6f61d606c2cccfe7eba432e8aafa2086.ppt
- Количество слайдов: 23

CRT 2013 Washington, DC, USA, Feb 23 -26, 2013 Postmarketing Evaluation: Does it Work and How Much Does it Cost? Horst Sievert, Ilona Hofmann, Laura Vaskelyte, Stefan Bertog Cardio. Vascular Center Frankfurt - CVC Frankfurt, Germany

Horst Sievert, MD Consulting: Access. Closure, Inc. , AGA Medical Corporation, Ardian, Inc. , Arstasis, Inc. , Atritech Atrium Medical Corporation, Avinger, Inc. , Bard Peripheral Vascular, Inc. , Boston Scientific Corporation, Bridgepoint, Cardio. Kinetix Inc. , Cardio. MEMS, Inc. , Coherex, Inc. , Contego, CSI, Endo. Cross, Endotex Interventional Systems, Epitek, Evalve, Inc. and ev 3, Inc.

Horst Sievert, MD Consulting: Flow. Cardia, Inc. , Gore, Guidant, Lumen Biomedical, Inc. , HLT, Kensey Nash Corporation, Kyoto Medical, Lifetech, Lutonix, Inc. , Medinol, Medtronic, Inc. , NDCNMT Medical, Inc. , OAS, Occlutech Osprey Medical, Inc. , Ovalis, Inc. , Pathway Medical Technologies, Inc. , Pendra. Care International B. V. , Pfm Medical, Inc. , Rox Medical, Recor, Sadra Medical, Sorin Biomedica Cardio S. R. L. , and Spectranetics Corporation, Trireme, Trivascular and Viacor, Inc.

Horst Sievert, MD Honoraria: Veryan, Ardian, Inc. , Atritech, Atrium Medical Corporation, Boston Scientific Corporation, Cardio. Kinetix Inc. , Cardio. MEMS, Inc. , Coherex, Inc. , Contego, Epitek, Evalve, Inc. , Guidant, Gore, Kyoto Medical, Lutonix, Inc. , Medinol, Medtronic, Inc. , Pfm Medical, Inc. , and Spectranetics Corporation.

Horst Sievert, MD Honoraria: Viacor, Inc. , HLT, Lifetech, Recor, Trivascular, Veryan, CVRx, GDS and In. Seal Medical.

Subject: CRT talk Date: Sun, 17 Feb 2013 16: 31 +0100 From: Horst Sievert <horstsievertmd@aol. com> To: Ron Waksman <rwaksman@gmail. com> Ron I just discovered this assignment: "Postmarketing Evaluation: Does it Work and How Much Does it Cost? " What do you expect from this talk? Could be a short one First slide: "Of course it works" 2 nd slide: "Don't know what it costs" Thanks Horst

On 18. 02. 2013 02: 55, Ron Waksman wrote: Horst I agree this is not a clear title The goal of this talk is to describe the postmarketing experince who gets it who pays reimbursment do patients continue take their drugs etc Descibe the practice with RDN post approval
On Feb 21, 2013, at 4: 03 PM, Horst Sievert <horstsievertmd@aol. com> wrote: I see When we lived in a free country, we could use new devices when we believed it was in the interest of our patients Then we could use them if an EC gave approval Thereafter we needed government approval And now something like CMS In the future there will be no problem because there will be no new devices anymore Will still be a short talk but I think that's what we will need at that time of the day

"What does it cost? " • Generator 25, 000 € • Symplicity catheter 5, 000 € - You have to buy 5 before you can start
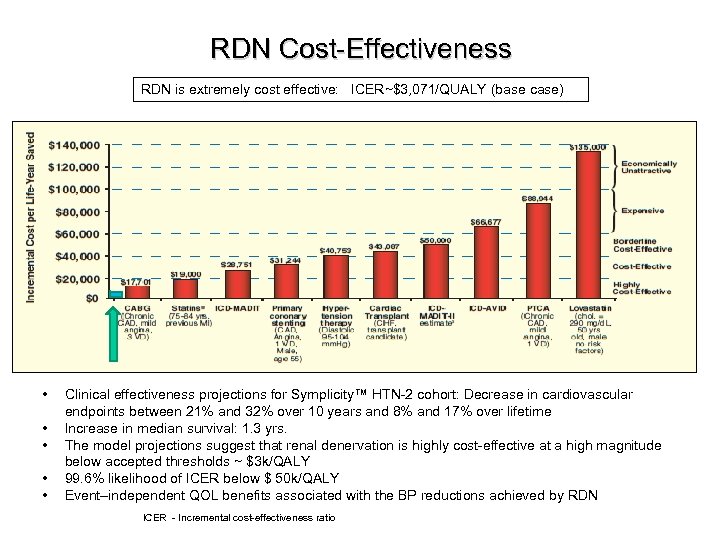
RDN Cost-Effectiveness RDN is extremely cost effective: ICER~$3, 071/QUALY (base case) • • • Clinical effectiveness projections for Symplicity™ HTN-2 cohort: Decrease in cardiovascular endpoints between 21% and 32% over 10 years and 8% and 17% over lifetime Increase in median survival: 1. 3 yrs. The model projections suggest that renal denervation is highly cost-effective at a high magnitude below accepted thresholds ~ $3 k/QALY 99. 6% likelihood of ICER below $ 50 k/QALY Event–independent QOL benefits associated with the BP reductions achieved by RDN ICER - Incremental cost-effectiveness ratio

"Who gets it? " • Medronic: - "The Symplicity system is approved in almost 80 countries. Essentially we have approval almost . everywhere except the US, Japan, China, India and Brazil where we are still awaiting regulatory approval" • I learned from this that the developing countries are developing into the wrong direction - "There is reimbursement in Germany. There is also some level of reimbursement in Austria, Denmark, Switzerland Italy" • I learned from this that it is still pretty good in Germany

"Who gets it in countries where the device is not approved? " • Those patients who can afford it go. abroad - Many US patients are coming to Europe • Very often physicians - I learned from this that it can not be that bad (yet) in the US for physicians

"Who gets it in countries where the device is approved? ".

The situation in Germany • 98% of patients are covered by some kind of insurrance • There is reimbursement but it is not sufficient - The DRG almost covers the device but overall hospitals loose money - Many hospitals offer the treatment because they want to compete with other hospitals - Many other do not because administration says "no" • Largest numbers of renal denervations worldwide • Although everybody agrees that it should be limited to resistant hypertension there is certainly a trend to treat patients with less severy hypertension

Case example • 50 y/o patient from far away (for example a famous lawyer from the north coast of Germany) - 24 hour BP recording at home: syst BP on average 170 mm. Hg • Many measurements > 190 mm. Hg - 5 antihypertensive drugs - Definitely resistant hypertension - Coming to your hospital because you are so famous • When patient arrives, office BP measured according to protocol is only 155 mm. Hg (despite all efforts) - Would not have qualified for HTN trials - But certainly gets treatment in daily practice • Insurances now are looking for ways to deny reimbursement in these cases - Retrospectively, of course! • I learned from this that it is not as nice in Germany anymore as it has been in the past
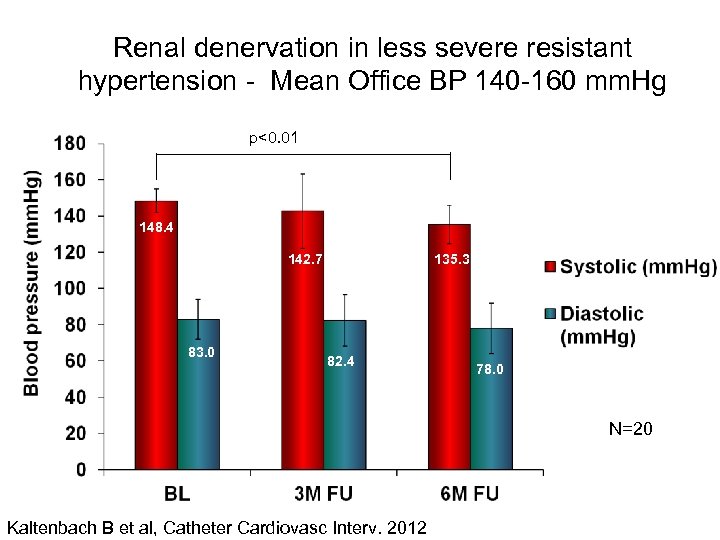
Renal denervation in less severe resistant hypertension - Mean Office BP 140 -160 mm. Hg p<0. 01 148. 4 135. 3 142. 7 83. 0 82. 4 78. 0 N=20 Kaltenbach B et al, Catheter Cardiovasc Interv. 2012

"Other indications than hypertension? " • Only within trials, very few offlabel uses - Heart failure - Sleep apnoea - Ventricular arrhythmias

Do patients continue take their drugs? • The main motivation for patients to undergo this procedure is to reduce their medication • That's why most of them try to reduce meds after renal denervation • That most often does not work well • Patients and referring physicians are frustrated • Extensive discussions with patients, family and referring physicians is essential

"etc" • Medtronic: - "As for sales, I would say that the uptake from interventionalists has been as strong as expected" - "The nephrology and hypertension specialist community has been relatively cautious as they continue to ask for additional data with longer term follow-up" - "We expect that much of that will be answered with Symplicity HTN-3, given the large patient cohort (530 patients)"

"etc" • Data collection post approval - Medtronic Global Registry - Many physician driven local registries - These registries are not published yet but some had been reported at conferences • Most of them with results comparable to the results in the trials • But not all of them. . .
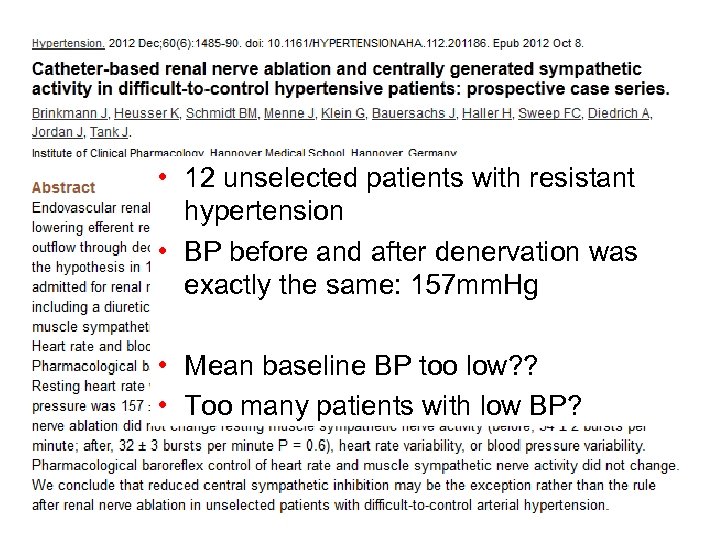
• 12 unselected patients with resistant hypertension • BP before and after denervation was exactly the same: 157 mm. Hg • Mean baseline BP too low? ? • Too many patients with low BP?

From: Ron Waksman <rwaksman@gmail. com> To: Horst Sievert <horstsievertmd@aol. com> Agree Short and sweet Looking forward to see u next week Ron

6f61d606c2cccfe7eba432e8aafa2086.ppt