df0af3c5eb4b30c9e1cb71720adb3d50.ppt
- Количество слайдов: 11

Creating a Conducive Environment for Biotechnology: The Cartagena Protocol as an Enabling Framework Drew L. Kershen Earl Sneed Centennial Professor University of Oklahoma College of Law Copyright 2008, Drew L. Kershen – all rights reserved
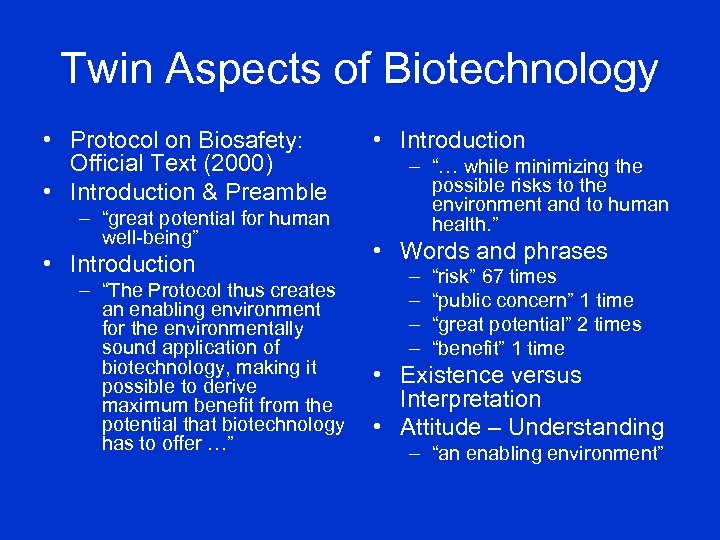
Twin Aspects of Biotechnology • Protocol on Biosafety: Official Text (2000) • Introduction & Preamble – “great potential for human well-being” • Introduction – “The Protocol thus creates an enabling environment for the environmentally sound application of biotechnology, making it possible to derive maximum benefit from the potential that biotechnology has to offer …” • Introduction – “… while minimizing the possible risks to the environment and to human health. ” • Words and phrases – – “risk” 67 times “public concern” 1 time “great potential” 2 times “benefit” 1 time • Existence versus Interpretation • Attitude – Understanding – “an enabling environment”

South East Asia: Objectives • Comply with International Obligations – World Trade Organization (WTO) Agreements – Cartagena Protocol on Biosafety (CPB) – Seek compatibility between both obligations • Focus on Agricultural Biotechnology – – – Encourage innovation, science and investment Develop the agricultural sector Insure food and feed safety Protect environmental values and resources Promote health and general welfare • Promote Science Based Regulatory Policy – Unless nations use science, cannot fulfill the two prior objectives – i. e. The comply and focus objectives
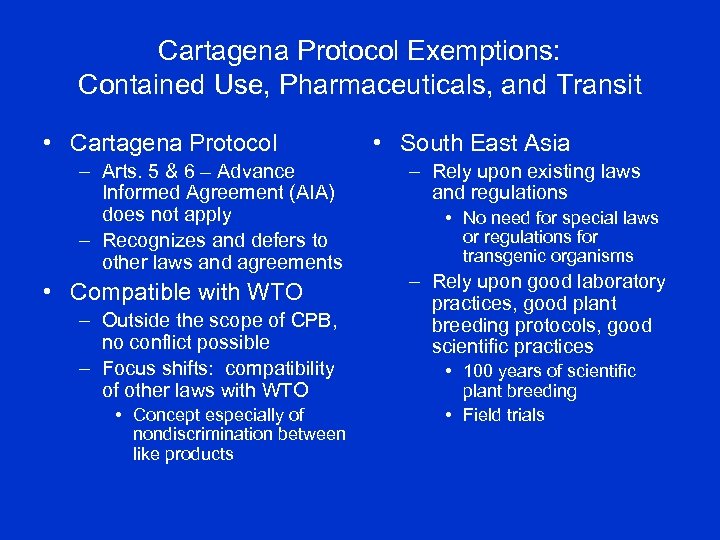
Cartagena Protocol Exemptions: Contained Use, Pharmaceuticals, and Transit • Cartagena Protocol – Arts. 5 & 6 – Advance Informed Agreement (AIA) does not apply – Recognizes and defers to other laws and agreements • Compatible with WTO – Outside the scope of CPB, no conflict possible – Focus shifts: compatibility of other laws with WTO • Concept especially of nondiscrimination between like products • South East Asia – Rely upon existing laws and regulations • No need for special laws or regulations for transgenic organisms – Rely upon good laboratory practices, good plant breeding protocols, good scientific practices • 100 years of scientific plant breeding • Field trials
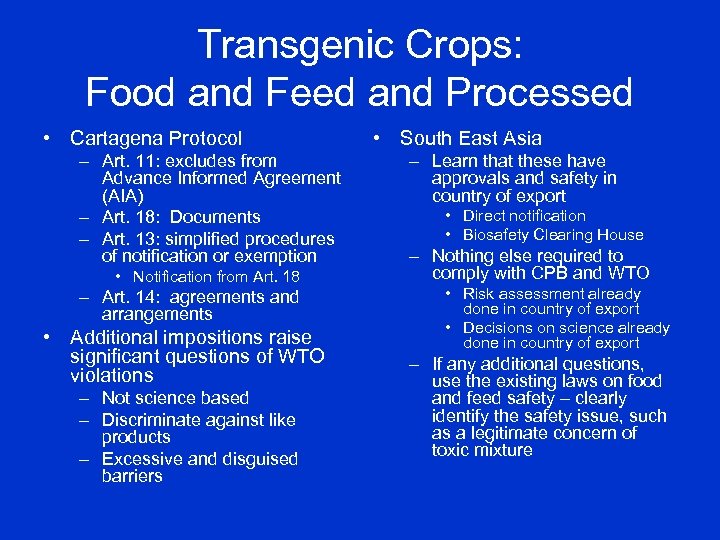
Transgenic Crops: Food and Feed and Processed • Cartagena Protocol – Art. 11: excludes from Advance Informed Agreement (AIA) – Art. 18: Documents – Art. 13: simplified procedures of notification or exemption • Notification from Art. 18 – Art. 14: agreements and arrangements • Additional impositions raise significant questions of WTO violations – Not science based – Discriminate against like products – Excessive and disguised barriers • South East Asia – Learn that these have approvals and safety in country of export • Direct notification • Biosafety Clearing House – Nothing else required to comply with CPB and WTO • Risk assessment already done in country of export • Decisions on science already done in country of export – If any additional questions, use the existing laws on food and feed safety – clearly identify the safety issue, such as a legitimate concern of toxic mixture

Transgenic Crops: Introduction into the Environment • Cartagena Protocol – (CBD) Convention on Biological Diversity: “conservation and sustainable use of biological diversity” – Arts. 7 -10, Arts. 15 -16, AIA procedures, risk assessment, and risk management – Core articles and core concern • Compatible with WTO if: – Based in science – Nondiscriminatory – Not excessive or disguised barriers to trade • South East Asia – equivalent AIA, risk assessment and risk management – Make nondiscriminatory as applying to domestic and nondomestic crops – CPB Arts 13 & 14: simplified procedures • Approvals in other nations • Rely on already done risk assessments and risk management plans • Only reconsider if a specific, legitimate additional concern about biodiversity • Approvals should be final – transparent, clear, and certain

Cartagena Concepts: Permissive not Mandatory • Socio-economic considerations • – CPB Art. 27 “… appropriate elaboration of international rules and procedures …” – CPB Art. 26: “… may take into account, consistent with their international obligations …” • Permissive, not mandatory • WTO obligations are based in science, not socio-economic considerations • Nations have no CPB obligation to include socioeconomic considerations in its biosafety laws. Liability and redress • The appropriate elaboration is no international rules and procedures • Nations have no CPB obligation to address liability and redress in its biosafety laws – Use existing laws for comparable harms and damages, if any – Environmental laws about damage should be nondiscriminatory – i. e. should be general laws that do not single out transgenic crops
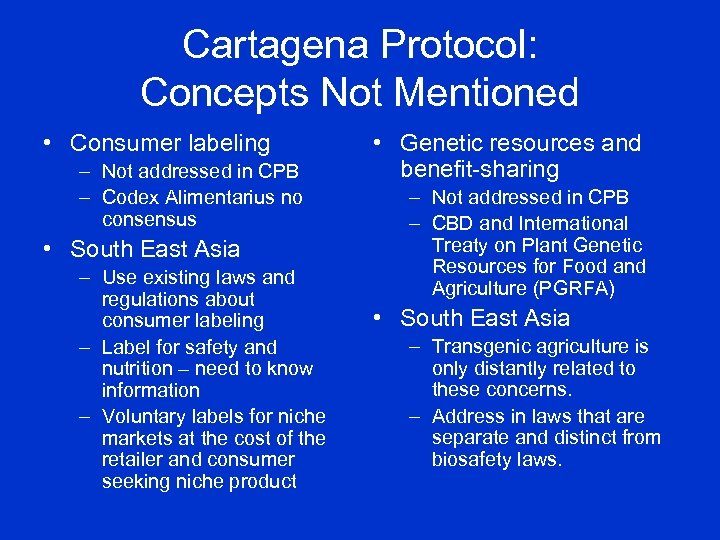
Cartagena Protocol: Concepts Not Mentioned • Consumer labeling – Not addressed in CPB – Codex Alimentarius no consensus • South East Asia – Use existing laws and regulations about consumer labeling – Label for safety and nutrition – need to know information – Voluntary labels for niche markets at the cost of the retailer and consumer seeking niche product • Genetic resources and benefit-sharing – Not addressed in CPB – CBD and International Treaty on Plant Genetic Resources for Food and Agriculture (PGRFA) • South East Asia – Transgenic agriculture is only distantly related to these concerns. – Address in laws that are separate and distinct from biosafety laws.

Way Forward: Experiences in other Nations • European Union (EU) – Spain – UK – France • Argentina – New WTO challenge to EU? • Brazil – Recent maize approvals • Australia – Canola – Wheat field trials • China – Top priority • India – Rethinking regulatory system • South Africa – Success with cotton and food (white) maize – Approvals pending • Burkina Faso – Bt cotton planted • Kenya & Uganda – Field trials banana – Bt cotton • Honduras & Guatemala – Good contrast – Bt maize for Honduras

Way Forward: General Ideas • Rely upon 20+ years research and 10+ years commercial use of agricultural biotechnology – Do not repeat what has already been done properly and correctly • Improve what has already functioned well – Use existing laws and regulations – Use Models that support, rather than undermine, the objectives of South East Asia • e. g. Biosafety Regulation Sourcebook, http: //www. arentfox. com/modelbiosafetyact. html – Pay attention to the experiences of other countries • ISAAA Annual Reports – best source of information

Thank you. I welcome questions and further discussions on these very important issues for the benefit of South East Asia and other developing nations.
df0af3c5eb4b30c9e1cb71720adb3d50.ppt