aa94c854c9b868b111c6cec38bbc9bab.ppt
- Количество слайдов: 17
 Creating ADa. M Friendly Analysis Data from SDTM Using Meta-data by Erik Brun & Rico Schiller (CD 10 - 2011) H. Lundbeck A/S 19 -Mar-18 1
Creating ADa. M Friendly Analysis Data from SDTM Using Meta-data by Erik Brun & Rico Schiller (CD 10 - 2011) H. Lundbeck A/S 19 -Mar-18 1
 Agenda The challenges The solution Conclusion Abreviations used: SADs 4 – HLu Statistical Analysis Data. Sets v. 4 DCD – HLu Meta Data Dictionary CDR – Clinical Data Repository H. Lundbeck A/S 19 -Mar-18 2
Agenda The challenges The solution Conclusion Abreviations used: SADs 4 – HLu Statistical Analysis Data. Sets v. 4 DCD – HLu Meta Data Dictionary CDR – Clinical Data Repository H. Lundbeck A/S 19 -Mar-18 2
 The Challenges The funnel and the trumpet SDTM data: Take data from a variety of sources and funnel it into a standard format Analysis data: Take data from a standard format and expand it into a variety of formats depeding on study design (and the statisticians) Data Flow H. Lundbeck A/S 19 -Mar-18 3
The Challenges The funnel and the trumpet SDTM data: Take data from a variety of sources and funnel it into a standard format Analysis data: Take data from a standard format and expand it into a variety of formats depeding on study design (and the statisticians) Data Flow H. Lundbeck A/S 19 -Mar-18 3
 The Challenges Lundbeck challenges with SADs v. 3 Time resolution was date not date-time Data model embedded in the code Peculiar error and warning messages Including reports on data issues Only one central lab was assumed used per study Very steep learning curve for new programmers Person dependent Insufficent for new study designs H. Lundbeck A/S 19 -Mar-18 4
The Challenges Lundbeck challenges with SADs v. 3 Time resolution was date not date-time Data model embedded in the code Peculiar error and warning messages Including reports on data issues Only one central lab was assumed used per study Very steep learning curve for new programmers Person dependent Insufficent for new study designs H. Lundbeck A/S 19 -Mar-18 4
 The Solution – SADs 4 Requirements Create the basis upon which the automated and validated production of consistent and standardised statistical analysis reports and listings for safety and efficacy data is possible. The system should allow for clear documentation of the configuration settings applied in a single study. The system should be easy to understand operate and yet flexible to handle a wide range of study designs. The system should be as CDISC-compliant as possible. Lundbeck pursues a strategy of applying CDISC standards, terminology, and concepts in all scientific data models. Provide together with CDR a validated and controlled environment for the collection and integration of clinical data across studies within a drug project. H. Lundbeck A/S 19 -Mar-18 5
The Solution – SADs 4 Requirements Create the basis upon which the automated and validated production of consistent and standardised statistical analysis reports and listings for safety and efficacy data is possible. The system should allow for clear documentation of the configuration settings applied in a single study. The system should be easy to understand operate and yet flexible to handle a wide range of study designs. The system should be as CDISC-compliant as possible. Lundbeck pursues a strategy of applying CDISC standards, terminology, and concepts in all scientific data models. Provide together with CDR a validated and controlled environment for the collection and integration of clinical data across studies within a drug project. H. Lundbeck A/S 19 -Mar-18 5
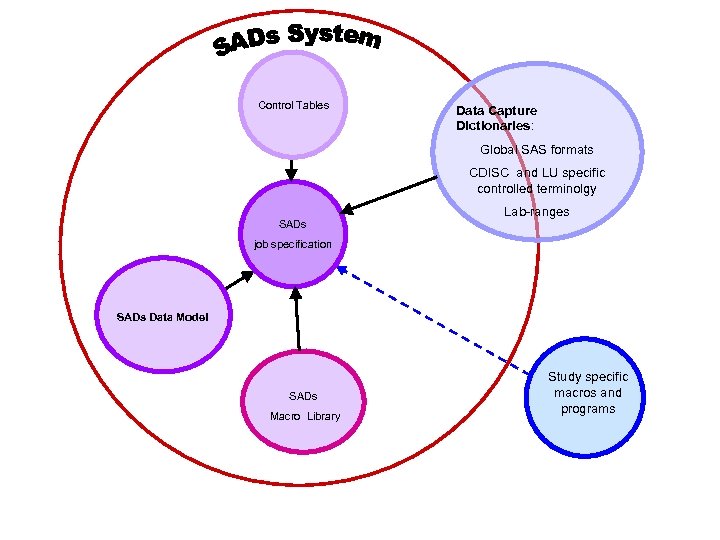 Control Tables Data Capture Dictionaries: Global SAS formats CDISC and LU specific controlled terminolgy SADs Lab-ranges job specification SADs Data Model SADs Macro Library Study specific macros and programs
Control Tables Data Capture Dictionaries: Global SAS formats CDISC and LU specific controlled terminolgy SADs Lab-ranges job specification SADs Data Model SADs Macro Library Study specific macros and programs
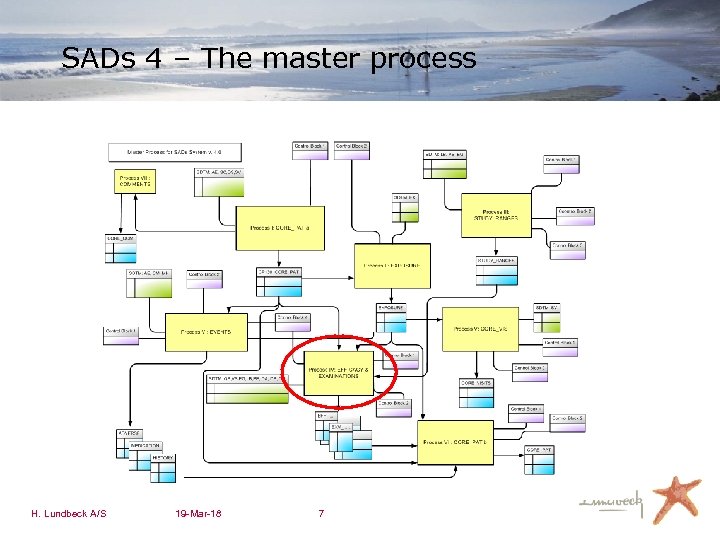 SADs 4 – The master process H. Lundbeck A/S 19 -Mar-18 7
SADs 4 – The master process H. Lundbeck A/S 19 -Mar-18 7
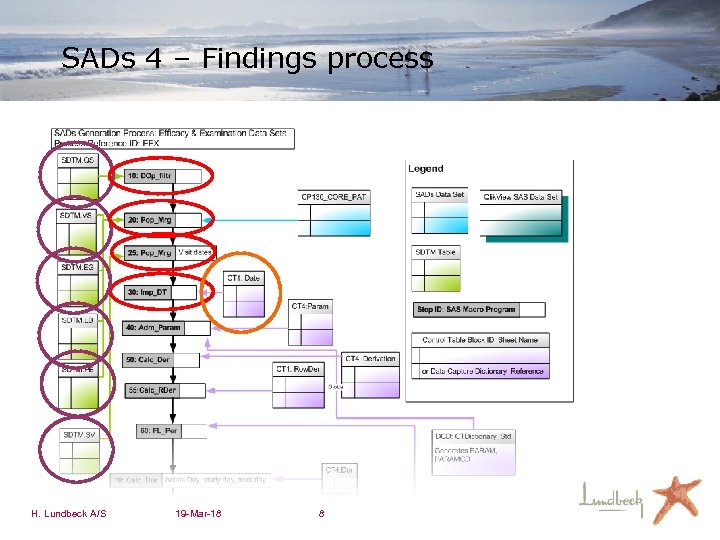 SADs 4 – Findings process H. Lundbeck A/S 19 -Mar-18 8
SADs 4 – Findings process H. Lundbeck A/S 19 -Mar-18 8
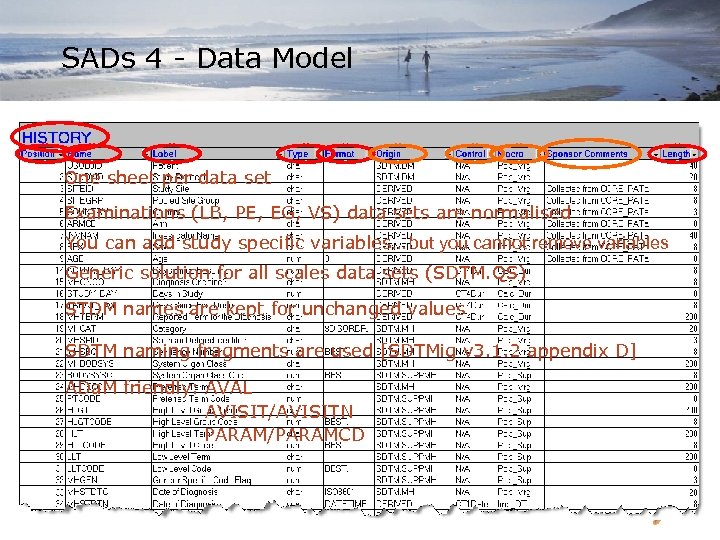 SADs 4 - Data Model One sheet per data set Examinations (LB, PE, EG, VS) data sets are normalised You can add study specific variables… but you cannot remove variables Generic solution for all scales data sets (SDTM. QS) STDM names are kept for unchanged values SDTM naming fragments are used [SDTMig v 3. 1. 2 appendix D] ADa. M friendly: AVAL AVISIT/AVISITN PARAM/PARAMCD H. Lundbeck A/S 19 -Mar-18 9
SADs 4 - Data Model One sheet per data set Examinations (LB, PE, EG, VS) data sets are normalised You can add study specific variables… but you cannot remove variables Generic solution for all scales data sets (SDTM. QS) STDM names are kept for unchanged values SDTM naming fragments are used [SDTMig v 3. 1. 2 appendix D] ADa. M friendly: AVAL AVISIT/AVISITN PARAM/PARAMCD H. Lundbeck A/S 19 -Mar-18 9
 SADs 4 – Control Tables Assign group centre Add treatment code Add population flags Rules for date imputations Derivations: Type casting Scale totals etc. Etc. Baseline definitions Windowing of Visits Sort order of output datasets Period definitions Study specific additions to the data model … and much more H. Lundbeck A/S 19 -Mar-18 10
SADs 4 – Control Tables Assign group centre Add treatment code Add population flags Rules for date imputations Derivations: Type casting Scale totals etc. Etc. Baseline definitions Windowing of Visits Sort order of output datasets Period definitions Study specific additions to the data model … and much more H. Lundbeck A/S 19 -Mar-18 10
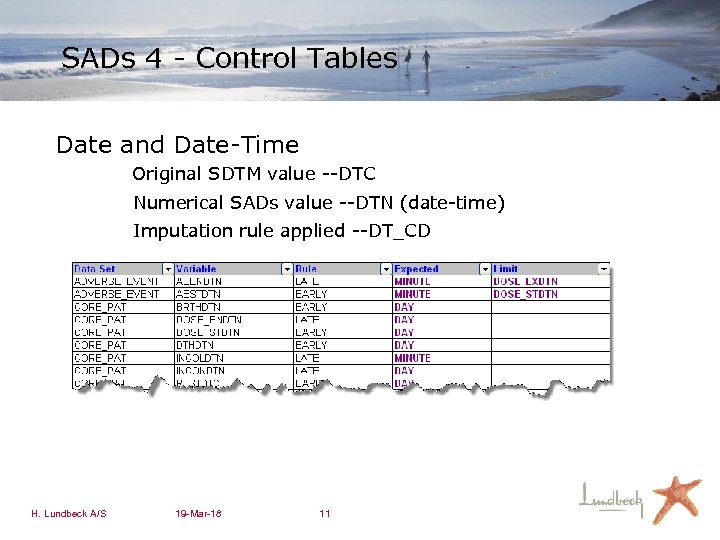 SADs 4 - Control Tables Date and Date-Time Original SDTM value --DTC Numerical SADs value --DTN (date-time) Imputation rule applied --DT_CD H. Lundbeck A/S 19 -Mar-18 11
SADs 4 - Control Tables Date and Date-Time Original SDTM value --DTC Numerical SADs value --DTN (date-time) Imputation rule applied --DT_CD H. Lundbeck A/S 19 -Mar-18 11
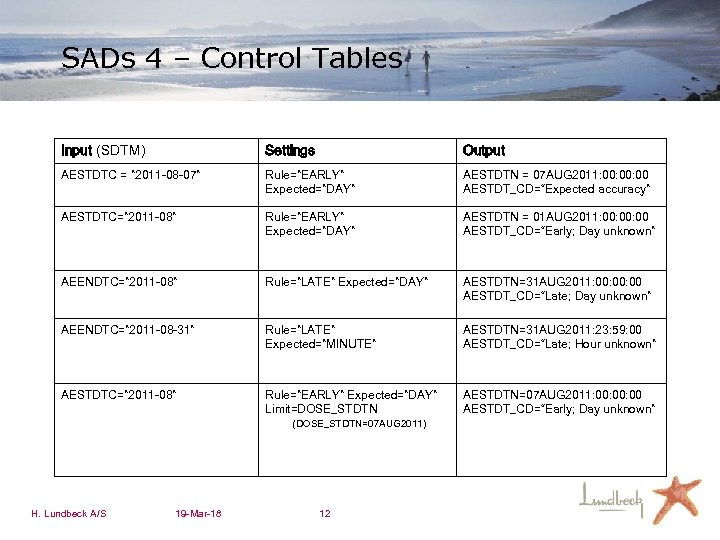 SADs 4 – Control Tables Input (SDTM) Settings Output AESTDTC = ” 2011 -08 -07” Rule=”EARLY” Expected=”DAY” AESTDTN = 07 AUG 2011: 00: 00 AESTDT_CD=“Expected accuracy” AESTDTC=” 2011 -08” Rule=”EARLY” Expected=”DAY” AESTDTN = 01 AUG 2011: 00: 00 AESTDT_CD=“Early; Day unknown” AEENDTC=” 2011 -08” Rule=”LATE” Expected=”DAY” AESTDTN=31 AUG 2011: 00: 00 AESTDT_CD=“Late; Day unknown” AEENDTC=” 2011 -08 -31” Rule=”LATE” Expected=”MINUTE” AESTDTN=31 AUG 2011: 23: 59: 00 AESTDT_CD=“Late; Hour unknown” AESTDTC=” 2011 -08” Rule=”EARLY” Expected=”DAY” Limit=DOSE_STDTN AESTDTN=07 AUG 2011: 00: 00 AESTDT_CD=“Early; Day unknown” (DOSE_STDTN=07 AUG 2011) H. Lundbeck A/S 19 -Mar-18 12
SADs 4 – Control Tables Input (SDTM) Settings Output AESTDTC = ” 2011 -08 -07” Rule=”EARLY” Expected=”DAY” AESTDTN = 07 AUG 2011: 00: 00 AESTDT_CD=“Expected accuracy” AESTDTC=” 2011 -08” Rule=”EARLY” Expected=”DAY” AESTDTN = 01 AUG 2011: 00: 00 AESTDT_CD=“Early; Day unknown” AEENDTC=” 2011 -08” Rule=”LATE” Expected=”DAY” AESTDTN=31 AUG 2011: 00: 00 AESTDT_CD=“Late; Day unknown” AEENDTC=” 2011 -08 -31” Rule=”LATE” Expected=”MINUTE” AESTDTN=31 AUG 2011: 23: 59: 00 AESTDT_CD=“Late; Hour unknown” AESTDTC=” 2011 -08” Rule=”EARLY” Expected=”DAY” Limit=DOSE_STDTN AESTDTN=07 AUG 2011: 00: 00 AESTDT_CD=“Early; Day unknown” (DOSE_STDTN=07 AUG 2011) H. Lundbeck A/S 19 -Mar-18 12
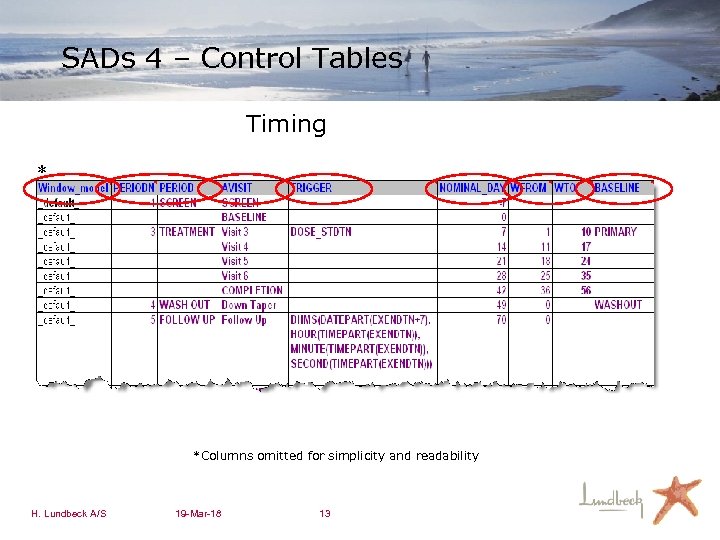 SADs 4 – Control Tables Timing * *Columns omitted for simplicity and readability H. Lundbeck A/S 19 -Mar-18 13
SADs 4 – Control Tables Timing * *Columns omitted for simplicity and readability H. Lundbeck A/S 19 -Mar-18 13
 Conclusions We have a validated system that works! It is flexible SDTM 3. 1. x can be used as source It has been used with success on a wide range of indications and study designs A junior programmer can make a good draft set-up of a study in 1½ day Easy to use Integration of studies made much easier The SADs data sets work for our standard reporting system ”Real” ADa. M data sets can easily be created from SADs 4 Renaming and type casting is all what is needed H. Lundbeck A/S 19 -Mar-18 14
Conclusions We have a validated system that works! It is flexible SDTM 3. 1. x can be used as source It has been used with success on a wide range of indications and study designs A junior programmer can make a good draft set-up of a study in 1½ day Easy to use Integration of studies made much easier The SADs data sets work for our standard reporting system ”Real” ADa. M data sets can easily be created from SADs 4 Renaming and type casting is all what is needed H. Lundbeck A/S 19 -Mar-18 14
 Conclusions A system generating SDTM has since been made applying the same methodologies, both in development and use SAS-DI can not be recommended as a tool for developing systems like this It requires not only dedicated and skilled resources to develop such a system. They must also be assigned wholehearted by their managers to the project The future: Move away from Excel as control tables CDISC PRM (Protocol Representation Model) , it could reduce and/or simplify the control tables, and the stat. prog. will not have to re-enter a lot of information H. Lundbeck A/S 19 -Mar-18 15
Conclusions A system generating SDTM has since been made applying the same methodologies, both in development and use SAS-DI can not be recommended as a tool for developing systems like this It requires not only dedicated and skilled resources to develop such a system. They must also be assigned wholehearted by their managers to the project The future: Move away from Excel as control tables CDISC PRM (Protocol Representation Model) , it could reduce and/or simplify the control tables, and the stat. prog. will not have to re-enter a lot of information H. Lundbeck A/S 19 -Mar-18 15
 SADs 4 ? ? ? H. Lundbeck A/S 19 -Mar-18 16
SADs 4 ? ? ? H. Lundbeck A/S 19 -Mar-18 16
 Contact Erik Brun, System & Process Specialist H. Lundbeck A/S Ottiliavej 9 2500 Valby Denmark erik@lundbeck. com Rico Schiller, Head of Section H. Lundbeck A/S Ottiliavej 9 2500 Valby Denmark rico@lundbeck. com H. Lundbeck A/S 19 -Mar-18 17
Contact Erik Brun, System & Process Specialist H. Lundbeck A/S Ottiliavej 9 2500 Valby Denmark erik@lundbeck. com Rico Schiller, Head of Section H. Lundbeck A/S Ottiliavej 9 2500 Valby Denmark rico@lundbeck. com H. Lundbeck A/S 19 -Mar-18 17


