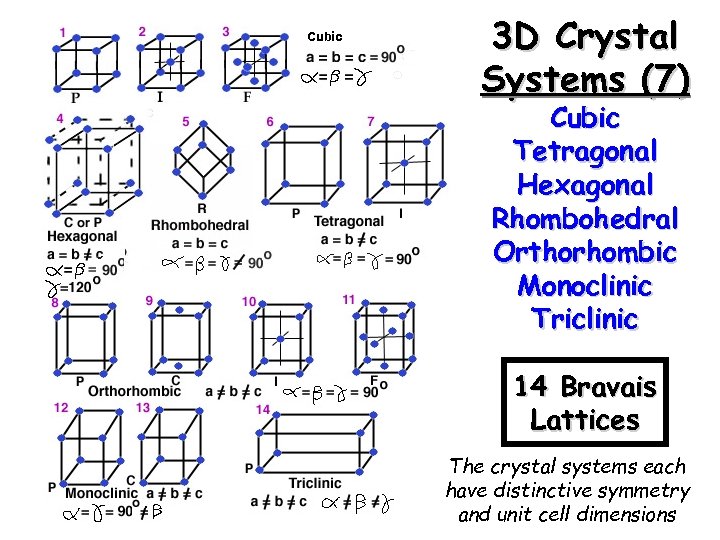 Cubic 3 D Crystal Systems (7) Cubic Tetragonal Hexagonal Rhombohedral Orthorhombic Monoclinic Triclinic 14 Bravais Lattices The crystal systems each have distinctive symmetry and unit cell dimensions
Cubic 3 D Crystal Systems (7) Cubic Tetragonal Hexagonal Rhombohedral Orthorhombic Monoclinic Triclinic 14 Bravais Lattices The crystal systems each have distinctive symmetry and unit cell dimensions
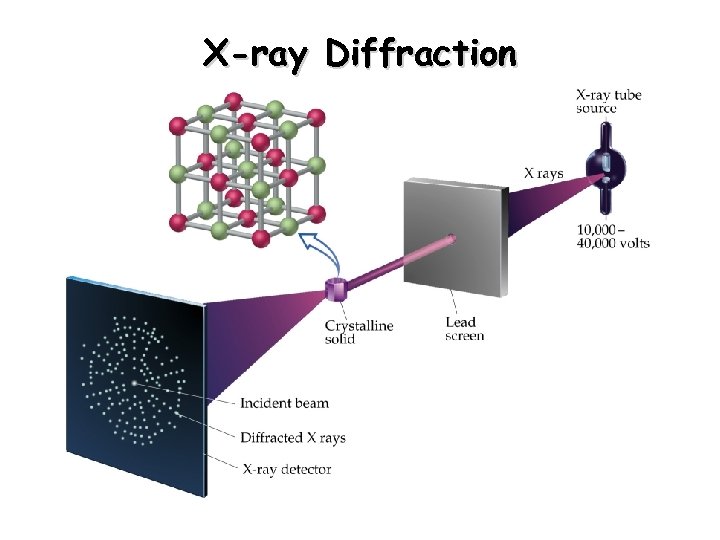 X-ray Diffraction
X-ray Diffraction
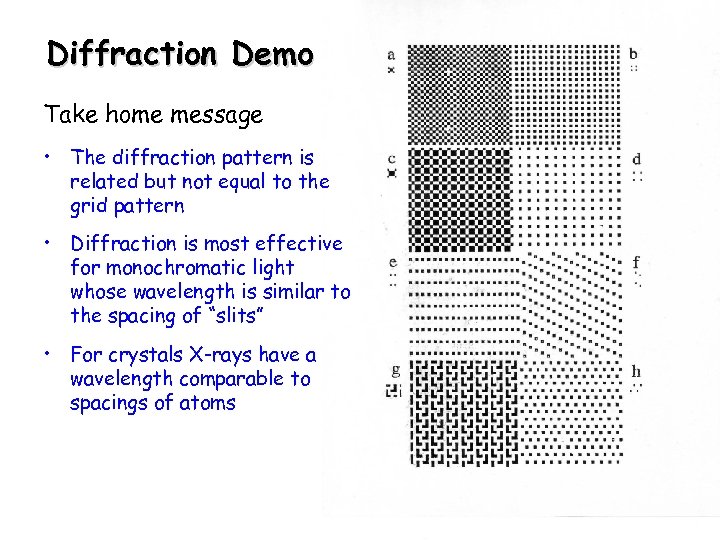 Diffraction Demo Take home message • The diffraction pattern is related but not equal to the grid pattern • Diffraction is most effective for monochromatic light whose wavelength is similar to the spacing of “slits” • For crystals X-rays have a wavelength comparable to spacings of atoms
Diffraction Demo Take home message • The diffraction pattern is related but not equal to the grid pattern • Diffraction is most effective for monochromatic light whose wavelength is similar to the spacing of “slits” • For crystals X-rays have a wavelength comparable to spacings of atoms
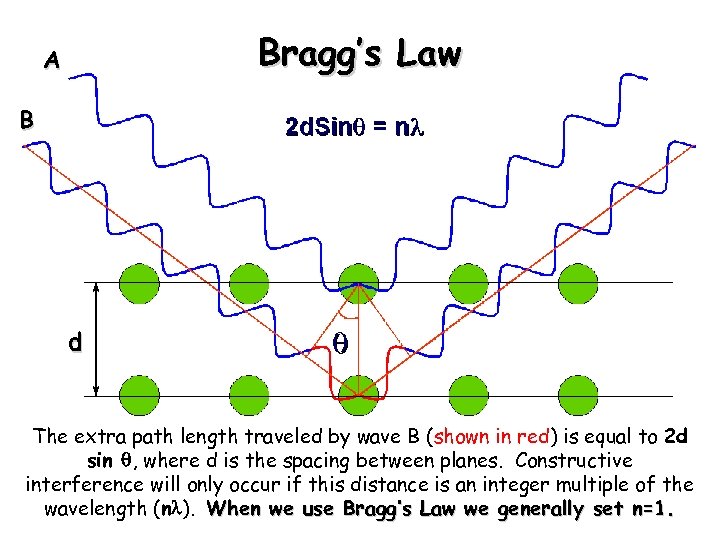 Bragg’s Law A B d The extra path length traveled by wave B (shown in red) is equal to 2 d sin q, where d is the spacing between planes. Constructive interference will only occur if this distance is an integer multiple of the wavelength (nl). When we use Bragg’s Law we generally set n=1.
Bragg’s Law A B d The extra path length traveled by wave B (shown in red) is equal to 2 d sin q, where d is the spacing between planes. Constructive interference will only occur if this distance is an integer multiple of the wavelength (nl). When we use Bragg’s Law we generally set n=1.
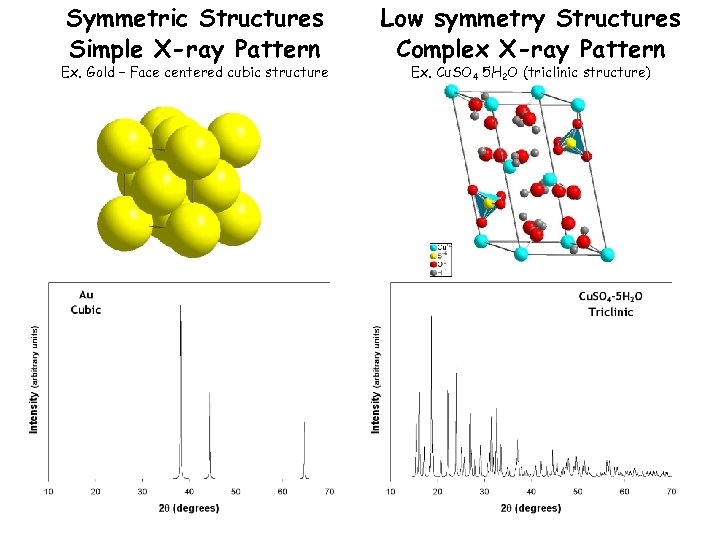 Symmetric Structures Simple X-ray Pattern Ex. Gold – Face centered cubic structure Low symmetry Structures Complex X-ray Pattern Ex. Cu. SO 4 5 H 2 O (triclinic structure)
Symmetric Structures Simple X-ray Pattern Ex. Gold – Face centered cubic structure Low symmetry Structures Complex X-ray Pattern Ex. Cu. SO 4 5 H 2 O (triclinic structure)
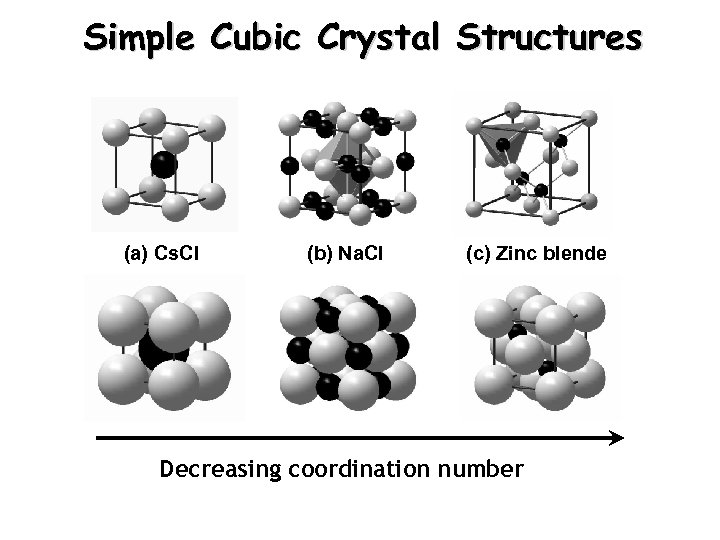 Simple Cubic Crystal Structures (a) Cs. Cl (b) Na. Cl (c) Zinc blende Decreasing coordination number
Simple Cubic Crystal Structures (a) Cs. Cl (b) Na. Cl (c) Zinc blende Decreasing coordination number
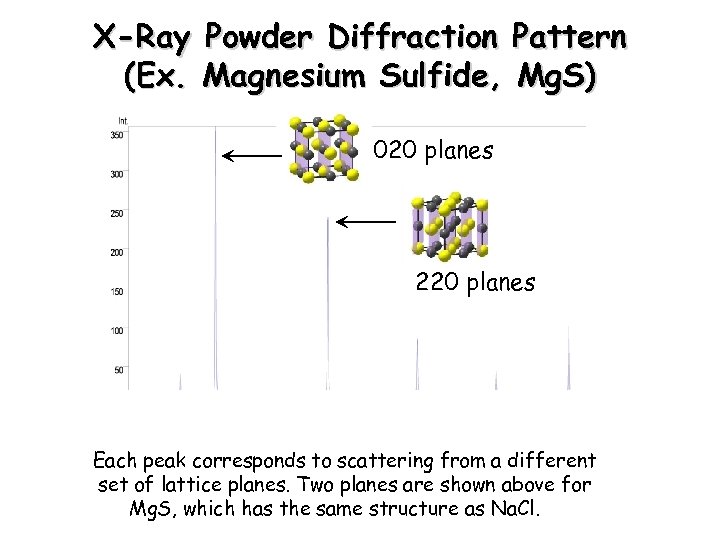 X-Ray Powder Diffraction Pattern (Ex. Magnesium Sulfide, Mg. S) 020 planes 220 planes Each peak corresponds to scattering from a different set of lattice planes. Two planes are shown above for Mg. S, which has the same structure as Na. Cl.
X-Ray Powder Diffraction Pattern (Ex. Magnesium Sulfide, Mg. S) 020 planes 220 planes Each peak corresponds to scattering from a different set of lattice planes. Two planes are shown above for Mg. S, which has the same structure as Na. Cl.
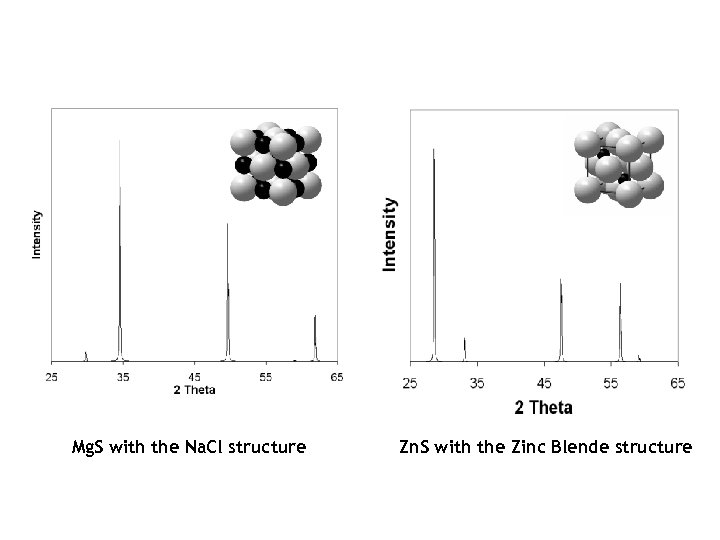 Mg. S with the Na. Cl structure Zn. S with the Zinc Blende structure
Mg. S with the Na. Cl structure Zn. S with the Zinc Blende structure
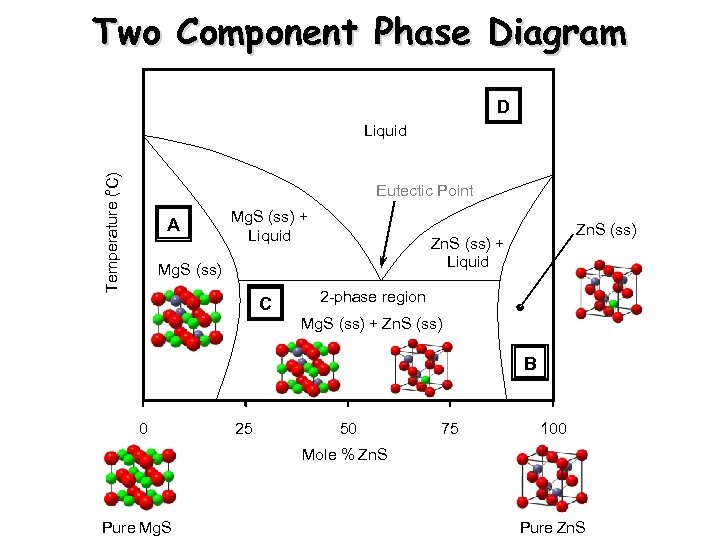 Two Component Phase Diagram D ° Temperature ( C) Liquid Eutectic Point A Mg. S (ss) + Liquid Zn. S (ss) + Liquid Mg. S (ss) C Zn. S (ss) 2 -phase region Mg. S (ss) + Zn. S (ss) B 0 25 50 75 100 Mole % Zn. S Pure Mg. S Pure Zn. S
Two Component Phase Diagram D ° Temperature ( C) Liquid Eutectic Point A Mg. S (ss) + Liquid Zn. S (ss) + Liquid Mg. S (ss) C Zn. S (ss) 2 -phase region Mg. S (ss) + Zn. S (ss) B 0 25 50 75 100 Mole % Zn. S Pure Mg. S Pure Zn. S
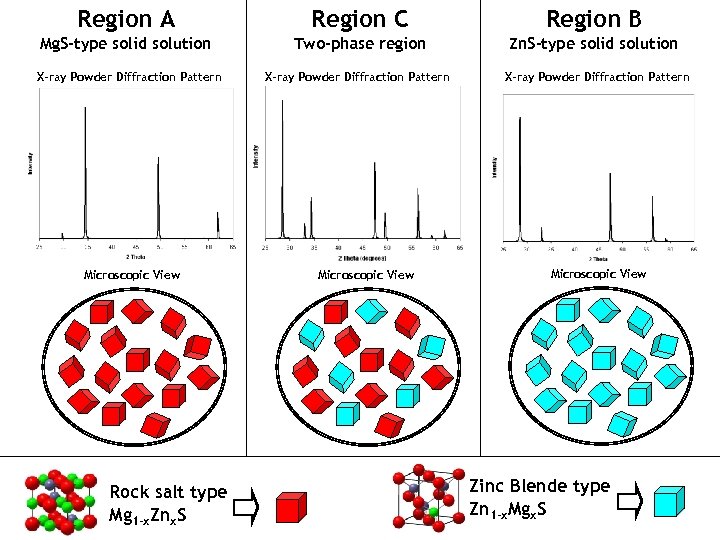 Region A Region C Region B Mg. S-type solid solution Two-phase region Zn. S-type solid solution X-ray Powder Diffraction Pattern Microscopic View Rock salt type Mg 1 -x. Znx. S Microscopic View Zinc Blende type Zn 1 -x. Mgx. S
Region A Region C Region B Mg. S-type solid solution Two-phase region Zn. S-type solid solution X-ray Powder Diffraction Pattern Microscopic View Rock salt type Mg 1 -x. Znx. S Microscopic View Zinc Blende type Zn 1 -x. Mgx. S