7dc6547a517870a065c7f4473be3a204.ppt
- Количество слайдов: 91
 CPPT 9010: Facility Design & Operation D. I. T. DT 275 Masters in Chemical and Pharmaceutical Process Technology 15 th December 2009 1 Clement Farrar BA BAI MSc MIEI
CPPT 9010: Facility Design & Operation D. I. T. DT 275 Masters in Chemical and Pharmaceutical Process Technology 15 th December 2009 1 Clement Farrar BA BAI MSc MIEI
 Lecture Overview 1) Organisational Structure 2) Safety & Environmental 3) Schedule, Start Up & Commercial 4) Preventative Maintenance 2
Lecture Overview 1) Organisational Structure 2) Safety & Environmental 3) Schedule, Start Up & Commercial 4) Preventative Maintenance 2
 1) Organisation Structure § § 3 Overall Site Structure Commercial Manufacturing Structure Overall Project, Start Up Structure Future (Shutdown Structure)
1) Organisation Structure § § 3 Overall Site Structure Commercial Manufacturing Structure Overall Project, Start Up Structure Future (Shutdown Structure)
 Overall Site Structure § § § 4 HR Finance Training IS Safety Occupational Health Calibrations Catering Cleaning Services Landscaping Mail Service Facilities
Overall Site Structure § § § 4 HR Finance Training IS Safety Occupational Health Calibrations Catering Cleaning Services Landscaping Mail Service Facilities
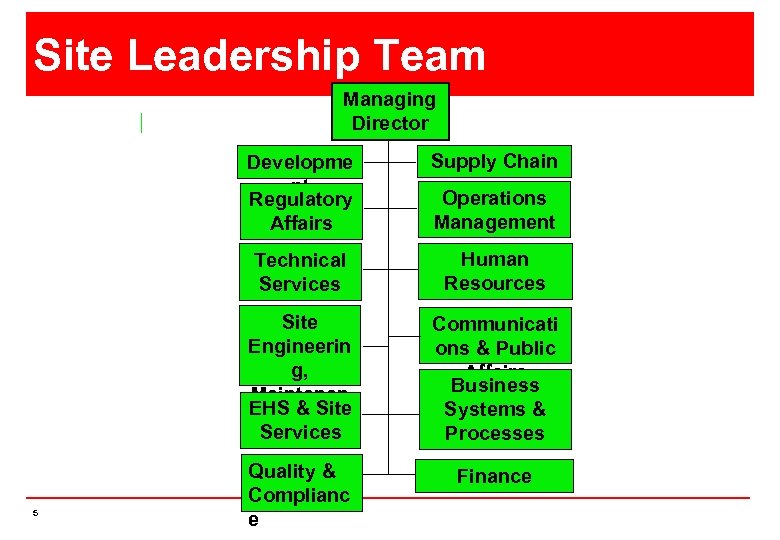 Site Leadership Team Managing Director Developme nt Regulatory Affairs Technical Services Human Resources Site Engineerin g, Maintenan EHSce Site & Services 5 Supply Chain Communicati ons & Public Affairs Business Systems & Processes Quality & Complianc e Finance Operations Management
Site Leadership Team Managing Director Developme nt Regulatory Affairs Technical Services Human Resources Site Engineerin g, Maintenan EHSce Site & Services 5 Supply Chain Communicati ons & Public Affairs Business Systems & Processes Quality & Complianc e Finance Operations Management
 Commercial Manufacturing Structure Consider the Actual Manufacturing itself: § § § § 6 Operations Engineering Quality Validation Training Manufacturing Support Technical Services
Commercial Manufacturing Structure Consider the Actual Manufacturing itself: § § § § 6 Operations Engineering Quality Validation Training Manufacturing Support Technical Services
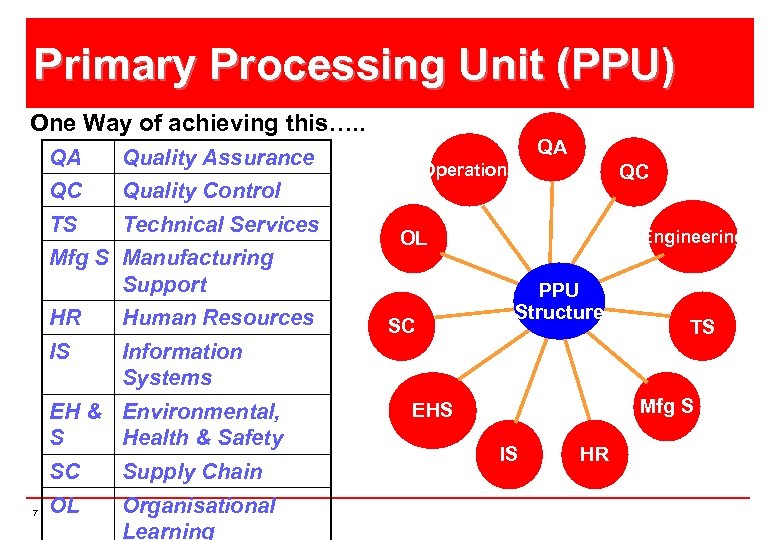 Primary Processing Unit (PPU) One Way of achieving this…. . QA QC Quality Control TS Technical Services Mfg S Manufacturing Support HR Human Resources IS Operations QC Engineering OL PPU Structure Information Systems EH & Environmental, S Health & Safety SC 7 QA Quality Assurance Supply Chain OL Organisational Learning SC TS Mfg S EHS IS HR
Primary Processing Unit (PPU) One Way of achieving this…. . QA QC Quality Control TS Technical Services Mfg S Manufacturing Support HR Human Resources IS Operations QC Engineering OL PPU Structure Information Systems EH & Environmental, S Health & Safety SC 7 QA Quality Assurance Supply Chain OL Organisational Learning SC TS Mfg S EHS IS HR
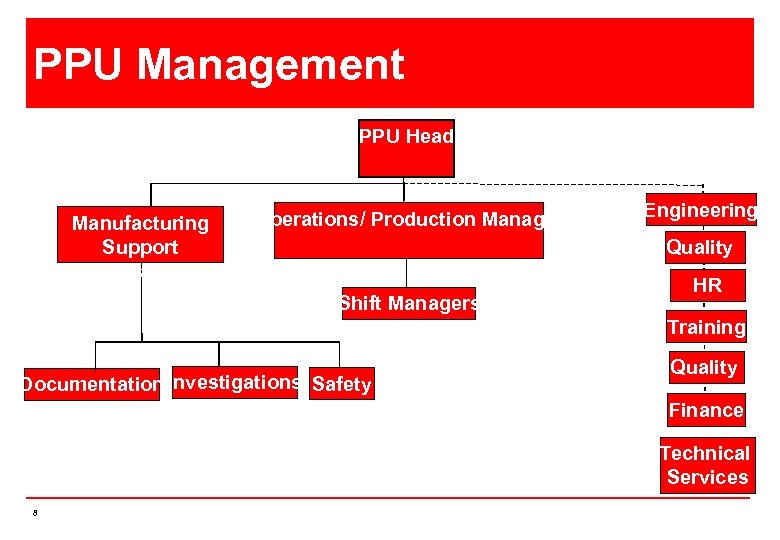 PPU Management PPU Head Manufacturing Support Manager Operations/ Production Manager Engineering Quality Shift Managers HR Training Documentation Investigations Safety Quality Finance Technical Services 8
PPU Management PPU Head Manufacturing Support Manager Operations/ Production Manager Engineering Quality Shift Managers HR Training Documentation Investigations Safety Quality Finance Technical Services 8
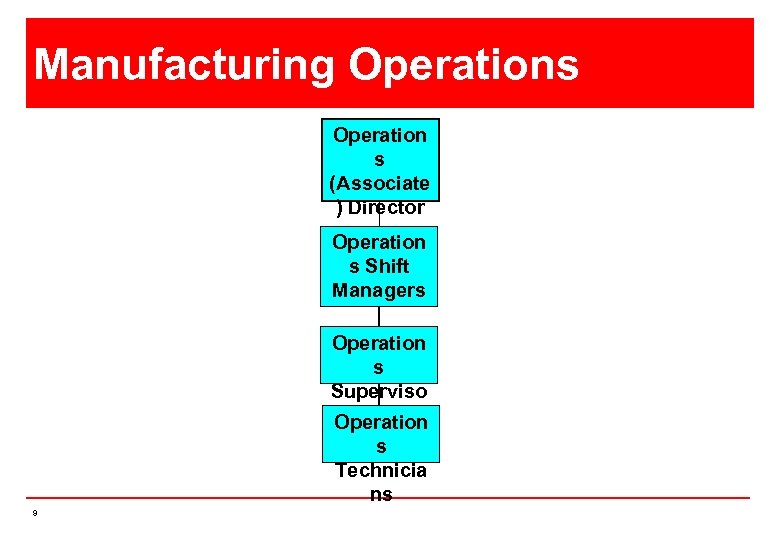 Manufacturing Operations Operation s (Associate ) Director Operation s Shift Managers Operation s Superviso rs Operation s Technicia ns 9
Manufacturing Operations Operation s (Associate ) Director Operation s Shift Managers Operation s Superviso rs Operation s Technicia ns 9
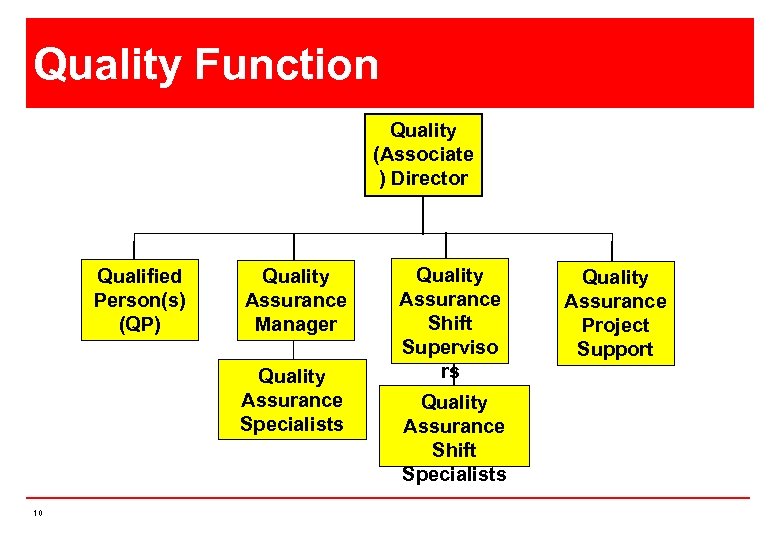 Quality Function Quality (Associate ) Director Qualified Person(s) (QP) Quality Assurance Manager Quality Assurance Specialists 10 Quality Assurance Shift Superviso rs Quality Assurance Shift Specialists Quality Assurance Project Support
Quality Function Quality (Associate ) Director Qualified Person(s) (QP) Quality Assurance Manager Quality Assurance Specialists 10 Quality Assurance Shift Superviso rs Quality Assurance Shift Specialists Quality Assurance Project Support
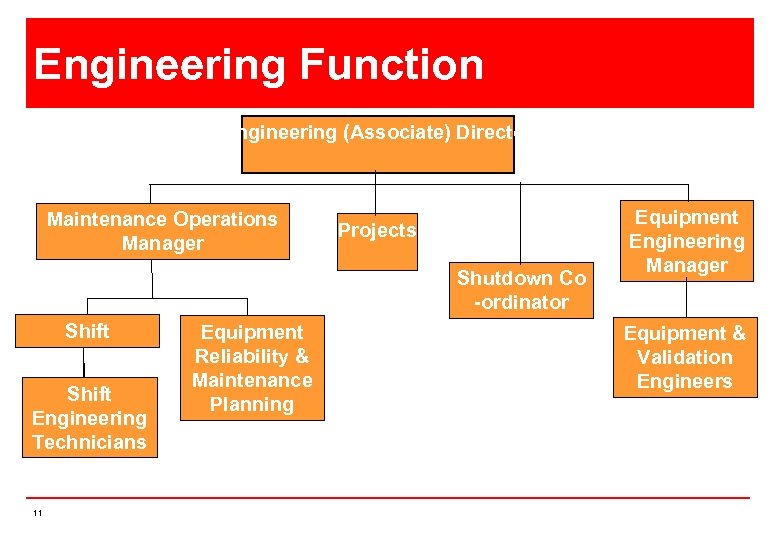 Engineering Function Engineering (Associate) Director Maintenance Operations Manager Projects Shutdown Co -ordinator Shift Engineers Shift Engineering Technicians 11 Equipment Reliability & Maintenance Planning Equipment Engineering Manager Equipment & Validation Engineers
Engineering Function Engineering (Associate) Director Maintenance Operations Manager Projects Shutdown Co -ordinator Shift Engineers Shift Engineering Technicians 11 Equipment Reliability & Maintenance Planning Equipment Engineering Manager Equipment & Validation Engineers
 Other Considerations for Organisation Audit Preparation n Documentation Group n Admin n 12
Other Considerations for Organisation Audit Preparation n Documentation Group n Admin n 12
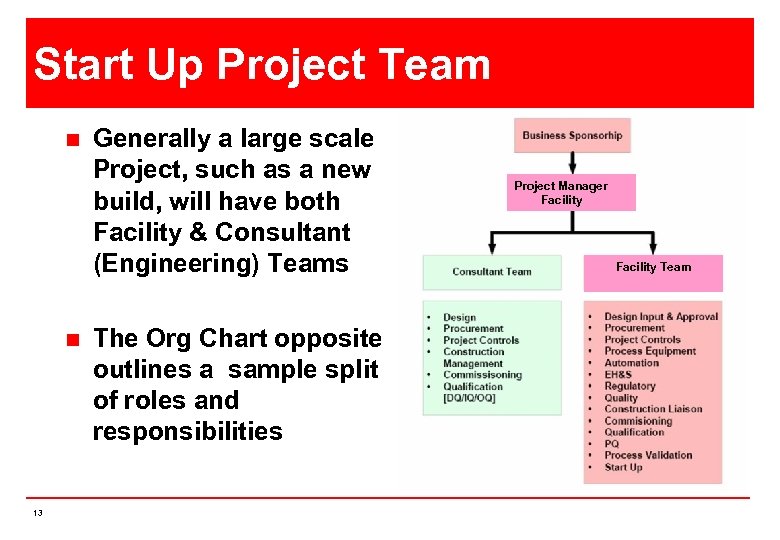 Start Up Project Team n n 13 Generally a large scale Project, such as a new build, will have both Facility & Consultant (Engineering) Teams The Org Chart opposite outlines a sample split of roles and responsibilities Project Manager Facility Team
Start Up Project Team n n 13 Generally a large scale Project, such as a new build, will have both Facility & Consultant (Engineering) Teams The Org Chart opposite outlines a sample split of roles and responsibilities Project Manager Facility Team
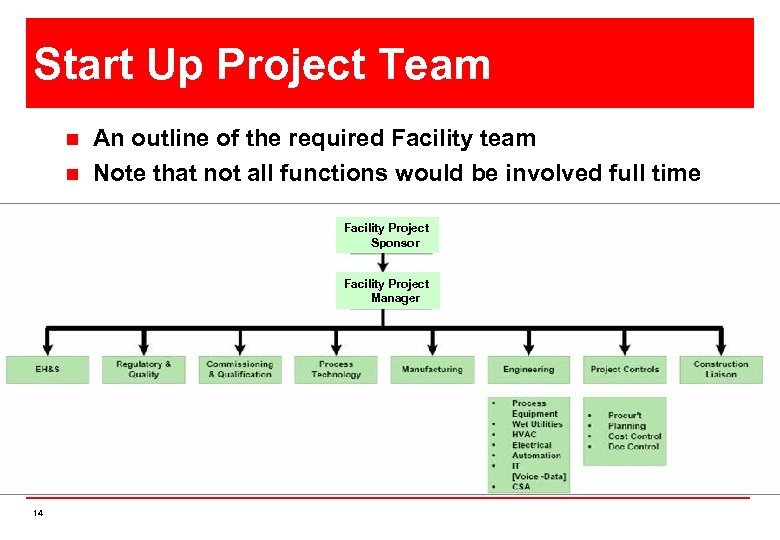 Start Up Project Team n n An outline of the required Facility team Note that not all functions would be involved full time Facility Project Sponsor Facility Project Manager 14
Start Up Project Team n n An outline of the required Facility team Note that not all functions would be involved full time Facility Project Sponsor Facility Project Manager 14
 Start Up Project Team At the start of the project implementation, the following must be established: n n 15 Organisation Charts for both Facility and Consultant Teams identifying all personnel with project responsibility and accountability Matrix of team roles and responsibilities – facility and consultant
Start Up Project Team At the start of the project implementation, the following must be established: n n 15 Organisation Charts for both Facility and Consultant Teams identifying all personnel with project responsibility and accountability Matrix of team roles and responsibilities – facility and consultant
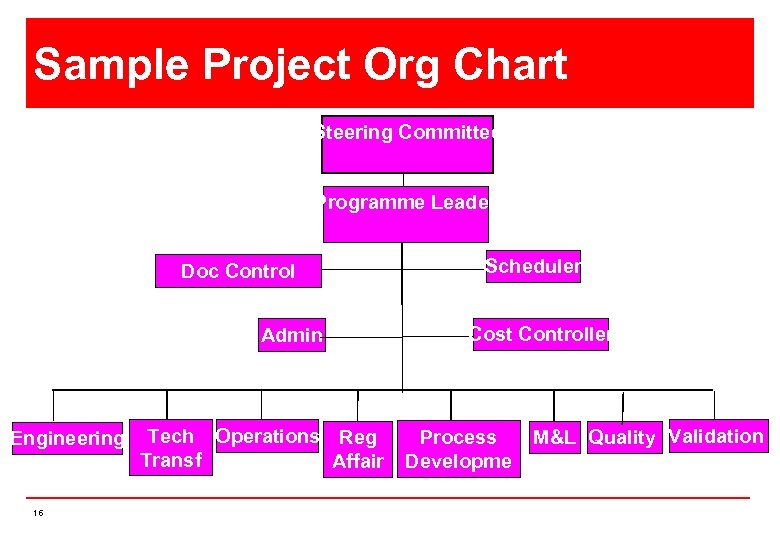 Sample Project Org Chart Steering Committee Programme Leader Doc Control Admin Engineering 16 Tech Operations Reg Transf Affair er s Scheduler Cost Controller Process Developme nt M&L Quality Validation
Sample Project Org Chart Steering Committee Programme Leader Doc Control Admin Engineering 16 Tech Operations Reg Transf Affair er s Scheduler Cost Controller Process Developme nt M&L Quality Validation
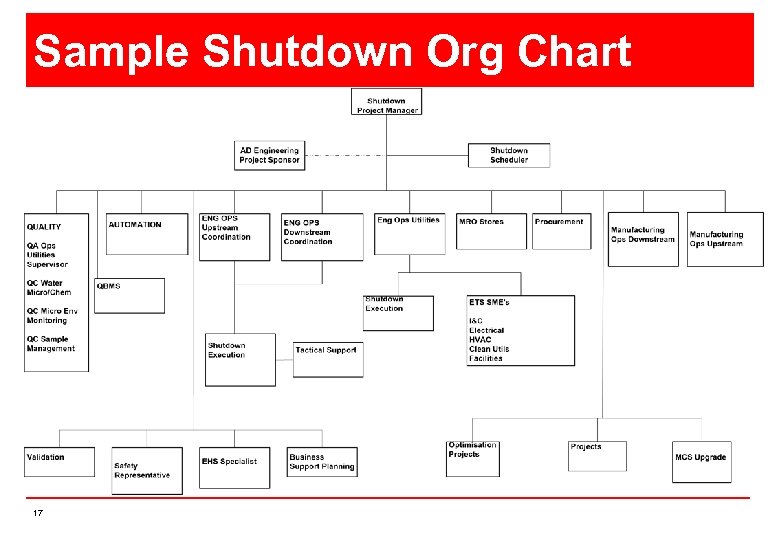 Sample Shutdown Org Chart 17
Sample Shutdown Org Chart 17
 2) Environment, Health & Safety 18
2) Environment, Health & Safety 18
 Environment, Health & Safety Introduction n Legislation n Key elements of BS OHSAS 18001: 2007 n Safety Statement n Injury Classification n 19
Environment, Health & Safety Introduction n Legislation n Key elements of BS OHSAS 18001: 2007 n Safety Statement n Injury Classification n 19
 Environment, Health and Safety Policy from EHS Sample Extracts Policy ‘World class EHS performance depends on management leadership and employee cooperation and conformance to company policy and ‘All employees are practices. ’ encouraged to actively participate in environment, health and safety matters and work in partnership with management to assure compliance and support 20 ‘We are dedicated to the principle that all workplace incidents, illnesses and adverse environmental impacts are preventable. We will continue to drive down the environment, health and safety impact of our operations by improving process safety, reducing waste, emissions and discharges and by using energy efficiently. ’
Environment, Health and Safety Policy from EHS Sample Extracts Policy ‘World class EHS performance depends on management leadership and employee cooperation and conformance to company policy and ‘All employees are practices. ’ encouraged to actively participate in environment, health and safety matters and work in partnership with management to assure compliance and support 20 ‘We are dedicated to the principle that all workplace incidents, illnesses and adverse environmental impacts are preventable. We will continue to drive down the environment, health and safety impact of our operations by improving process safety, reducing waste, emissions and discharges and by using energy efficiently. ’
 Early Site Development Environmental Considerations An Environmental Impact Assessment (EIA) should be conducted prior to construction of a Facility. This assessment identifies potential environmental impacts and as a result measures can be put in place to mitigate these impacts. The primary impacts assessed are: Ø Wastewater Treatment Ø Noise Control Ø Air Emissions 21 Ø Waste Generation
Early Site Development Environmental Considerations An Environmental Impact Assessment (EIA) should be conducted prior to construction of a Facility. This assessment identifies potential environmental impacts and as a result measures can be put in place to mitigate these impacts. The primary impacts assessed are: Ø Wastewater Treatment Ø Noise Control Ø Air Emissions 21 Ø Waste Generation
 Early Site Development Environmental Considerations n n 22 CONSULTATION is key during early site design and development Planning Permission can be granted quickly and without any objections as a result of effective design to minimise potential environmental impacts and also excellent consultation with local residents and interest groups.
Early Site Development Environmental Considerations n n 22 CONSULTATION is key during early site design and development Planning Permission can be granted quickly and without any objections as a result of effective design to minimise potential environmental impacts and also excellent consultation with local residents and interest groups.
 Early Site Development Environmental Considerations n n 23 The facility must maintain a good environmental performance and continually meet and improve on the early message delivered for the facility i. e. the site will not cause an adverse environmental impact or create a nuisance for local residents. The environmental context of the site remains as important e. g. surface waters from the site generally discharge to a local river - and generally these water bodies have a high amenity value and need to be protected.
Early Site Development Environmental Considerations n n 23 The facility must maintain a good environmental performance and continually meet and improve on the early message delivered for the facility i. e. the site will not cause an adverse environmental impact or create a nuisance for local residents. The environmental context of the site remains as important e. g. surface waters from the site generally discharge to a local river - and generally these water bodies have a high amenity value and need to be protected.
 Exercise What environmental programmes are in place at your current/ a previous place of employment? Is there good Employee Awareness Ø Are there good/ novel Environmental Initiatives Ø Ø 24 What could be improved upon?
Exercise What environmental programmes are in place at your current/ a previous place of employment? Is there good Employee Awareness Ø Are there good/ novel Environmental Initiatives Ø Ø 24 What could be improved upon?
 Legal Obligations Corporate EHS Department Integrated Pollution Control Licence Local Authority 25
Legal Obligations Corporate EHS Department Integrated Pollution Control Licence Local Authority 25
 Legal Obligations IPCL Licence to prevent or solve pollution problems rather than transferring them from one part of the environment to another. The IPC License regulates the entire Site and ensures that all processes, procedures and materials are managed in a way that does not cause environmental pollution. 26 The next slide demonstrates the interaction of a Facility with key Environmental Aspects
Legal Obligations IPCL Licence to prevent or solve pollution problems rather than transferring them from one part of the environment to another. The IPC License regulates the entire Site and ensures that all processes, procedures and materials are managed in a way that does not cause environmental pollution. 26 The next slide demonstrates the interaction of a Facility with key Environmental Aspects
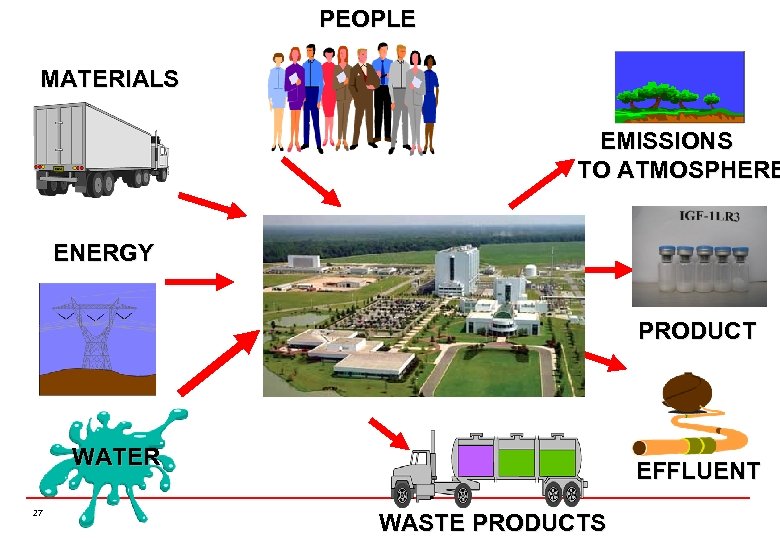 PEOPLE MATERIALS EMISSIONS TO ATMOSPHERE ENERGY PRODUCT WATER 27 EFFLUENT WASTE PRODUCTS
PEOPLE MATERIALS EMISSIONS TO ATMOSPHERE ENERGY PRODUCT WATER 27 EFFLUENT WASTE PRODUCTS
 EHS Management System “Say what you do and do what you say you do” n Ensure 28 all tasks are RISK ASSESSED and controls implemented to: Ø Minimise Waste Generated Ø Minimise Water and Energy Usage Ø Prevent Spillage n Generate STANDARD OPERATING PROCEDURES (SOPs) n Personnel must be TRAINED on relevant procedures: Ø e. g. Waste Management & Spill Control Procedures
EHS Management System “Say what you do and do what you say you do” n Ensure 28 all tasks are RISK ASSESSED and controls implemented to: Ø Minimise Waste Generated Ø Minimise Water and Energy Usage Ø Prevent Spillage n Generate STANDARD OPERATING PROCEDURES (SOPs) n Personnel must be TRAINED on relevant procedures: Ø e. g. Waste Management & Spill Control Procedures
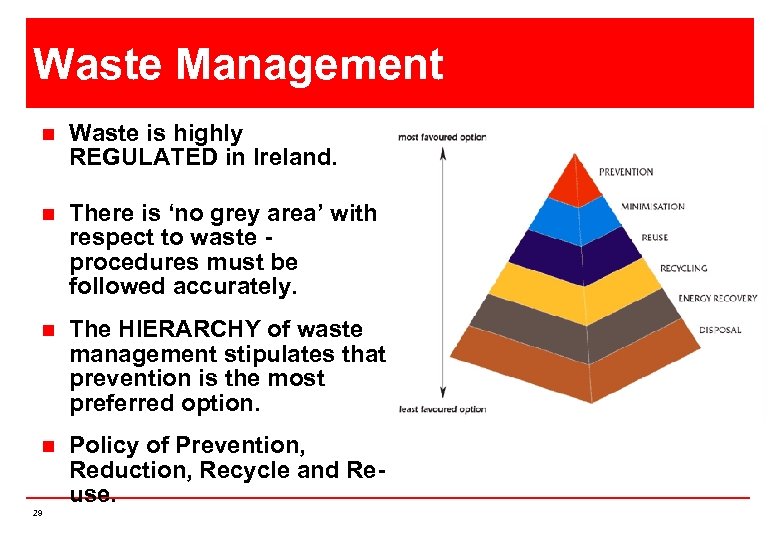 Waste Management n Waste is highly REGULATED in Ireland. n There is ‘no grey area’ with respect to waste procedures must be followed accurately. n The HIERARCHY of waste management stipulates that prevention is the most preferred option. n Policy of Prevention, Reduction, Recycle and Reuse. 29
Waste Management n Waste is highly REGULATED in Ireland. n There is ‘no grey area’ with respect to waste procedures must be followed accurately. n The HIERARCHY of waste management stipulates that prevention is the most preferred option. n Policy of Prevention, Reduction, Recycle and Reuse. 29
 Waste Management n n ENSURE correct segregation procedures in place HAZARDOUS Waste must NOT be mixed with Non-Hazardous Waste BIOHAZARDOUS Waste must be segregated Utilise approved containers/ bins for hazardous waste § SOPs must ensure: Ø Full and accurate labeling Ø Full logbook entry 30
Waste Management n n ENSURE correct segregation procedures in place HAZARDOUS Waste must NOT be mixed with Non-Hazardous Waste BIOHAZARDOUS Waste must be segregated Utilise approved containers/ bins for hazardous waste § SOPs must ensure: Ø Full and accurate labeling Ø Full logbook entry 30
 Environmental Protection Employee Responsibility n n 31 Spill Control (ALWAYS Notify Supervisor where a spill occurs) Waste Segregation and Disposal Minimise Water and Energy Usage Adherence to Procedures
Environmental Protection Employee Responsibility n n 31 Spill Control (ALWAYS Notify Supervisor where a spill occurs) Waste Segregation and Disposal Minimise Water and Energy Usage Adherence to Procedures
 Simple Key Points n n n n 32 Watch what you put down the sink Solvent/ detergents - use the proper amount Turn off equipment, lights, PC’s etc when not in use Minimize waste - eliminate, reuse and recycle Know your spill procedures Report faulty Plumbing Follow recycling procedures for paper, card, foil, cans & batteries Create a Culture
Simple Key Points n n n n 32 Watch what you put down the sink Solvent/ detergents - use the proper amount Turn off equipment, lights, PC’s etc when not in use Minimize waste - eliminate, reuse and recycle Know your spill procedures Report faulty Plumbing Follow recycling procedures for paper, card, foil, cans & batteries Create a Culture
 Simple Environment Friendly Initiatives n n n 33 Consider car sharing initiatives - halve your costs Consider Cycle-to-work initiatives Promote the use of e-mail Use double side when Photocopying Promote Recycling
Simple Environment Friendly Initiatives n n n 33 Consider car sharing initiatives - halve your costs Consider Cycle-to-work initiatives Promote the use of e-mail Use double side when Photocopying Promote Recycling
 Safety Programmes to Consider 1. Occupational Health & Safety Industrial Hygiene Program Ø Radiation Safety Program Ø Bio-safety Program Ø Occupational Health Program 2. Operational Safety and Fatality Prevention: Ø Work at Height Ø Confined Space Entry Ø Control of Hazardous Energy Ø Electrical Safety Ø Material Handling Equipment Ø ATEX Ø Slips Trips and Falls Ø Manual Handling and Ergonomics Ø 34
Safety Programmes to Consider 1. Occupational Health & Safety Industrial Hygiene Program Ø Radiation Safety Program Ø Bio-safety Program Ø Occupational Health Program 2. Operational Safety and Fatality Prevention: Ø Work at Height Ø Confined Space Entry Ø Control of Hazardous Energy Ø Electrical Safety Ø Material Handling Equipment Ø ATEX Ø Slips Trips and Falls Ø Manual Handling and Ergonomics Ø 34
 Safety Programmes to Consider 3. Loss Prevention and Life Safety: Ø Fire Detection and Protection Systems Program 4. Emergency Preparedness: Ø Ø Emergency Response Team First Aid Management 5. Contractor Management: Ø 35 Craft and Sustaining
Safety Programmes to Consider 3. Loss Prevention and Life Safety: Ø Fire Detection and Protection Systems Program 4. Emergency Preparedness: Ø Ø Emergency Response Team First Aid Management 5. Contractor Management: Ø 35 Craft and Sustaining
 What does Health & Safety Legislation mean for the Company and its & Safety legislation defines the minimum Employees? § Health regulatory requirements that must be adhered to in order to ensure the safety, health and welfare at work of all site personnel and contractors § Legislation imposes specific duties/ obligations on: Ø The company as an employer including directors, managers, and supervisors of personnel Ø The employees Ø Contractors and their employees § Failure to comply with the legislation can constitute an offence under the legislation and lead to a fine or imprisonment or both on conviction in court § Everyone must comply with their legal obligations and 36 conduct their activities in a manner which complies with the
What does Health & Safety Legislation mean for the Company and its & Safety legislation defines the minimum Employees? § Health regulatory requirements that must be adhered to in order to ensure the safety, health and welfare at work of all site personnel and contractors § Legislation imposes specific duties/ obligations on: Ø The company as an employer including directors, managers, and supervisors of personnel Ø The employees Ø Contractors and their employees § Failure to comply with the legislation can constitute an offence under the legislation and lead to a fine or imprisonment or both on conviction in court § Everyone must comply with their legal obligations and 36 conduct their activities in a manner which complies with the
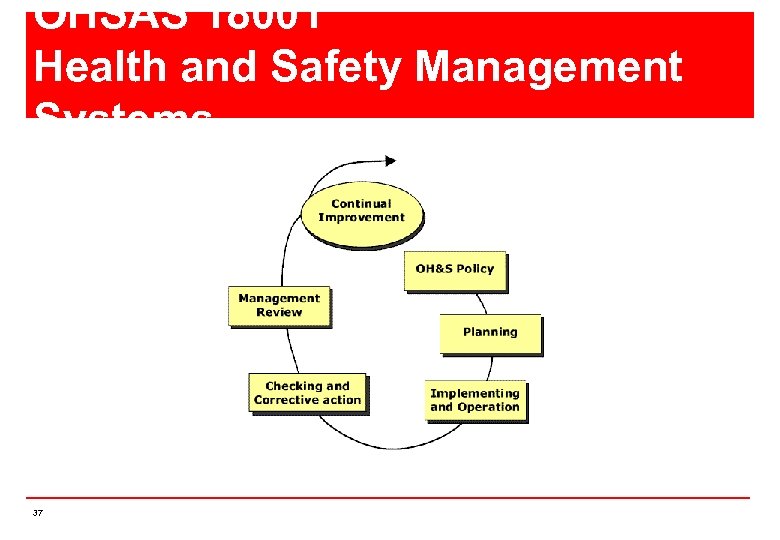 OHSAS 18001 Health and Safety Management Systems 37
OHSAS 18001 Health and Safety Management Systems 37
 BS OHSAS 18001: 2007 What is OHSAS 18001? n International Occupational, Health and Safety (OH&S) Management System Standard n Defines the requirements for establishing, implementing and operating an OH&S Management System n Structured approach to OH&S management n Usually a corporate business objective 38
BS OHSAS 18001: 2007 What is OHSAS 18001? n International Occupational, Health and Safety (OH&S) Management System Standard n Defines the requirements for establishing, implementing and operating an OH&S Management System n Structured approach to OH&S management n Usually a corporate business objective 38
 OHSAS 18001 What is OHSAS 18001 n n 39 Traditional OH&S management usually meant reacting to work related incidents rather than planning for the control of work related risks. The emphasis is placed on practices being proactive and preventative by the indication of hazards and evaluation
OHSAS 18001 What is OHSAS 18001 n n 39 Traditional OH&S management usually meant reacting to work related incidents rather than planning for the control of work related risks. The emphasis is placed on practices being proactive and preventative by the indication of hazards and evaluation
 OHSAS 18001 What does it mean for Facilities? n n It will help to ensure that there is a continual cycle of planning, implementing, reviewing, and improving the actions that the facility takes to meet its OH&S obligations. n It will prevent incidents and injuries and protect those who work at the facility. n It will help to ensure a consistently high rate of compliance is achieved. n 40 The facility should operate an Occupational Health & Safety management system (OH&SMS) that meets the full requirements of an international and highly recognised standard. It will provide a framework for continual improvement in OH&S performance
OHSAS 18001 What does it mean for Facilities? n n It will help to ensure that there is a continual cycle of planning, implementing, reviewing, and improving the actions that the facility takes to meet its OH&S obligations. n It will prevent incidents and injuries and protect those who work at the facility. n It will help to ensure a consistently high rate of compliance is achieved. n 40 The facility should operate an Occupational Health & Safety management system (OH&SMS) that meets the full requirements of an international and highly recognised standard. It will provide a framework for continual improvement in OH&S performance
 OHSAS 18001 Features include the following elements: n n 41 OH&S Policy Planning Implementation & Operation Checking & Corrective Action
OHSAS 18001 Features include the following elements: n n 41 OH&S Policy Planning Implementation & Operation Checking & Corrective Action
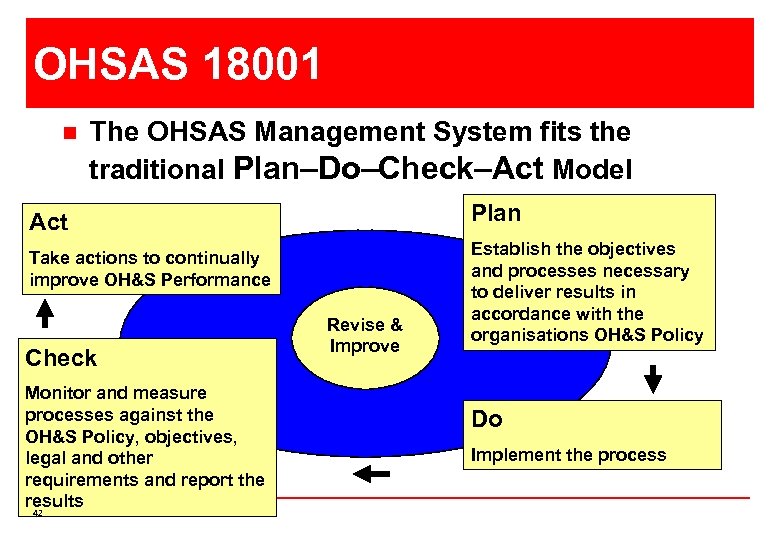 OHSAS 18001 n The OHSAS Management System fits the traditional Plan–Do–Check–Act Model Plan Act Take actions to continually improve OH&S Performance Check Monitor and measure processes against the OH&S Policy, objectives, legal and other requirements and report the results 42 Revise & Improve Establish the objectives and processes necessary to deliver results in Plan accordance with the organisations OH&S Policy Do Implement the process
OHSAS 18001 n The OHSAS Management System fits the traditional Plan–Do–Check–Act Model Plan Act Take actions to continually improve OH&S Performance Check Monitor and measure processes against the OH&S Policy, objectives, legal and other requirements and report the results 42 Revise & Improve Establish the objectives and processes necessary to deliver results in Plan accordance with the organisations OH&S Policy Do Implement the process
 BS OHSAS 18001: 2007 Standard Methodology n The Plan-Do-Check-Act (PDCA) model can also be briefly described as follows: n n 43 Plan: establish the objectives and processes necessary to deliver results in accordance with the organization’s OH&S policy Do: implement the processes Check: monitor and measure processes against OH&S policy, objectives, legal and other requirements, and report the results Act: take actions to continually improve OH&S performance
BS OHSAS 18001: 2007 Standard Methodology n The Plan-Do-Check-Act (PDCA) model can also be briefly described as follows: n n 43 Plan: establish the objectives and processes necessary to deliver results in accordance with the organization’s OH&S policy Do: implement the processes Check: monitor and measure processes against OH&S policy, objectives, legal and other requirements, and report the results Act: take actions to continually improve OH&S performance
 Legal Requirement for Safety Statement: SHWW Act, 2005 n Employer shall prepare a written ‘Safety Statement’ based on the risk assessments carried out, specifying the manner in which the SHWW of his or her employees shall be secured and managed including: Ø Ø Ø 44 The hazards identified and the risks assessed The protective and preventive measures taken and the resources provided for protecting SHWW Emergency plans and procedures The duties of his or her employees The roles and responsibilities of personnel with respect to SHWW The arrangements made regarding safety representatives and consultation with employees
Legal Requirement for Safety Statement: SHWW Act, 2005 n Employer shall prepare a written ‘Safety Statement’ based on the risk assessments carried out, specifying the manner in which the SHWW of his or her employees shall be secured and managed including: Ø Ø Ø 44 The hazards identified and the risks assessed The protective and preventive measures taken and the resources provided for protecting SHWW Emergency plans and procedures The duties of his or her employees The roles and responsibilities of personnel with respect to SHWW The arrangements made regarding safety representatives and consultation with employees
 Legal Requirement for Safety Statement: SHWW Act, 2005 n n Employer shall bring to the attention of persons exposed to specific risks the relevant extracts of the safety statement including the risk assessment and the protective and preventive measures taken n 45 Employer shall bring the safety statement to the attention of: Ø His or her employees, at least annually and following any amendments Ø New employees upon commencement employment Ø Other persons at the place of work who may be exposed to any risks A copy of a safety statement, or relevant extract of it, shall be kept available for inspection at or near the
Legal Requirement for Safety Statement: SHWW Act, 2005 n n Employer shall bring to the attention of persons exposed to specific risks the relevant extracts of the safety statement including the risk assessment and the protective and preventive measures taken n 45 Employer shall bring the safety statement to the attention of: Ø His or her employees, at least annually and following any amendments Ø New employees upon commencement employment Ø Other persons at the place of work who may be exposed to any risks A copy of a safety statement, or relevant extract of it, shall be kept available for inspection at or near the
 Overview of Safety Statement n n n A facility's Safety Statement must be located in an easy access location It is a summary document describing the main elements of the OHS management system at the facility It also constitutes the OHS Manual as required by the BS OSHAS 18001: 2007 standard and therefore the contents of the safety statement are arranged to replicate the content of the standard, namely: n n n 46 Introduction EHS Policy Planning Implementation and Operation Checking and Corrective Action Management Review
Overview of Safety Statement n n n A facility's Safety Statement must be located in an easy access location It is a summary document describing the main elements of the OHS management system at the facility It also constitutes the OHS Manual as required by the BS OSHAS 18001: 2007 standard and therefore the contents of the safety statement are arranged to replicate the content of the standard, namely: n n n 46 Introduction EHS Policy Planning Implementation and Operation Checking and Corrective Action Management Review
 Safety Statement: Introduction Safety Principles 1. All occupational illness and injuries are preventable. 2. We are all directly responsible for preventing illness and injury. 3. Working safely is a condition of employment. 4. All personnel must be trained in safety. 5. Safety audits must be conducted. 6. All deficiencies must be corrected promptly. 7. All injuries and incidents with injury potential must be investigated. 8. It is good business to prevent illnesses and injuries. 9. Line management must own and role model good safety practices and behaviours. 10. You are our most important asset and the most critical element in the success of a safety and health programme. 47
Safety Statement: Introduction Safety Principles 1. All occupational illness and injuries are preventable. 2. We are all directly responsible for preventing illness and injury. 3. Working safely is a condition of employment. 4. All personnel must be trained in safety. 5. Safety audits must be conducted. 6. All deficiencies must be corrected promptly. 7. All injuries and incidents with injury potential must be investigated. 8. It is good business to prevent illnesses and injuries. 9. Line management must own and role model good safety practices and behaviours. 10. You are our most important asset and the most critical element in the success of a safety and health programme. 47
 Safety Statement: Introduction Safety Rules 1. Never work on live electrical equipment or uncontrolled hazardous energy. 2. Never work at a height without the approved fall protection. 3. Never dispose of hazardous waste as non-hazardous waste. 4. Never breach Confined Space Entry requirements. 5. Never by-pass machine guards or safety interlocks. 6. Never use unapproved equipment in explosion rated (Ex) areas. 7. Never operate powered industrial vehicles or equipment without the required training. 8. Never ignore evacuation alarms or interfere with life safety and emergency equipment. 9. Never disregard warning label instructions on chemicals. 10. Always report incidents to line management. 48
Safety Statement: Introduction Safety Rules 1. Never work on live electrical equipment or uncontrolled hazardous energy. 2. Never work at a height without the approved fall protection. 3. Never dispose of hazardous waste as non-hazardous waste. 4. Never breach Confined Space Entry requirements. 5. Never by-pass machine guards or safety interlocks. 6. Never use unapproved equipment in explosion rated (Ex) areas. 7. Never operate powered industrial vehicles or equipment without the required training. 8. Never ignore evacuation alarms or interfere with life safety and emergency equipment. 9. Never disregard warning label instructions on chemicals. 10. Always report incidents to line management. 48
 Injuries n n 49 Outline the legislative and corporate requirements for reporting injuries and illnesses (EHS) Define & distinguish between First Aid, Lost Workday Case, Restricted Work Case and Medical Treatment (EHS)
Injuries n n 49 Outline the legislative and corporate requirements for reporting injuries and illnesses (EHS) Define & distinguish between First Aid, Lost Workday Case, Restricted Work Case and Medical Treatment (EHS)
 General - Corporate & Legal n n 50 All company locations should have a programme established to promptly investigate, record and report all occupational injuries and illnesses to ensure national regulatory requirements are maintained All employers have an obligation to prevent accidents at work. Where accidents occur the employer must investigate the causes and implement action to prevent reoccurrence of the accident Source - 2007 General Application Regulations
General - Corporate & Legal n n 50 All company locations should have a programme established to promptly investigate, record and report all occupational injuries and illnesses to ensure national regulatory requirements are maintained All employers have an obligation to prevent accidents at work. Where accidents occur the employer must investigate the causes and implement action to prevent reoccurrence of the accident Source - 2007 General Application Regulations
 General - Corporate & Legal Injury Reporting Guidelines n Corporate Requirements Ø Ø n OSHA Requirements (Occ Health & Safety Administration) Ø n 51 All Lost Time and Restricted work cases should be communicated to Corporate Leadership Team on a weekly basis by EHS This information should be collated in a SPRS (Safety Performance Reporting System) for Injury data management All Lost Time, Restricted Work and Medical Treatment cases must be reported monthly to Corporate for inclusion in the company Injury data OSHA Report Irish Statutory Requirements Ø All lost time cases of 3 days or more ( including days off) must be reported to H. S. A ( IR 1 Form) by EHS
General - Corporate & Legal Injury Reporting Guidelines n Corporate Requirements Ø Ø n OSHA Requirements (Occ Health & Safety Administration) Ø n 51 All Lost Time and Restricted work cases should be communicated to Corporate Leadership Team on a weekly basis by EHS This information should be collated in a SPRS (Safety Performance Reporting System) for Injury data management All Lost Time, Restricted Work and Medical Treatment cases must be reported monthly to Corporate for inclusion in the company Injury data OSHA Report Irish Statutory Requirements Ø All lost time cases of 3 days or more ( including days off) must be reported to H. S. A ( IR 1 Form) by EHS
 Definitions of Occupational injury & illness § An injury is a sudden trauma to the body resulting from physical damage Ø Cuts, sprains, burns etc § An occupational illness is a medical condition that results from exposure in the workplace. It includes acute and chronic illnesses or diseases which may be caused by inhalation, absorption, ingestion, or direct contact Ø Asthma , dermatitis etc 52
Definitions of Occupational injury & illness § An injury is a sudden trauma to the body resulting from physical damage Ø Cuts, sprains, burns etc § An occupational illness is a medical condition that results from exposure in the workplace. It includes acute and chronic illnesses or diseases which may be caused by inhalation, absorption, ingestion, or direct contact Ø Asthma , dermatitis etc 52
 Work Related versus Non-Work Related? n n Any contribution from the workplace is sufficient to make a case work-related. If the employee is in the work environment, even if they are not engaged in a workrelated task, the case is ‘PRESUMED’ to be work-related. An accident that occurs off the premises is considered work-related if the employee is engaged in, or present to do work. If an employee leaves the work area for other purposes, then these activities are not work-related Ø 53 E. g. Employees who travel on Company business are considered to be engaged in work-related activities. However, an accident would not be reportable if the employee deviates from a reasonable route of travel such as a side trip for vacation or visiting friends
Work Related versus Non-Work Related? n n Any contribution from the workplace is sufficient to make a case work-related. If the employee is in the work environment, even if they are not engaged in a workrelated task, the case is ‘PRESUMED’ to be work-related. An accident that occurs off the premises is considered work-related if the employee is engaged in, or present to do work. If an employee leaves the work area for other purposes, then these activities are not work-related Ø 53 E. g. Employees who travel on Company business are considered to be engaged in work-related activities. However, an accident would not be reportable if the employee deviates from a reasonable route of travel such as a side trip for vacation or visiting friends
 How to determine if an injury or illness is work-related An injury or illness is determined to be work-related if: n An event or exposure in the work environment either caused or contributed to the resulting condition OR n n Ø Ø 54 Significantly aggravated a pre-existing illness or injury. In all injury classifications an OH opinion is required. There are some exemptions to this rule (e. g. , injury/ illness arising from voluntary participation in flu vaccine, blood donation or recreational activity) A clear medical diagnosis is necessary to identify these exemptions and referral to Occupational Health for clarification is appropriate.
How to determine if an injury or illness is work-related An injury or illness is determined to be work-related if: n An event or exposure in the work environment either caused or contributed to the resulting condition OR n n Ø Ø 54 Significantly aggravated a pre-existing illness or injury. In all injury classifications an OH opinion is required. There are some exemptions to this rule (e. g. , injury/ illness arising from voluntary participation in flu vaccine, blood donation or recreational activity) A clear medical diagnosis is necessary to identify these exemptions and referral to Occupational Health for clarification is appropriate.
 How are work-related injuries/ illnesses classified ? First Aid n Medical treatment n Work restricted case n Day away/ Lost Time case n Fatal case n 55
How are work-related injuries/ illnesses classified ? First Aid n Medical treatment n Work restricted case n Day away/ Lost Time case n Fatal case n 55
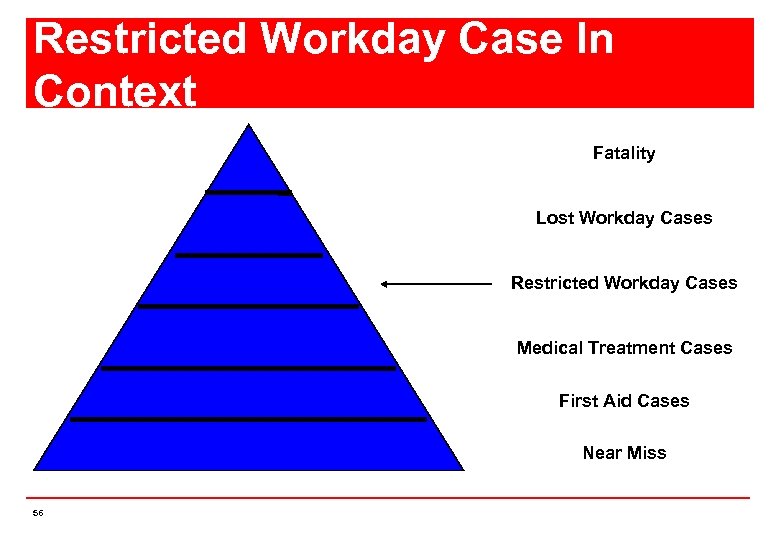 Restricted Workday Case In Context Fatality Lost Workday Cases Restricted Workday Cases Medical Treatment Cases First Aid Cases Near Miss 56
Restricted Workday Case In Context Fatality Lost Workday Cases Restricted Workday Cases Medical Treatment Cases First Aid Cases Near Miss 56
 OSHA Definition of First Aid Case Any injury or illness which can be treated on site by a trained First Aider or OHA Ø Ø Ø 57 Cleaning / flushing or dressing wounds Using hot or cold therapy (for treatment of burns/ soft tissue injuries) Using eye patches Removing foreign bodies from the eye using only irrigation Removing splinters or foreign bodies Drinking fluids for relief of heat stress
OSHA Definition of First Aid Case Any injury or illness which can be treated on site by a trained First Aider or OHA Ø Ø Ø 57 Cleaning / flushing or dressing wounds Using hot or cold therapy (for treatment of burns/ soft tissue injuries) Using eye patches Removing foreign bodies from the eye using only irrigation Removing splinters or foreign bodies Drinking fluids for relief of heat stress
 OSHA Definition of Medical Treatment Medical treatment is any treatment other than first aid treatment. Ø 58 The definition focuses on the nature of the treatment given and not on the person administering the treatment (e. g. physician, physiotherapist etc) E. g. a doctor prescribes medication or applies sutures or surgery to treat a condition arising from a work related injury/illness
OSHA Definition of Medical Treatment Medical treatment is any treatment other than first aid treatment. Ø 58 The definition focuses on the nature of the treatment given and not on the person administering the treatment (e. g. physician, physiotherapist etc) E. g. a doctor prescribes medication or applies sutures or surgery to treat a condition arising from a work related injury/illness
 Restricted Workday Case An injury is considered to be a Restricted Work Case if an employee cannot perform all of the ‘routine functions’ of their job. Ø Ø Ø 59 Departmental assignments cannot be altered so that employee’s injury is not classified as a ‘restricted work case’. For recordkeeping purposes, an employee’s routine functions are those work activities the employee regularly performs at least once per week E. g. If a medical recommendation advises that a person avoids lifting and it is a requirement for his/her role then it must be logged as a Restricted Work Case
Restricted Workday Case An injury is considered to be a Restricted Work Case if an employee cannot perform all of the ‘routine functions’ of their job. Ø Ø Ø 59 Departmental assignments cannot be altered so that employee’s injury is not classified as a ‘restricted work case’. For recordkeeping purposes, an employee’s routine functions are those work activities the employee regularly performs at least once per week E. g. If a medical recommendation advises that a person avoids lifting and it is a requirement for his/her role then it must be logged as a Restricted Work Case
 Lost Workday Case A Lost Workday Case is an occupational injury that is serious enough to result in an employee being away from work for one day or more. The day of the injury is not counted Ø Shift work days off should be considered Ø 60
Lost Workday Case A Lost Workday Case is an occupational injury that is serious enough to result in an employee being away from work for one day or more. The day of the injury is not counted Ø Shift work days off should be considered Ø 60
 OSHA Injury/ Illness Classification Team A team will meet to confirm the workrelatedness of the condition and determine the classification of the injury where there is ambiguity This team will usually consist of: Ø Head of Department Ø Supervisor/ Manager of employee injured Ø OH Advisor Ø EHS specialist Ø EHS Manager Ø Subject Matter Expert (e. g. Occupational Hygienist, Ergonomist) as required 61
OSHA Injury/ Illness Classification Team A team will meet to confirm the workrelatedness of the condition and determine the classification of the injury where there is ambiguity This team will usually consist of: Ø Head of Department Ø Supervisor/ Manager of employee injured Ø OH Advisor Ø EHS specialist Ø EHS Manager Ø Subject Matter Expert (e. g. Occupational Hygienist, Ergonomist) as required 61
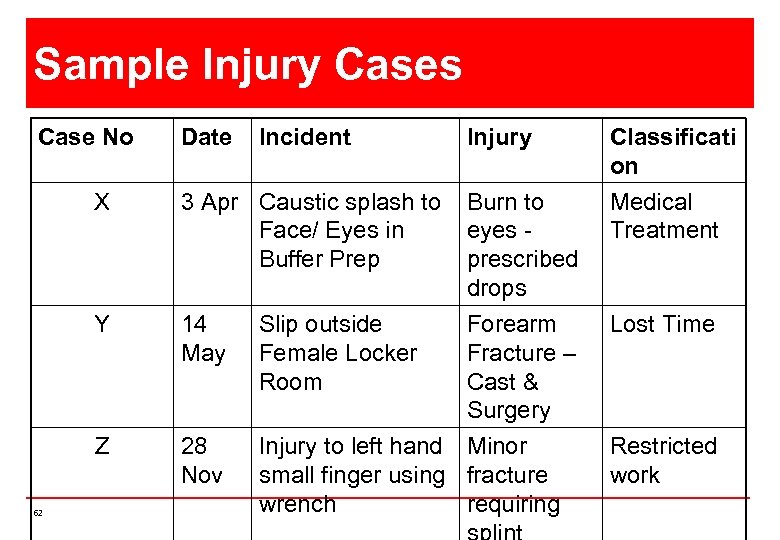 Sample Injury Cases Case No Date Incident Injury X Y 14 May Slip outside Female Locker Room Z 62 3 Apr Caustic splash to Face/ Eyes in Buffer Prep Burn to eyes prescribed drops Forearm Fracture – Cast & Surgery 28 Nov Injury to left hand Minor small finger using fracture wrench requiring Classificati on Medical Treatment Lost Time Restricted work
Sample Injury Cases Case No Date Incident Injury X Y 14 May Slip outside Female Locker Room Z 62 3 Apr Caustic splash to Face/ Eyes in Buffer Prep Burn to eyes prescribed drops Forearm Fracture – Cast & Surgery 28 Nov Injury to left hand Minor small finger using fracture wrench requiring Classificati on Medical Treatment Lost Time Restricted work
 3) Schedule Phases n High Level Milestones n High Level Schedule n Schedule Breakdown n Integration of Project Phases n Schedule Optimisation n Assumptions & Risks n 63
3) Schedule Phases n High Level Milestones n High Level Schedule n Schedule Breakdown n Integration of Project Phases n Schedule Optimisation n Assumptions & Risks n 63
 Schedule (Design to Sustaining Production) Design n Construction n Qualification n Start-Up n Regulatory Submission n Commercial Production n Long Term Outlook (Ramp Up, Optimisations, Shutdowns, New Product Introduction) n 64
Schedule (Design to Sustaining Production) Design n Construction n Qualification n Start-Up n Regulatory Submission n Commercial Production n Long Term Outlook (Ramp Up, Optimisations, Shutdowns, New Product Introduction) n 64
 High Level Milestones Break up Work into Milestones - easy way of viewing overall schedule to ensure on track § Sample List of Milestones: Prelim Design Report (Issue Wk 1) Ø Detailed Design Phase Proposal (Issue Wk 6) Ø Value Engineering & Scope Revision (Wk 10) Ø Start Detailed Design (Wk 11) Ø Long Lead Equipment & Cleanroom Panel System ordered (Wk 11) Ø Plant shutdown (Wk 16 - Wk 38) Ø Process Start-up (Wk 35 - Wk 38) Ø All systems operational (Wk 52) Ø 65
High Level Milestones Break up Work into Milestones - easy way of viewing overall schedule to ensure on track § Sample List of Milestones: Prelim Design Report (Issue Wk 1) Ø Detailed Design Phase Proposal (Issue Wk 6) Ø Value Engineering & Scope Revision (Wk 10) Ø Start Detailed Design (Wk 11) Ø Long Lead Equipment & Cleanroom Panel System ordered (Wk 11) Ø Plant shutdown (Wk 16 - Wk 38) Ø Process Start-up (Wk 35 - Wk 38) Ø All systems operational (Wk 52) Ø 65
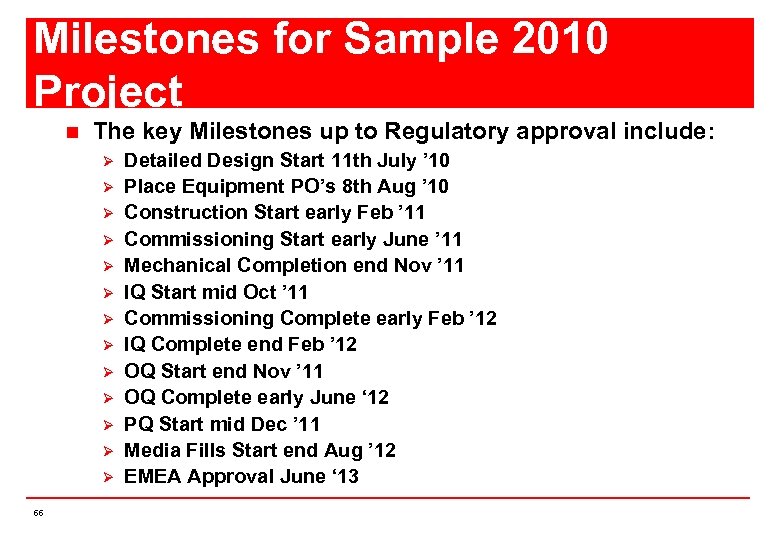 Milestones for Sample 2010 Project n The key Milestones up to Regulatory approval include: Ø Ø Ø Ø 66 Detailed Design Start 11 th July ’ 10 Place Equipment PO’s 8 th Aug ’ 10 Construction Start early Feb ’ 11 Commissioning Start early June ’ 11 Mechanical Completion end Nov ’ 11 IQ Start mid Oct ’ 11 Commissioning Complete early Feb ’ 12 IQ Complete end Feb ’ 12 OQ Start end Nov ’ 11 OQ Complete early June ‘ 12 PQ Start mid Dec ’ 11 Media Fills Start end Aug ’ 12 EMEA Approval June ‘ 13
Milestones for Sample 2010 Project n The key Milestones up to Regulatory approval include: Ø Ø Ø Ø 66 Detailed Design Start 11 th July ’ 10 Place Equipment PO’s 8 th Aug ’ 10 Construction Start early Feb ’ 11 Commissioning Start early June ’ 11 Mechanical Completion end Nov ’ 11 IQ Start mid Oct ’ 11 Commissioning Complete early Feb ’ 12 IQ Complete end Feb ’ 12 OQ Start end Nov ’ 11 OQ Complete early June ‘ 12 PQ Start mid Dec ’ 11 Media Fills Start end Aug ’ 12 EMEA Approval June ‘ 13
 High Level Milestones - Sample 67
High Level Milestones - Sample 67
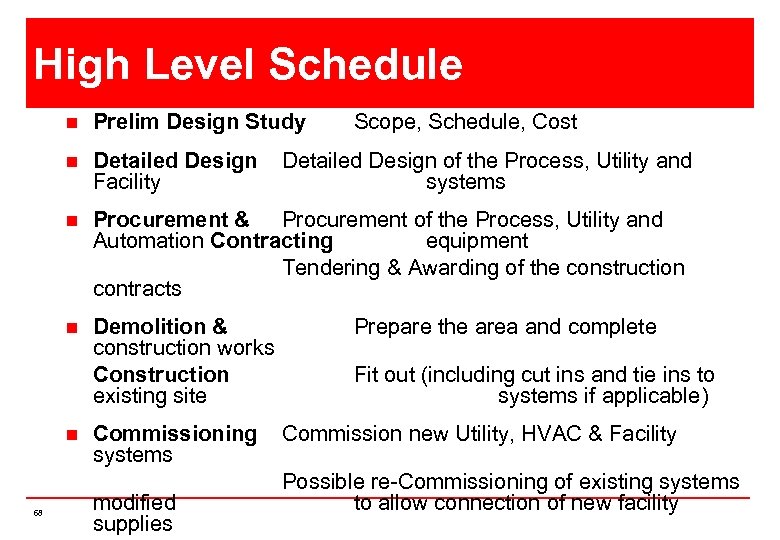 High Level Schedule n Prelim Design Study n Detailed Design Facility n Procurement & Procurement of the Process, Utility and Automation Contracting equipment Tendering & Awarding of the construction contracts n Demolition & construction works Construction existing site n 68 Commissioning systems modified supplies Scope, Schedule, Cost Detailed Design of the Process, Utility and systems Prepare the area and complete Fit out (including cut ins and tie ins to systems if applicable) Commission new Utility, HVAC & Facility Possible re-Commissioning of existing systems to allow connection of new facility
High Level Schedule n Prelim Design Study n Detailed Design Facility n Procurement & Procurement of the Process, Utility and Automation Contracting equipment Tendering & Awarding of the construction contracts n Demolition & construction works Construction existing site n 68 Commissioning systems modified supplies Scope, Schedule, Cost Detailed Design of the Process, Utility and systems Prepare the area and complete Fit out (including cut ins and tie ins to systems if applicable) Commission new Utility, HVAC & Facility Possible re-Commissioning of existing systems to allow connection of new facility
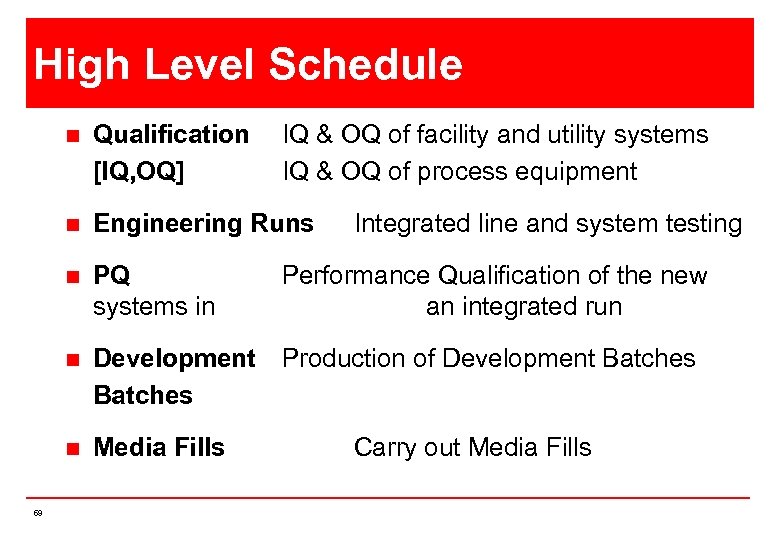 High Level Schedule n n Engineering Runs n PQ systems in n Development Production of Development Batches n 69 Qualification [IQ, OQ] IQ & OQ of facility and utility systems IQ & OQ of process equipment Media Fills Integrated line and system testing Performance Qualification of the new an integrated run Carry out Media Fills
High Level Schedule n n Engineering Runs n PQ systems in n Development Production of Development Batches n 69 Qualification [IQ, OQ] IQ & OQ of facility and utility systems IQ & OQ of process equipment Media Fills Integrated line and system testing Performance Qualification of the new an integrated run Carry out Media Fills
 High Level Schedule n n Regulatory Filing Prepare & submit Regulatory Filing and & Approval achieve approval n 70 Stability Maintain filled material for a 6 mth period under controlled conditions with stability testing to prove integrity of filling process Commercial Production of product for sale
High Level Schedule n n Regulatory Filing Prepare & submit Regulatory Filing and & Approval achieve approval n 70 Stability Maintain filled material for a 6 mth period under controlled conditions with stability testing to prove integrity of filling process Commercial Production of product for sale
 High Level Schedule - Sample 1 These phases are organised generally in the following sequence: 71
High Level Schedule - Sample 1 These phases are organised generally in the following sequence: 71
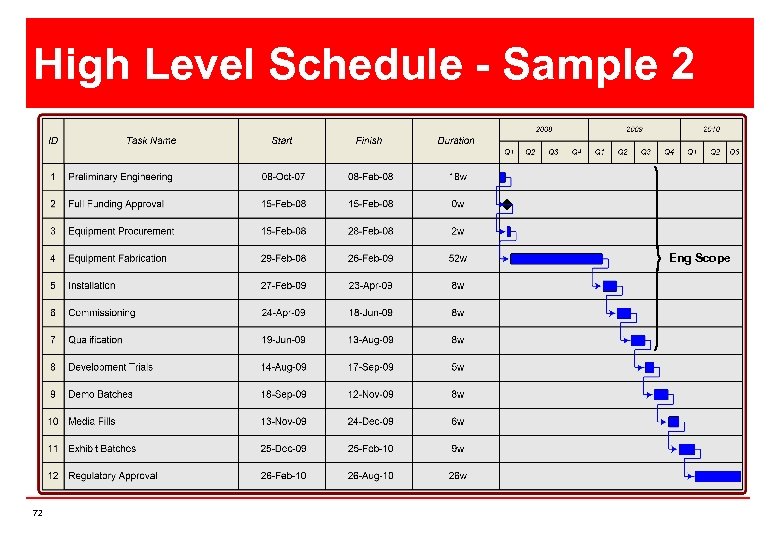 High Level Schedule - Sample 2 Eng Scope 72
High Level Schedule - Sample 2 Eng Scope 72
 Schedule Breakdown Schedule can broken down into chunks n Each piece of Equipment will have it’s own individual schedule n Allows individuals to focus on their own particular area of interest n All these individual schedules are part of one overall master schedule n 73
Schedule Breakdown Schedule can broken down into chunks n Each piece of Equipment will have it’s own individual schedule n Allows individuals to focus on their own particular area of interest n All these individual schedules are part of one overall master schedule n 73
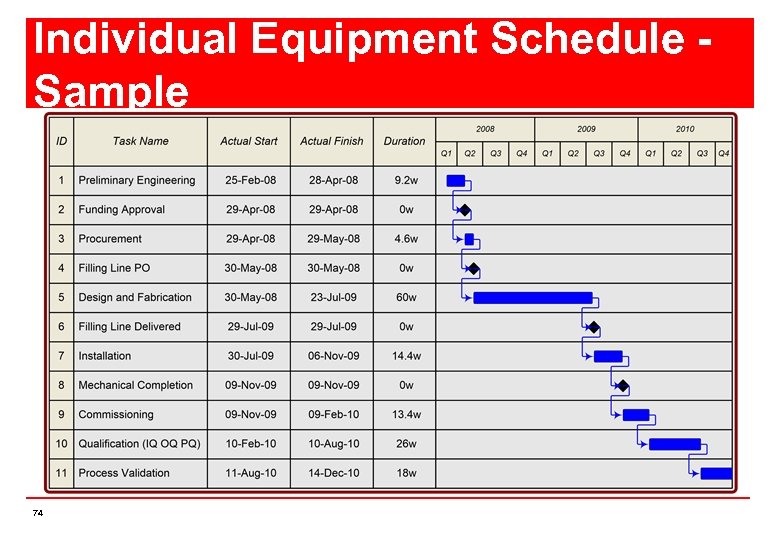 Individual Equipment Schedule Sample 74
Individual Equipment Schedule Sample 74
 Integration of Project Phases A Project needs Project Phases to be well Integrated: n n This places a significant emphasis on developing an integrated project life cycle to ensure that activities carried out in each phase are aligned and supportive of the activities in the subsequent phases n 75 No phase of the project can be executed without consideration of the subsequent phases For successful project integration the following needs to happen early in the implementation phase:
Integration of Project Phases A Project needs Project Phases to be well Integrated: n n This places a significant emphasis on developing an integrated project life cycle to ensure that activities carried out in each phase are aligned and supportive of the activities in the subsequent phases n 75 No phase of the project can be executed without consideration of the subsequent phases For successful project integration the following needs to happen early in the implementation phase:
 Integration of Project Phases Ø Hold a series of Project Implementation Workshops to ensure that the: − Project scope is understood − Project ‘life cycle’ is understood − Key implementation issues are reviewed and understood − Clear ‘buy in’ and ownership of the implementation strategy is achieved − Implementation schedule is tested, understood and owned by all parties § Some of the key ‘integration’ issues and philosophies that will be key include: Ø 76 Qualification − Early agreement on Qualification & Validation strategy − Calibration − Information leveraging to support Qualification − Scope of OQ versus PQ
Integration of Project Phases Ø Hold a series of Project Implementation Workshops to ensure that the: − Project scope is understood − Project ‘life cycle’ is understood − Key implementation issues are reviewed and understood − Clear ‘buy in’ and ownership of the implementation strategy is achieved − Implementation schedule is tested, understood and owned by all parties § Some of the key ‘integration’ issues and philosophies that will be key include: Ø 76 Qualification − Early agreement on Qualification & Validation strategy − Calibration − Information leveraging to support Qualification − Scope of OQ versus PQ
 Integration of Project Phases Ø Factory Acceptance Testing (FAT) − Engineering or Qualification activity − Information leveraging to support Qualification Ø Process Equipment Vendors − Roles onsite − Extent of time required onsite Ø Other projects that would affect the main project − Other Projects in the building e. g. new Clean Steam Generators − Other Projects on the wider Site e. g. new Warehouse 77
Integration of Project Phases Ø Factory Acceptance Testing (FAT) − Engineering or Qualification activity − Information leveraging to support Qualification Ø Process Equipment Vendors − Roles onsite − Extent of time required onsite Ø Other projects that would affect the main project − Other Projects in the building e. g. new Clean Steam Generators − Other Projects on the wider Site e. g. new Warehouse 77
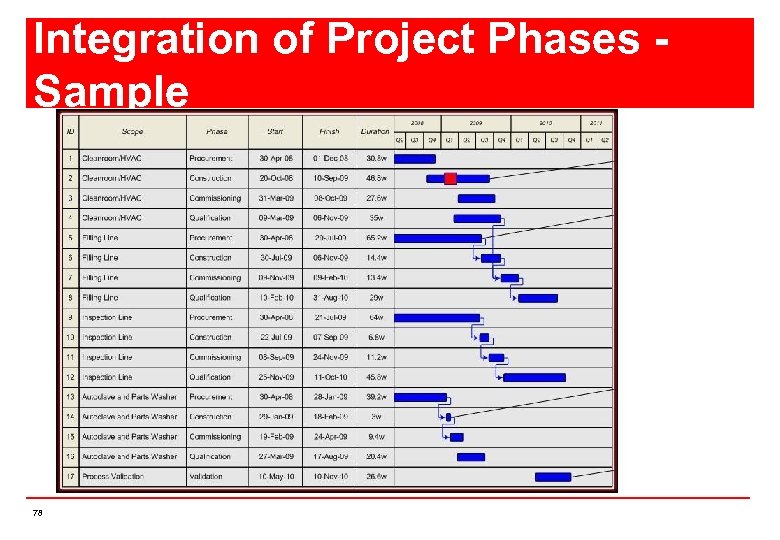 Integration of Project Phases Sample 78
Integration of Project Phases Sample 78
 Schedule Optimisation Aggressive Schedule n n A facility start up schedule is always very aggressive, particularly with pharmaceutical facilities given the type of facility and the technology involved. For example the complexity of the formulation process and the use of filling lines are key schedule challenges. Various options can be reviewed to reduce / significantly improve the schedule; some of which are: Ø Ø Ø 79 Need to have a structured and ‘controlled’ project Design phase must have qualification life cycle requirements agreed and integrated from the start Need to have a controlled procurement phase where vendors are brought on board early with clear scope definition and project execution life cycles to hold them to
Schedule Optimisation Aggressive Schedule n n A facility start up schedule is always very aggressive, particularly with pharmaceutical facilities given the type of facility and the technology involved. For example the complexity of the formulation process and the use of filling lines are key schedule challenges. Various options can be reviewed to reduce / significantly improve the schedule; some of which are: Ø Ø Ø 79 Need to have a structured and ‘controlled’ project Design phase must have qualification life cycle requirements agreed and integrated from the start Need to have a controlled procurement phase where vendors are brought on board early with clear scope definition and project execution life cycles to hold them to
 Schedule Optimisation Ø Ø 80 Lead times for modular cleanroom components Construction period to allow practical sequencing and a good quality of construction Allowance for dedicated commissioning periods per system [commissioning is a critical phase in the project, it must be carried out in a structured fashion to ensure a successful subsequent qualification] Benchmarking from recent similar projects
Schedule Optimisation Ø Ø 80 Lead times for modular cleanroom components Construction period to allow practical sequencing and a good quality of construction Allowance for dedicated commissioning periods per system [commissioning is a critical phase in the project, it must be carried out in a structured fashion to ensure a successful subsequent qualification] Benchmarking from recent similar projects
 Assumptions & Risks § 81 There are many risks to the facility start up schedules that have to be monitored and managed
Assumptions & Risks § 81 There are many risks to the facility start up schedules that have to be monitored and managed
 Assumptions & Risks Examples of Key risks to schedules are: Fixed shutdown constraints - typically there is virtually no float within the given shutdown period n Long Lead items must be procured by certain date n Reliance on Package Vendors for validation documentation must be managed closely n Design review & HAZOP approach to be agreed n Validation document review will require n 82
Assumptions & Risks Examples of Key risks to schedules are: Fixed shutdown constraints - typically there is virtually no float within the given shutdown period n Long Lead items must be procured by certain date n Reliance on Package Vendors for validation documentation must be managed closely n Design review & HAZOP approach to be agreed n Validation document review will require n 82
 Assumptions & Risks § Key assumptions underpinning the project schedule are: Ø Ø Ø Ø 83 Integrated ‘one team’ approach between Vendors (particularly engineering design) and Facility ‘Can do’ / ‘make it happen’ attitude Integrated Design / C&V team PO’s will be placed as early a possible to allow vendor design information to flow as early as possible - this will allow a more robust design URS’s content will be focused on user requirements - they will not be detailed engineering specifications Quick and efficient turn around of documents from Facility Automation ‘sub projects’ [MCS & QBMS] are fully integrated and controlled as a fundamental part of
Assumptions & Risks § Key assumptions underpinning the project schedule are: Ø Ø Ø Ø 83 Integrated ‘one team’ approach between Vendors (particularly engineering design) and Facility ‘Can do’ / ‘make it happen’ attitude Integrated Design / C&V team PO’s will be placed as early a possible to allow vendor design information to flow as early as possible - this will allow a more robust design URS’s content will be focused on user requirements - they will not be detailed engineering specifications Quick and efficient turn around of documents from Facility Automation ‘sub projects’ [MCS & QBMS] are fully integrated and controlled as a fundamental part of
 Assumptions & Risks Ø Ø Ø 84 No significant scope changes - for this type of project the impact of a change is significant given the significant knock -on to design documentation, vendor documentation, automation and commissioning/ qualification protocols Cleanroom package will be negotiated to allow detailed cleanroom design to be fully integrated with the full design Commissioning will be completed per system prior to qualification starting on that system Commissioning of process equipment can progress with commissioned clean utilities Commissioning & qualification logic will be as follows: Systems will move directly from mechanical completion to commissioning to qualification with minimal constraints / holds imposed from other systems
Assumptions & Risks Ø Ø Ø 84 No significant scope changes - for this type of project the impact of a change is significant given the significant knock -on to design documentation, vendor documentation, automation and commissioning/ qualification protocols Cleanroom package will be negotiated to allow detailed cleanroom design to be fully integrated with the full design Commissioning will be completed per system prior to qualification starting on that system Commissioning of process equipment can progress with commissioned clean utilities Commissioning & qualification logic will be as follows: Systems will move directly from mechanical completion to commissioning to qualification with minimal constraints / holds imposed from other systems
 Assumptions & Risks Ø Ø 85 Facility System Owners identified and available during the detailed design phase to attend FAT’s, coordinate Facility input to system snagging, provide Facility lead for systems through completion, commissioning & qualification phases Sufficient Facility Operations & Maintenance personnel allocated to the project from construction completion / FAT phase Progress from IQ to OQ and OQ to PQ is based on minor deviations outstanding Dedicated project change control ‘sub committee’ for the project [to review and approve change controls arising post the start of IQ]
Assumptions & Risks Ø Ø 85 Facility System Owners identified and available during the detailed design phase to attend FAT’s, coordinate Facility input to system snagging, provide Facility lead for systems through completion, commissioning & qualification phases Sufficient Facility Operations & Maintenance personnel allocated to the project from construction completion / FAT phase Progress from IQ to OQ and OQ to PQ is based on minor deviations outstanding Dedicated project change control ‘sub committee’ for the project [to review and approve change controls arising post the start of IQ]
 4) Preventive Maintenance Objective Ensure routine maintenance work is performed so that GMP equipment and associated ancillary systems shall be maintained to continue to perform in a qualified/ validated state 86
4) Preventive Maintenance Objective Ensure routine maintenance work is performed so that GMP equipment and associated ancillary systems shall be maintained to continue to perform in a qualified/ validated state 86
 Maintenance Management System n Preventive Maintenance Work Ø All Relevant Areas in the facility n Maintenance Management System (SAP) n Engineering Stores/ Spare Parts (SAP) n Relevant SOPs - possible examples: Ø Ø Ø 87 Maintenance Work Procedure Maintenance Management System Procedure Engineering and Operations Stores Procedure
Maintenance Management System n Preventive Maintenance Work Ø All Relevant Areas in the facility n Maintenance Management System (SAP) n Engineering Stores/ Spare Parts (SAP) n Relevant SOPs - possible examples: Ø Ø Ø 87 Maintenance Work Procedure Maintenance Management System Procedure Engineering and Operations Stores Procedure
 Maintenance Management System n Manage Equipment Register & associated Maintenance History Records n Run a Preventive Maintenance program Ø Ø n Direct the Corrective Maintenance program Ø Ø n 88 Maintenance Task List Maintenance Plan Track equipment malfunction Facilitate analysis of maintenance information Facilitate asset Spare Parts replacement, storage, control and records
Maintenance Management System n Manage Equipment Register & associated Maintenance History Records n Run a Preventive Maintenance program Ø Ø n Direct the Corrective Maintenance program Ø Ø n 88 Maintenance Task List Maintenance Plan Track equipment malfunction Facilitate analysis of maintenance information Facilitate asset Spare Parts replacement, storage, control and records
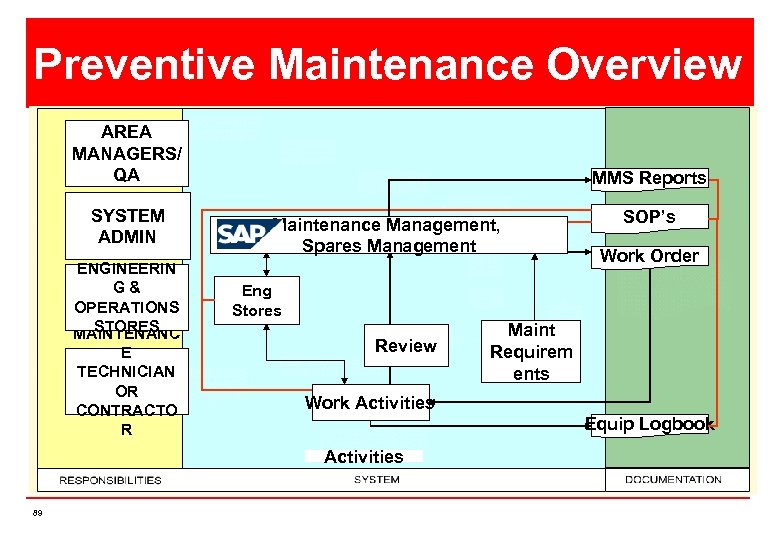 Preventive Maintenance Overview AREA MANAGERS/ QA SYSTEM ADMIN ENGINEERIN G& OPERATIONS STORES MAINTENANC E TECHNICIAN OR CONTRACTO R MMS Reports Maintenance Management, Spares Management Work Order Eng Stores Review Maint Requirem ents Work Activities Equip Logbook Activities 89 SOP’s
Preventive Maintenance Overview AREA MANAGERS/ QA SYSTEM ADMIN ENGINEERIN G& OPERATIONS STORES MAINTENANC E TECHNICIAN OR CONTRACTO R MMS Reports Maintenance Management, Spares Management Work Order Eng Stores Review Maint Requirem ents Work Activities Equip Logbook Activities 89 SOP’s
 MMS (SAP Plant Maintenance) Roles n MMS Administration Ø Ø Ø n MMS Operator Ø Ø Ø 90 Create Corrective Maintenance Work Orders Print Work Orders Display & Print Reports Feedback Information from completed Work Orders Alter Details on Assets, Maintenance Task Lists & Plans Ø Create Corrective Maintenance Work Orders Print Work Orders Display & Print Reports Feedback Information from completed Work Orders
MMS (SAP Plant Maintenance) Roles n MMS Administration Ø Ø Ø n MMS Operator Ø Ø Ø 90 Create Corrective Maintenance Work Orders Print Work Orders Display & Print Reports Feedback Information from completed Work Orders Alter Details on Assets, Maintenance Task Lists & Plans Ø Create Corrective Maintenance Work Orders Print Work Orders Display & Print Reports Feedback Information from completed Work Orders
 QUESTIONS? ? ? n 91 clement. farrar@gmail. com
QUESTIONS? ? ? n 91 clement. farrar@gmail. com


