4ea502df1409c4098fab75d9cab7d39a.ppt
- Количество слайдов: 45

Criteri SPRINT e terapia di combinazione nel controllo dell’ipertensione arteriosa Stefano Taddei Dipartimento di Medicina Clinica e Sperimentale Università di Pisa

Conflitto di interessi negli ultimi 24 mesi 1. Grant: Università di Pisa 2. Contratti di ricerca: Boheringer Ingelheim, Novartis, Servier, Menarini 3. Relatore in convegni: Servier, Pfizer, Boheringer Ingelheim, Sigma Tau
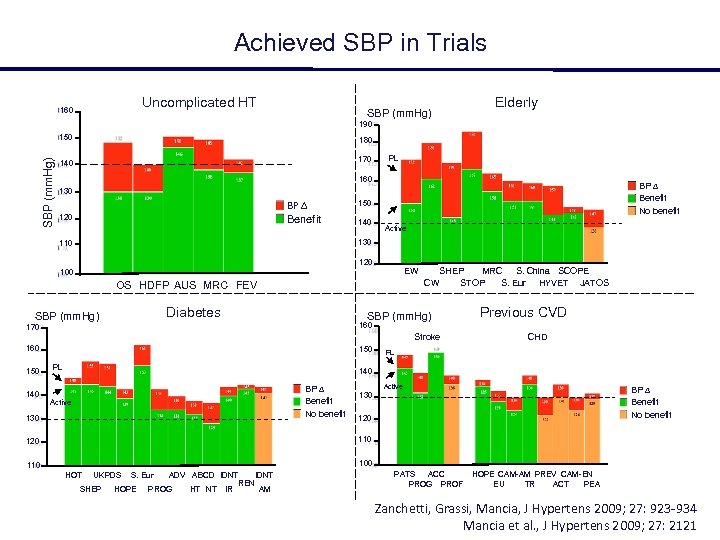
Achieved SBP in Trials Uncomplicated HT 160 SBP (mm. Hg) Elderly 190 SBP (mm. Hg) 150 180 140 170 PL 160 BP Benefit No benefit 130 BP Benefit 120 150 140 Active 130 110 120 EW 100 OS HDFP AUS MRC FEV Diabetes SBP (mm. Hg) SHEP MRC S. China SCOPE CW STOP S. Eur HYVET JATOS SBP (mm. Hg) 170 160 150 Previous CVD 160 Stroke 150 140 PL CHD PL 140 BP Benefit No benefit Active 130 Active BP Benefit No benefit 130 120 110 120 100 110 HOT UKPDS SHEP S. Eur HOPE ADV ABCD IDNT PROG HT NT IDNT REN IR AM PATS ACC PROG PROF HOPE CAM-AM PREV CAM-EN EU TR ACT PEA Zanchetti, Grassi, Mancia, J Hypertens 2009; 27: 923 -934 Mancia et al. , J Hypertens 2009; 27: 2121

La terapia del paziente iperteso • Obiettivi pressori del trattamento: • ESH/ESC 2013: • JNC 8 2014: <140/90 mm Hg <150/90 mm Hg
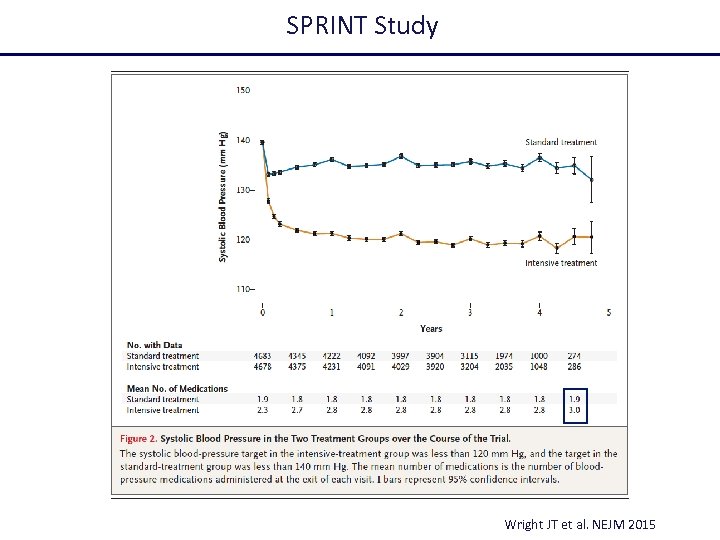
SPRINT Study Wright JT et al. NEJM 2015
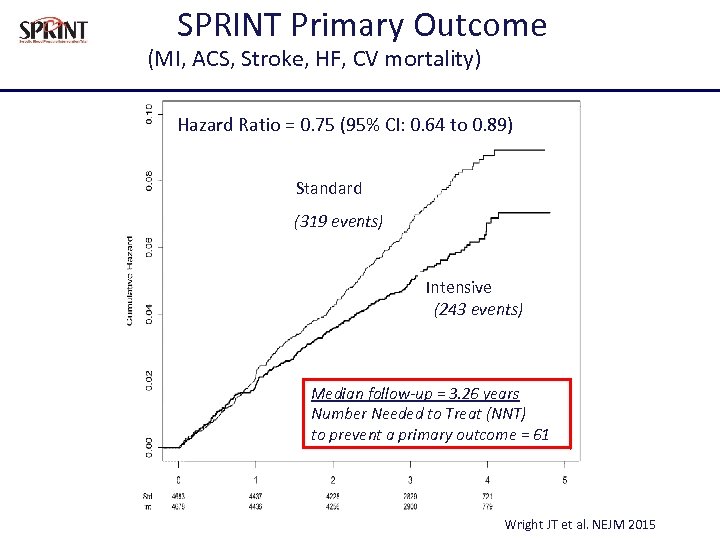
SPRINT Primary Outcome (MI, ACS, Stroke, HF, CV mortality) Hazard Ratio = 0. 75 (95% CI: 0. 64 to 0. 89) Standard (319 events) Intensive (243 events) Median follow-up = 3. 26 years Number Needed to Treat (NNT) to prevent a primary outcome = 61 Wright JT et al. NEJM 2015
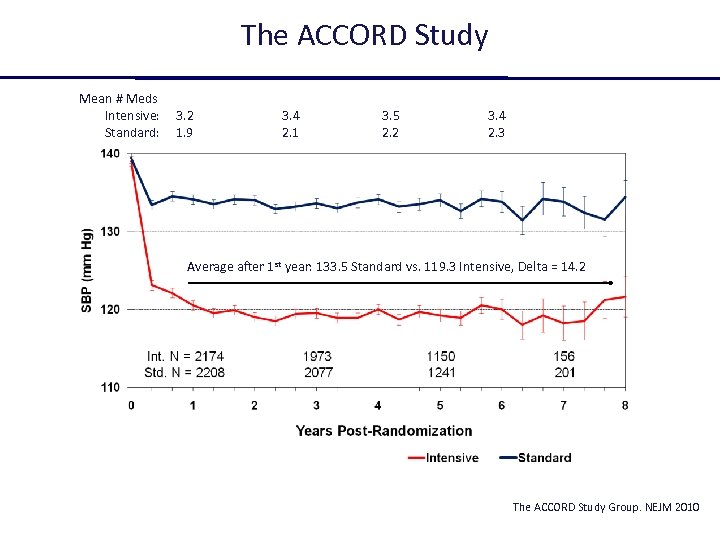
The ACCORD Study Mean # Meds Intensive: Standard: 3. 2 1. 9 3. 4 2. 1 3. 5 2. 2 3. 4 2. 3 Average after 1 st year: 133. 5 Standard vs. 119. 3 Intensive, Delta = 14. 2 The ACCORD Study Group. NEJM 2010
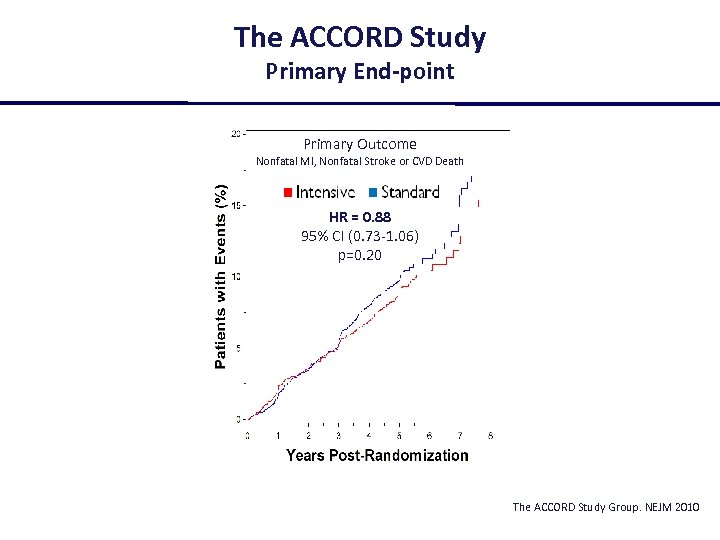
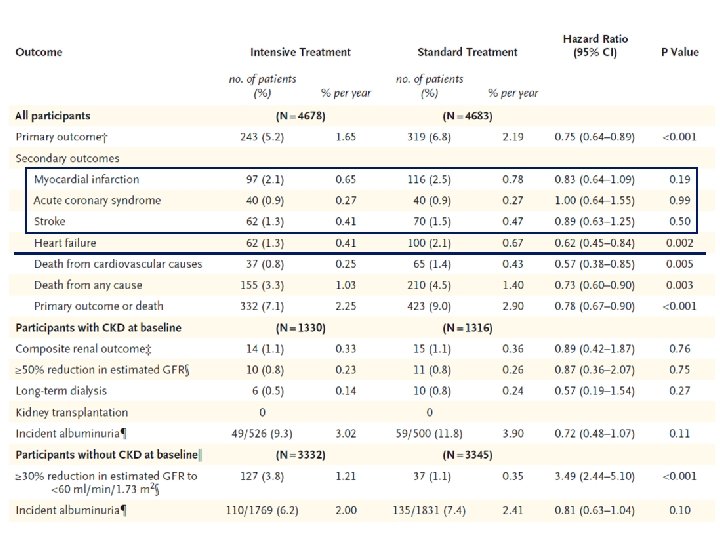
The ACCORD Study Primary End-point Primary Outcome Nonfatal MI, Nonfatal Stroke or CVD Death HR = 0. 88 95% CI (0. 73 -1. 06) p=0. 20 The ACCORD Study Group. NEJM 2010
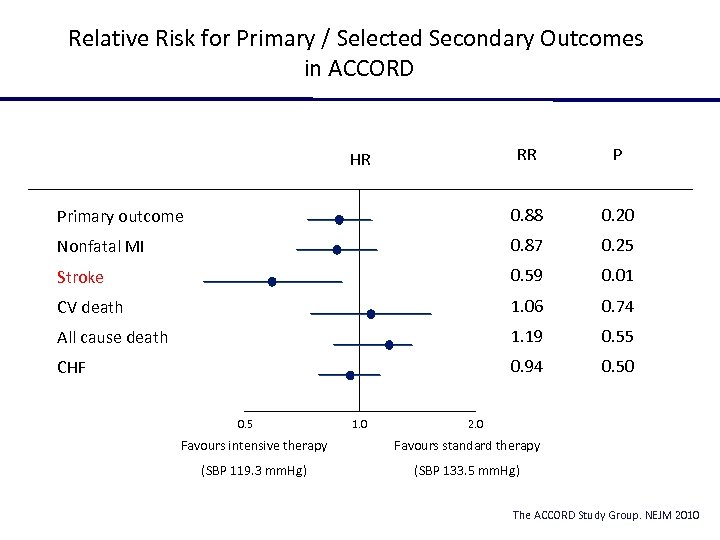
Relative Risk for Primary / Selected Secondary Outcomes in ACCORD RR P Primary outcome 0. 88 0. 20 Nonfatal MI 0. 87 0. 25 Stroke 0. 59 0. 01 CV death 1. 06 0. 74 All cause death 1. 19 0. 55 CHF 0. 94 0. 50 HR 0. 5 1. 0 2. 0 Favours intensive therapy Favours standard therapy (SBP 119. 3 mm. Hg) (SBP 133. 5 mm. Hg) The ACCORD Study Group. NEJM 2010
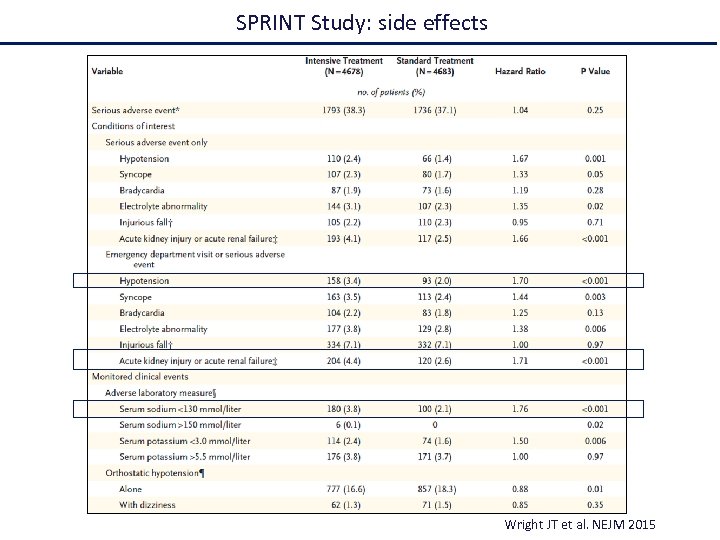
SPRINT Study: side effects Wright JT et al. NEJM 2015

Ma esiste un’altra spiegazione per i risultati dello studio SPRINT In questo studio, a differenza di tutti gli altri studi clinici controllati, i valori pressori erano misurati da un apparecchio automatico con il paziente lasciato da solo in un stanza isolata: è una misurazione molto simile alla “home blood pressure” e non può essere paragonata alla “clinic blood pressure”.
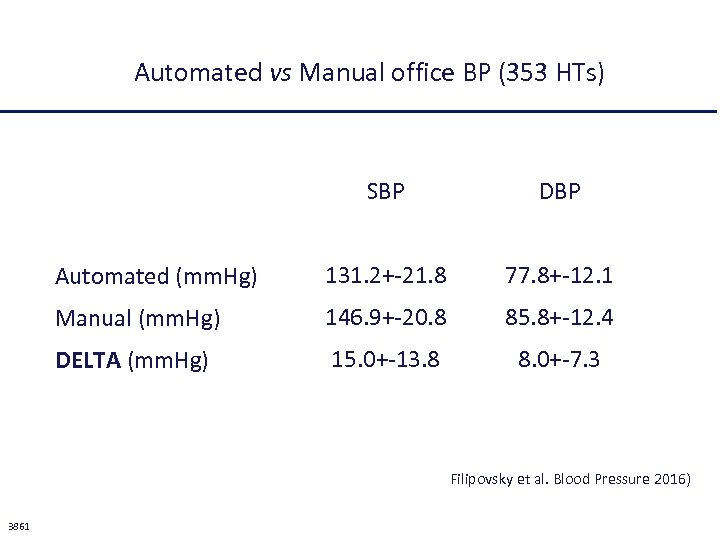
Automated vs Manual office BP (353 HTs) SBP DBP Automated (mm. Hg) 131. 2+-21. 8 77. 8+-12. 1 Manual (mm. Hg) 146. 9+-20. 8 85. 8+-12. 4 DELTA (mm. Hg) 15. 0+-13. 8 8. 0+-7. 3 Filipovsky et al. Blood Pressure 2016) 3861

Efficacia della monoterapia nella pratica clinica La monoterapia normalizza la pressione arteriosa in non più del 30% dei pazienti con ipertensione di grado 1 -2 ed è inefficace nei pazienti con ipertensione di grado 3.
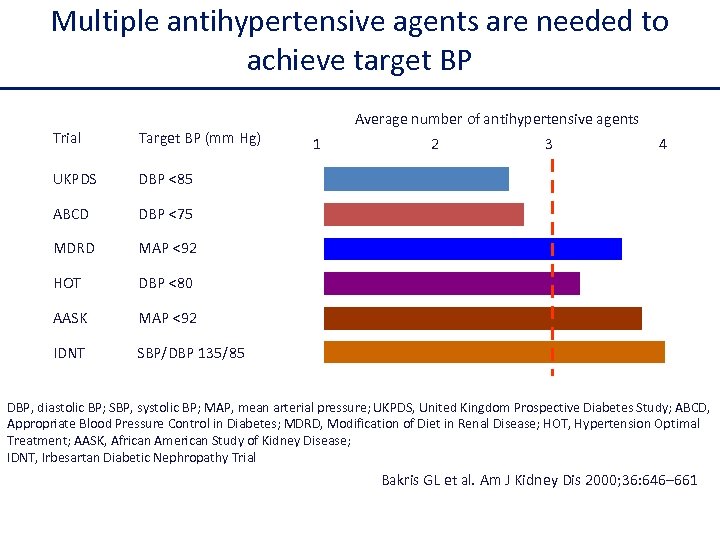
Multiple antihypertensive agents are needed to achieve target BP Trial Target BP (mm Hg) UKPDS DBP <85 ABCD DBP <75 MDRD MAP <92 HOT DBP <80 AASK MAP <92 IDNT Average number of antihypertensive agents SBP/DBP 135/85 1 2 3 4 DBP, diastolic BP; SBP, systolic BP; MAP, mean arterial pressure; UKPDS, United Kingdom Prospective Diabetes Study; ABCD, Appropriate Blood Pressure Control in Diabetes; MDRD, Modification of Diet in Renal Disease; HOT, Hypertension Optimal Treatment; AASK, African American Study of Kidney Disease; IDNT, Irbesartan Diabetic Nephropathy Trial Bakris GL et al. Am J Kidney Dis 2000; 36: 646– 661
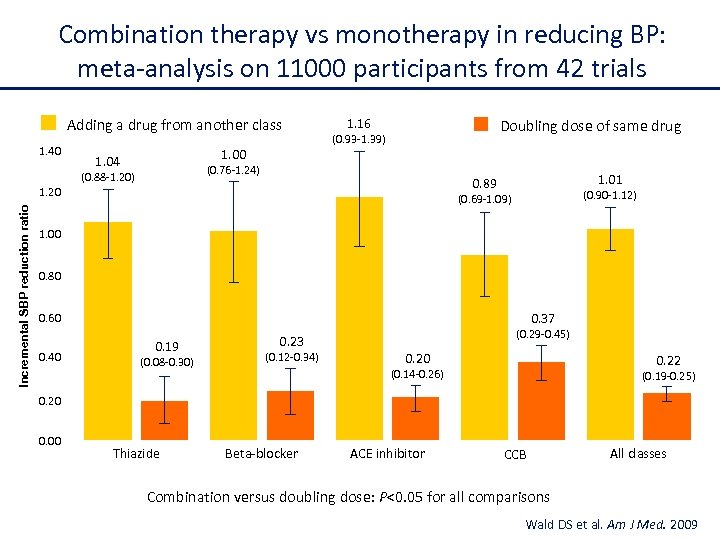
Combination therapy vs monotherapy in reducing BP: meta-analysis on 11000 participants from 42 trials Adding a drug from another class 1. 40 1. 04 1. 16 Doubling dose of same drug (0. 93 -1. 39) (0. 76 -1. 24) (0. 88 -1. 20) Incremental SBP reduction ratio 1. 01 0. 89 1. 20 (0. 90 -1. 12) (0. 69 -1. 09) 1. 00 0. 80 0. 37 0. 60 0. 40 0. 19 (0. 08 -0. 30) 0. 23 (0. 12 -0. 34) (0. 29 -0. 45) 0. 20 0. 22 (0. 14 -0. 26) (0. 19 -0. 25) 0. 20 0. 00 Thiazide Beta-blocker ACE inhibitor CCB All classes Combination versus doubling dose: P<0. 05 for all comparisons Wald DS et al. Am J Med. 2009
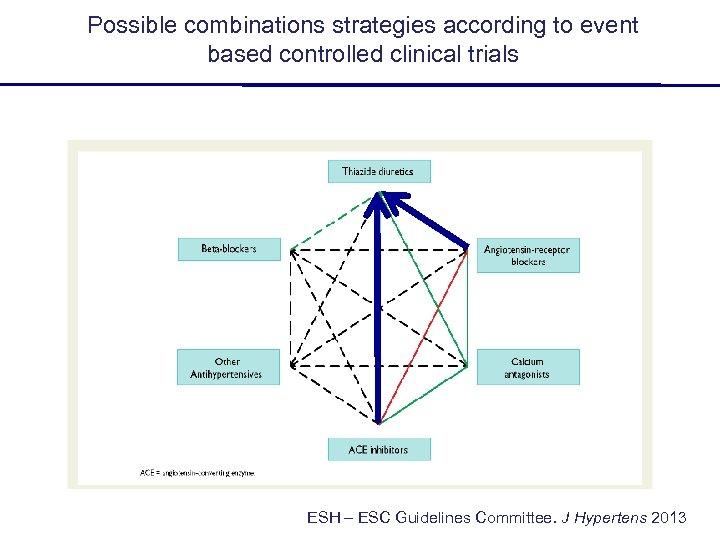
Possible combinations strategies according to event based controlled clinical trials ESH – ESC Guidelines Committee. J Hypertens 2013

Equivalenza tra ACE-I e ARBs
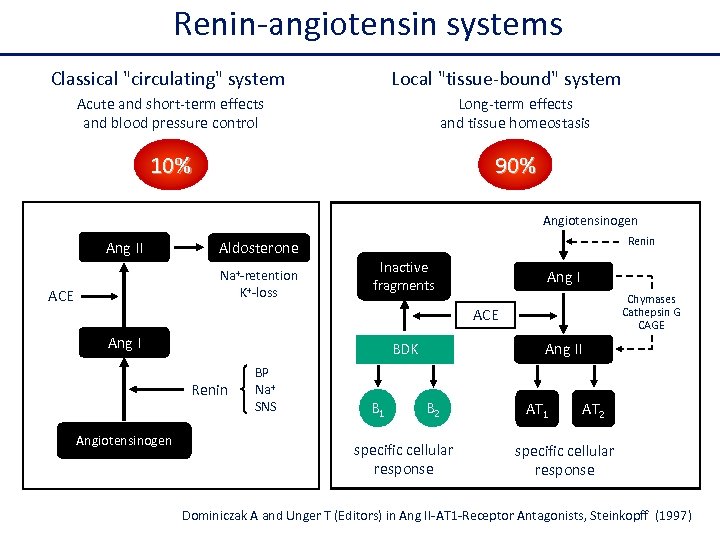
Renin-angiotensin systems Classical "circulating" system Local "tissue-bound" system Acute and short-term effects and blood pressure control Long-term effects and tissue homeostasis 10% 90% Angiotensinogen Ang II Na+-retention K+-loss ACE Renin Aldosterone Inactive fragments Ang I Chymases Cathepsin G CAGE ACE Ang I BDK Renin Angiotensinogen BP Na+ SNS B 1 Ang II B 2 specific cellular response AT 1 AT 2 specific cellular response Dominiczak A and Unger T (Editors) in Ang II-AT 1 -Receptor Antagonists, Steinkopff (1997)
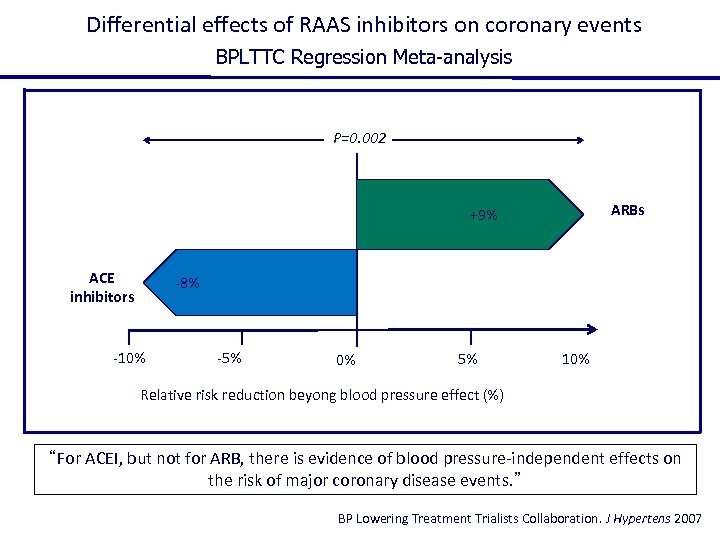
Differential effects of RAAS inhibitors on coronary events BPLTTC Regression Meta-analysis P=0. 002 ARBs +9% ACE inhibitors -8% -10% -5% 0% 5% 10% Relative risk reduction beyong blood pressure effect (%) “For ACEI, but not for ARB, there is evidence of blood pressure-independent effects on the risk of major coronary disease events. ” BP Lowering Treatment Trialists Collaboration. J Hypertens 2007

The finding that ARBs are not effective on coronary artery disease is not unexpected since there is no one controlled clinical trial who has demonstrate a beneficial effect of ARBs on this specific end point!!
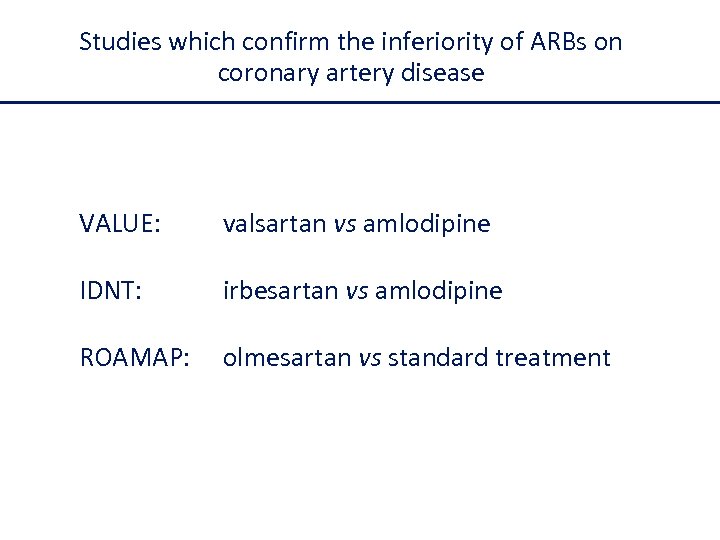
Studies which confirm the inferiority of ARBs on coronary artery disease VALUE: valsartan vs amlodipine IDNT: irbesartan vs amlodipine ROAMAP: olmesartan vs standard treatment
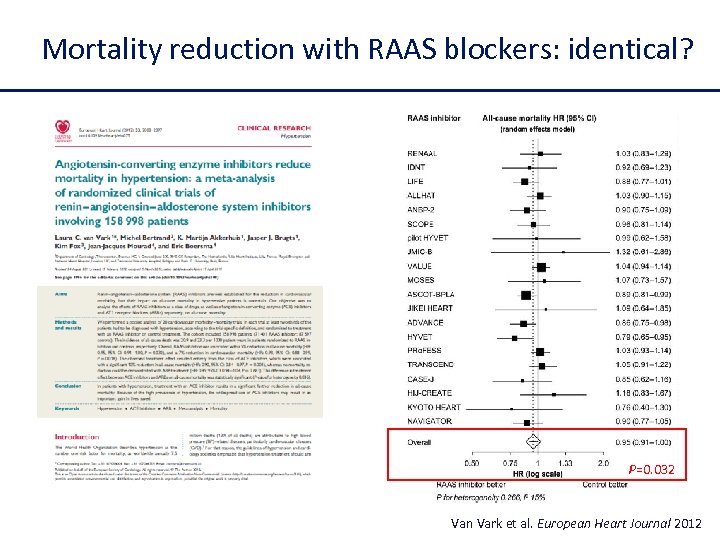
Mortality reduction with RAAS blockers: identical? P=0. 032 Van Vark et al. European Heart Journal 2012
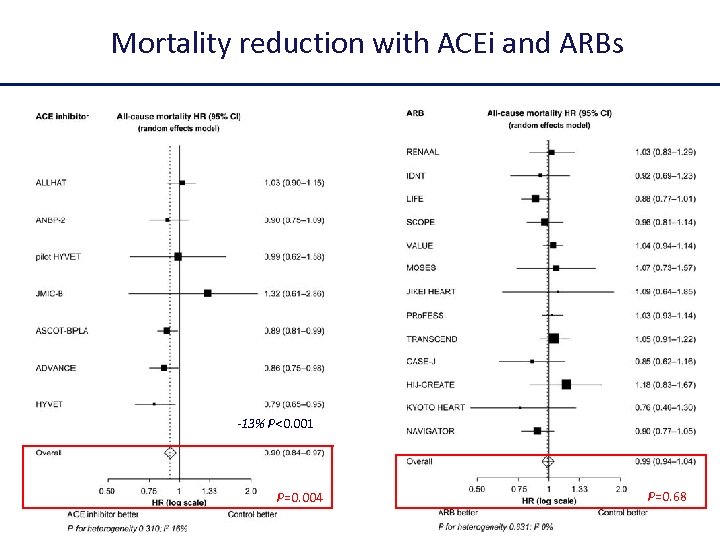
Mortality reduction with ACEi and ARBs -13% P<0. 001 P=0. 004 P=0. 68
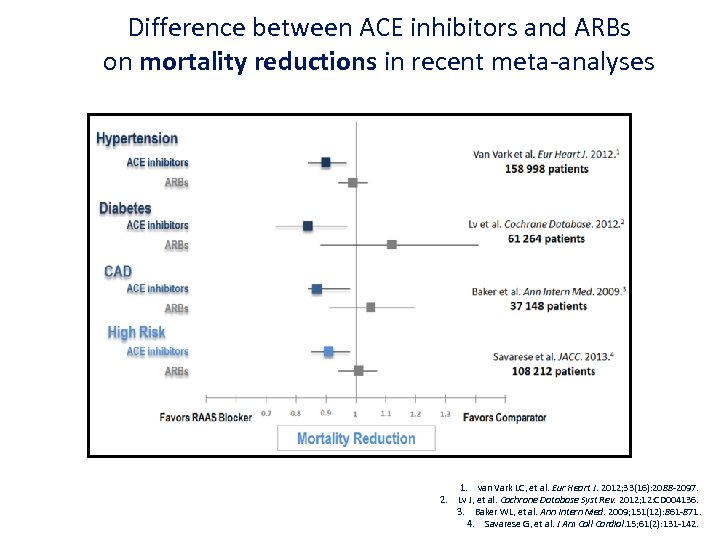
Difference between ACE inhibitors and ARBs on mortality reductions in recent meta-analyses 1. van Vark LC, et al. Eur Heart J. 2012; 33(16): 2088 -2097. 2. Lv J, et al. Cochrane Database Syst Rev. 2012; 12: CD 004136. 3. Baker WL, et al. Ann Intern Med. 2009; 151(12): 861 -871. 4. Savarese G, et al. J Am Coll Cardiol. 15; 61(2): 131 -142.
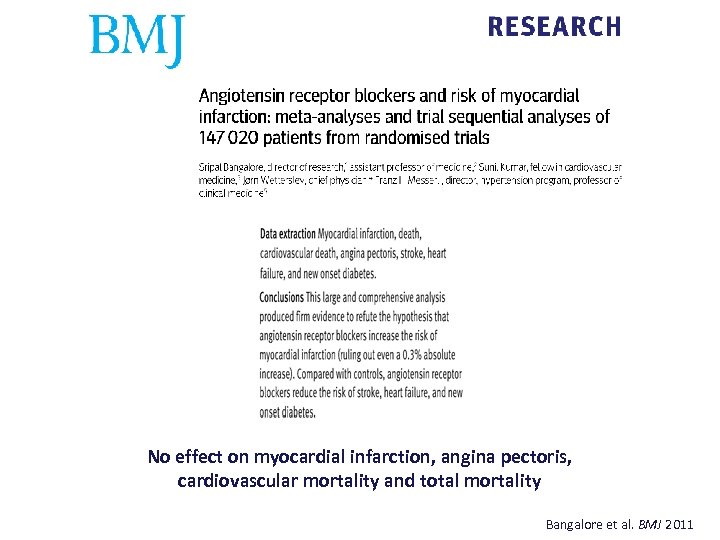
No effect on myocardial infarction, angina pectoris, cardiovascular mortality and total mortality Bangalore et al. BMJ 2011
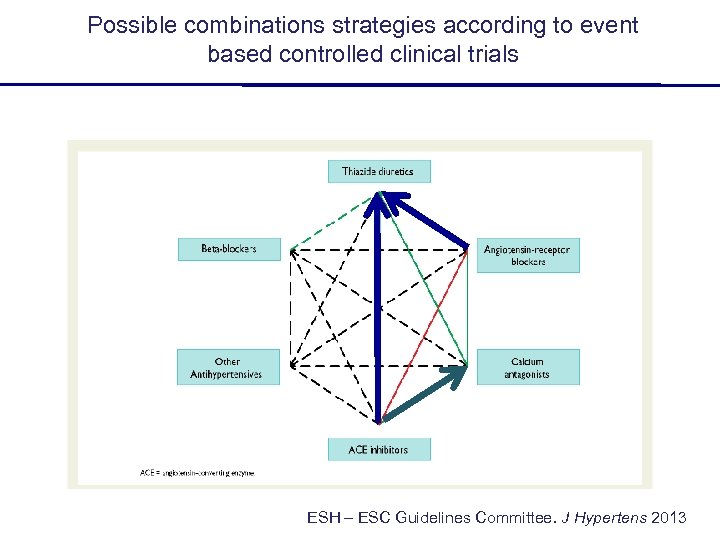
Possible combinations strategies according to event based controlled clinical trials ESH – ESC Guidelines Committee. J Hypertens 2013
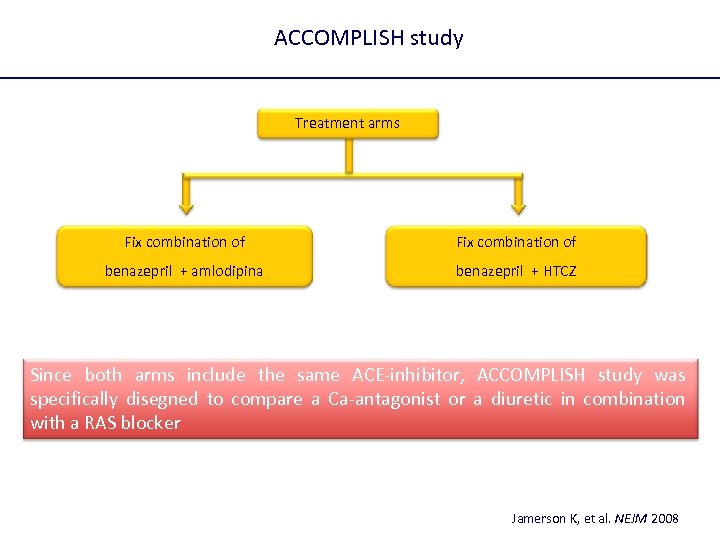
ACCOMPLISH study Treatment arms Fix combination of benazepril + amlodipina benazepril + HTCZ Since both arms include the same ACE-inhibitor, ACCOMPLISH study was specifically disegned to compare a Ca-antagonist or a diuretic in combination with a RAS blocker Jamerson K, et al. NEJM 2008
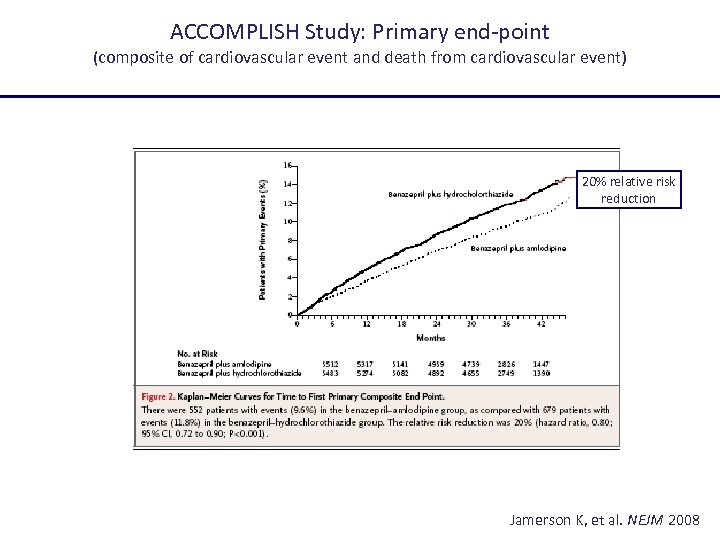
ACCOMPLISH Study: Primary end-point (composite of cardiovascular event and death from cardiovascular event) 20% relative risk reduction Jamerson K, et al. NEJM 2008

Quale ACE-inibitore? Quale Calcio-antagonista?
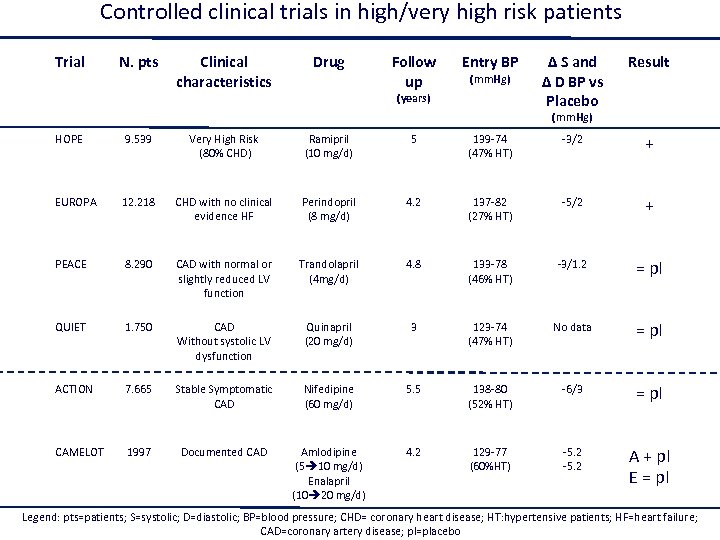
Controlled clinical trials in high/very high risk patients Trial N. pts Clinical characteristics Drug Follow up Entry BP (mm. Hg) (years) Δ S and Δ D BP vs Placebo Result (mm. Hg) HOPE 9. 539 Very High Risk (80% CHD) Ramipril (10 mg/d) 5 139 -74 (47% HT) -3/2 + EUROPA 12. 218 CHD with no clinical evidence HF Perindopril (8 mg/d) 4. 2 137 -82 (27% HT) -5/2 + PEACE 8. 290 CAD with normal or slightly reduced LV function Trandolapril (4 mg/d) 4. 8 133 -78 (46% HT) -3/1. 2 = pl QUIET 1. 750 CAD Without systolic LV dysfunction Quinapril (20 mg/d) 3 123 -74 (47% HT) No data = pl ACTION 7. 665 Stable Symptomatic CAD Nifedipine (60 mg/d) 5. 5 138 -80 (52% HT) -6/3 = pl CAMELOT 1997 Documented CAD Amlodipine (5 10 mg/d) Enalapril (10 20 mg/d) 4. 2 129 -77 (60%HT) -5. 2 A + pl E = pl Legend: pts=patients; S=systolic; D=diastolic; BP=blood pressure; CHD= coronary heart disease; HT: hypertensive patients; HF=heart failure; CAD=coronary artery disease; pl=placebo
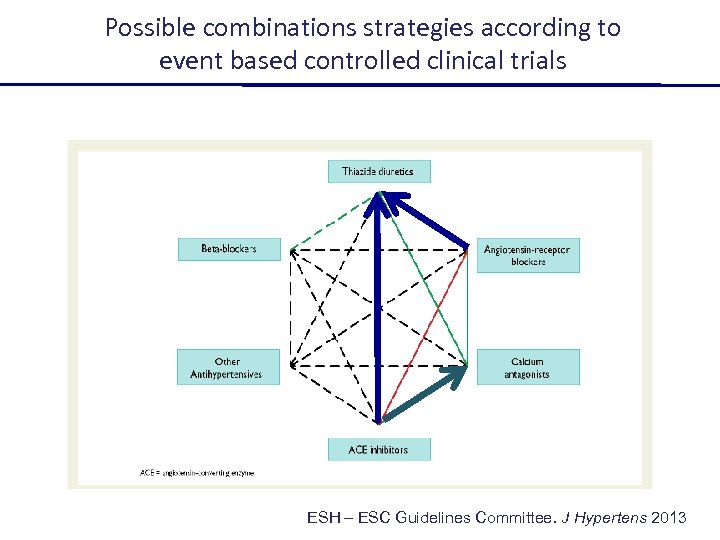
Possible combinations strategies according to event based controlled clinical trials ESH – ESC Guidelines Committee. J Hypertens 2013
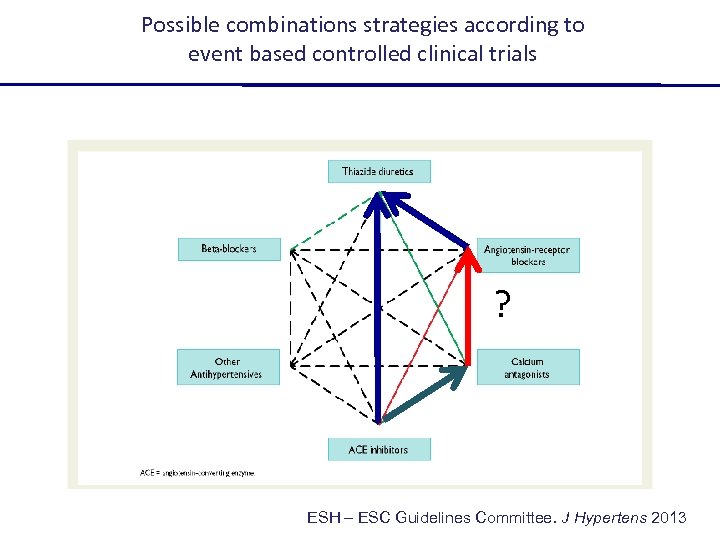
Possible combinations strategies according to event based controlled clinical trials ? ESH – ESC Guidelines Committee. J Hypertens 2013
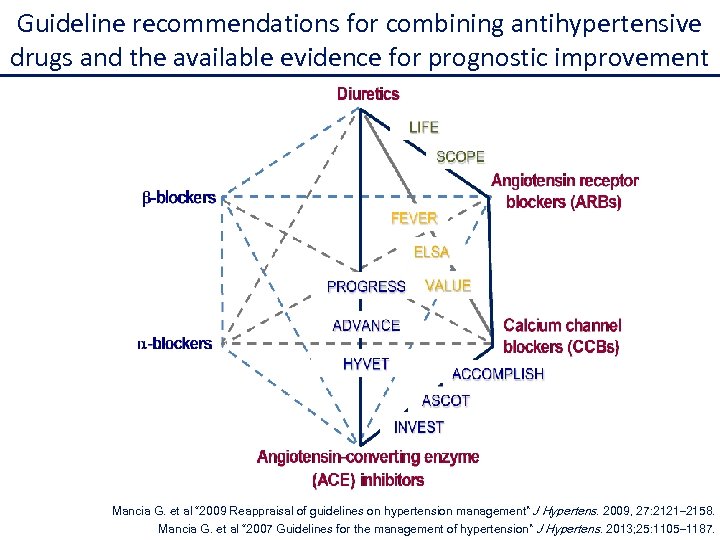
Guideline recommendations for combining antihypertensive drugs and the available evidence for prognostic improvement Mancia G. et al “ 2009 Reappraisal of guidelines on hypertension management” J Hypertens. 2009, 27: 2121– 2158. Mancia G. et al “ 2007 Guidelines for the management of hypertension” J Hypertens. 2013; 25: 1105– 1187.
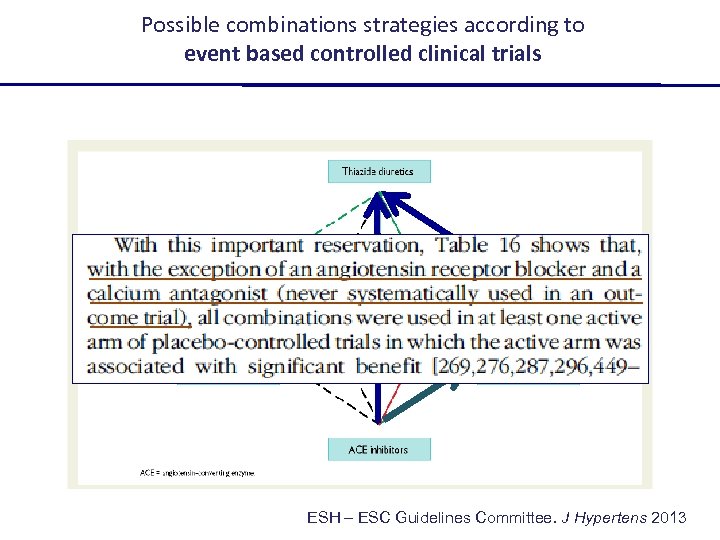
Possible combinations strategies according to event based controlled clinical trials ? ESH – ESC Guidelines Committee. J Hypertens 2013
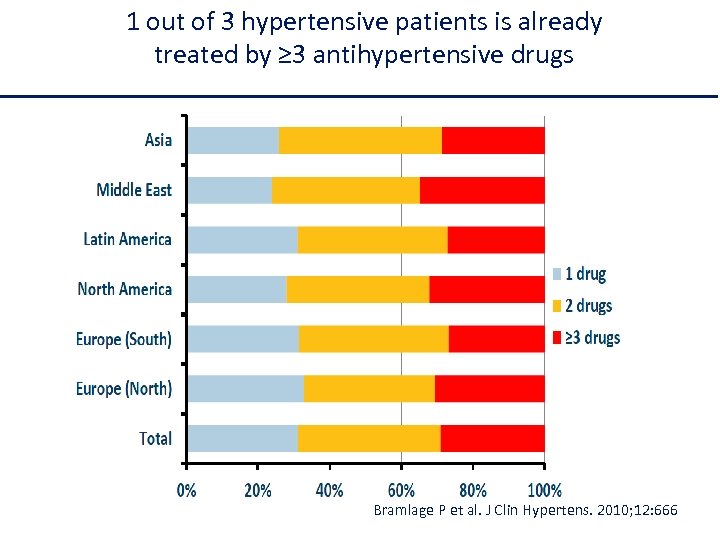
1 out of 3 hypertensive patients is already treated by ≥ 3 antihypertensive drugs Bramlage P et al. J Clin Hypertens. 2010; 12: 666

The evidence supporting the utilization of a triple combination including Perindopril, Indapamide and Amlodipine
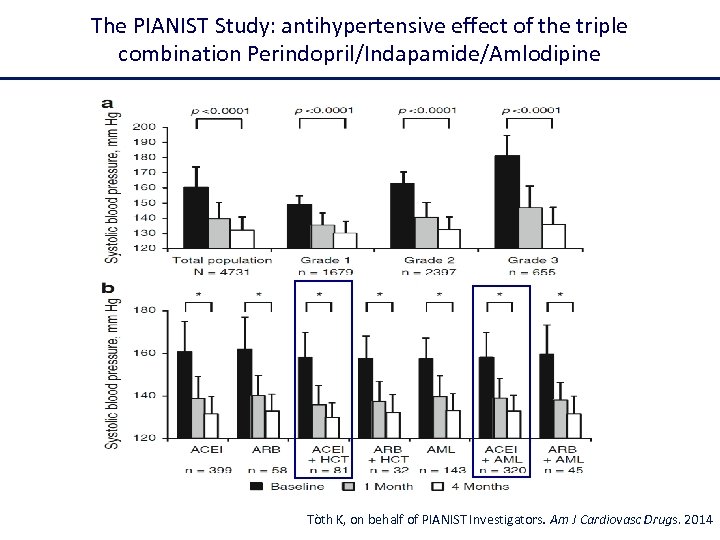
The PIANIST Study: antihypertensive effect of the triple combination Perindopril/Indapamide/Amlodipine Tòth K, on behalf of PIANIST Investigators. Am J Cardiovasc Drugs. 2014
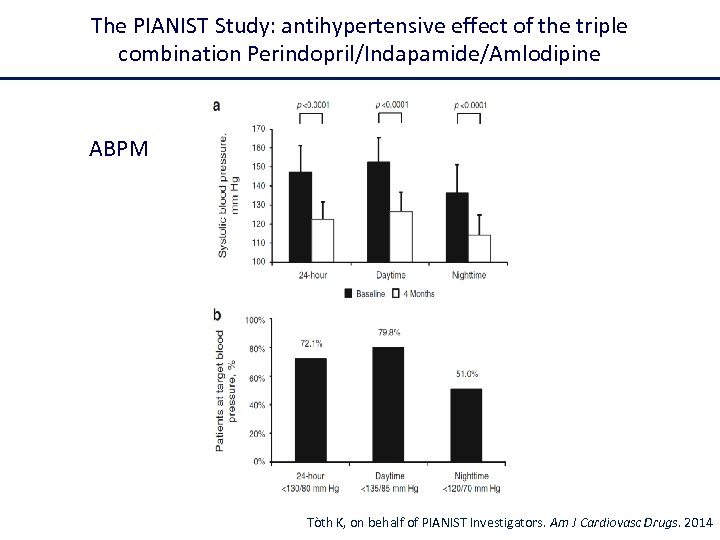
The PIANIST Study: antihypertensive effect of the triple combination Perindopril/Indapamide/Amlodipine ABPM Tòth K, on behalf of PIANIST Investigators. Am J Cardiovasc Drugs. 2014
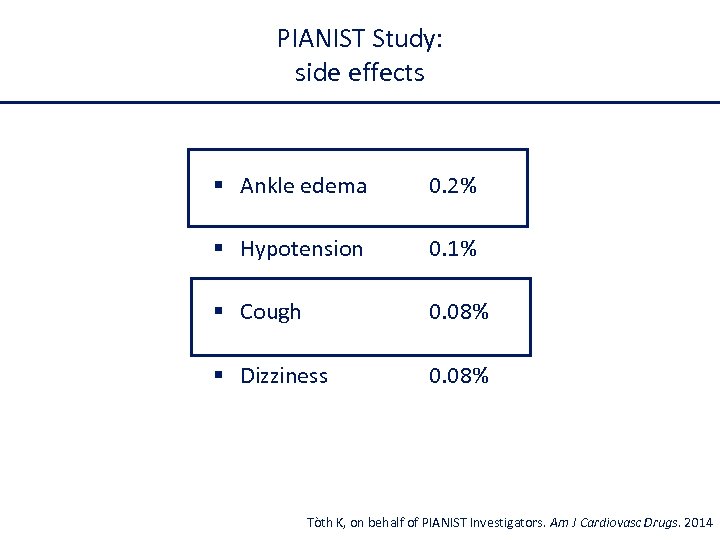
PIANIST Study: side effects § Ankle edema 0. 2% § Hypotension 0. 1% § Cough 0. 08% § Dizziness 0. 08% Tòth K, on behalf of PIANIST Investigators. Am J Cardiovasc Drugs. 2014
This analysis compares the effect of the combination perindopril/indapamide in the absence and presence of calcium antagonist treatment at baseline. Chalmers J et al. Hypertension 2014
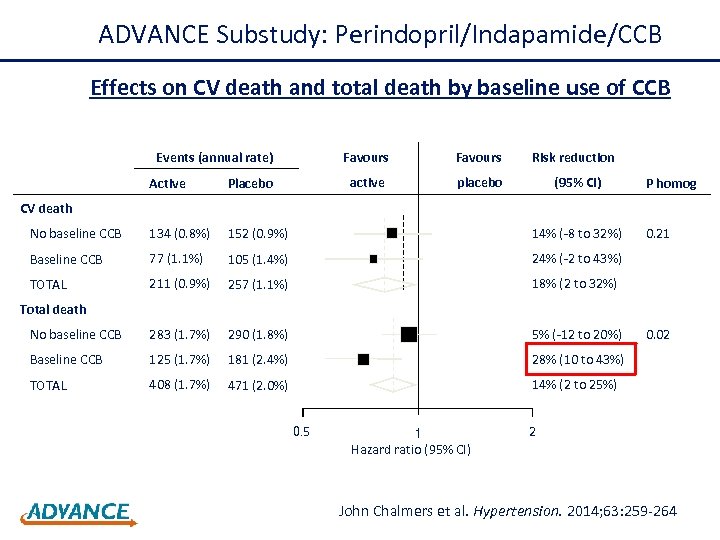
ADVANCE Substudy: Perindopril/Indapamide/CCB Effects on CV death and total death by baseline use of CCB Events (annual rate) Favours active placebo Risk reduction (95% CI) Active Placebo No baseline CCB 134 (0. 8%) 152 (0. 9%) 14% (-8 to 32%) Baseline CCB 77 (1. 1%) 105 (1. 4%) 24% (-2 to 43%) TOTAL 211 (0. 9%) 257 (1. 1%) 18% (2 to 32%) No baseline CCB 283 (1. 7%) 290 (1. 8%) 5% (-12 to 20%) Baseline CCB 125 (1. 7%) 181 (2. 4%) 28% (10 to 43%) TOTAL 408 (1. 7%) 471 (2. 0%) P homog 14% (2 to 25%) CV death 0. 21 Total death 0. 5 1 Hazard ratio (95% CI) 0. 02 2 John Chalmers et al. Hypertension. 2014; 63: 259 -264
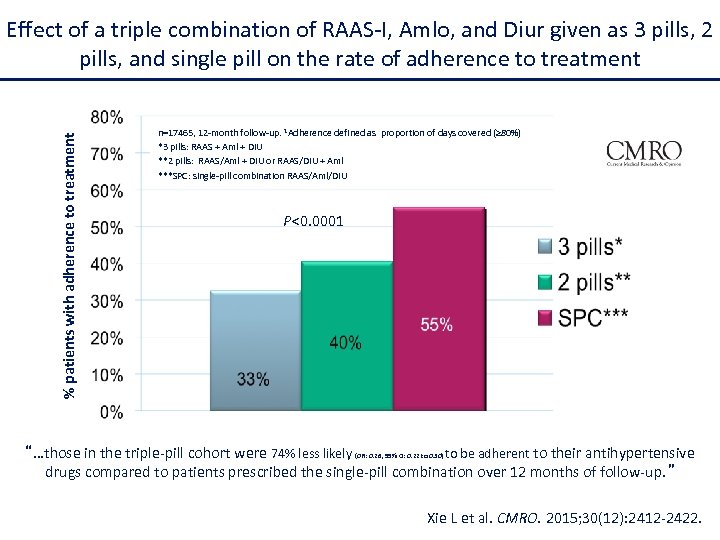
% patients with adherence to treatment Effect of a triple combination of RAAS-I, Amlo, and Diur given as 3 pills, 2 pills, and single pill on the rate of adherence to treatment n=17465, 12 -month follow-up. 1 Adherence defined as proportion of days covered (≥ 80%) *3 pills: RAAS + Aml + DIU **2 pills: RAAS/Aml + DIU or RAAS/DIU + Aml ***SPC: single-pill combination RAAS/Aml/DIU P<0. 0001 “…those in the triple-pill cohort were 74% less likely (OR: 0. 26, 95% CI: 0. 22 to 0. 30) to be adherent to their antihypertensive drugs compared to patients prescribed the single-pill combination over 12 months of follow-up. ” Xie L et al. CMRO. 2015; 30(12): 2412 -2422.

Valori target nel paziente iperteso 1. Ridurre la pressione arteriosa sotto 140 -90 mm. Hg 2. Possibilmente raggiungere valori intorno a 130 -80 mm. Hg (considerando anche la variabilità della misurazione della pressione arteriosa). 3. Valori ancora più bassi sono ben accetti purchè siano ottenuti senza problemi di compliance (numero di farmaci) e di effetti collaterali.

… la vita reale … Grazie per l’attenzione! Courtesy of A. Ungar
4ea502df1409c4098fab75d9cab7d39a.ppt