436aafc5d9bbbb62028073dfca9e4c4b.ppt
- Количество слайдов: 32
 Country Report for India M. R. Rajagopal, Chairman, Pallium India, Trivandrum Nandini Vallath – Palliative Care Physician, Bangalore Priyadarshini Kulkarni – Medical Director, Cipla Palliative Care Centre, Pune Rajesh Nandan Srivastava – Director NC - DOR Shalini Vallabhan – Trustee, Pallium India, Mumbai Sudhir Gupta – Additional Deputy Director General, MOH 16 -03 -2018 1
Country Report for India M. R. Rajagopal, Chairman, Pallium India, Trivandrum Nandini Vallath – Palliative Care Physician, Bangalore Priyadarshini Kulkarni – Medical Director, Cipla Palliative Care Centre, Pune Rajesh Nandan Srivastava – Director NC - DOR Shalini Vallabhan – Trustee, Pallium India, Mumbai Sudhir Gupta – Additional Deputy Director General, MOH 16 -03 -2018 1
 Cancer pain and palliative care 16 -03 -2018 2
Cancer pain and palliative care 16 -03 -2018 2
 a. What is the estimated prevalence and types of cancer in your country, mortality, and the prevalence of pain? Estimated prevalence and type of cancer in India incidence ~ 11 lakh per year, prevalence 27 -28 lakh per year, deaths about 5 lakh per year 2. 5 million cancer cases at any given point of time Age-standardized death for cancer is 78. 8 Most Prevalent forms in men are lung, oral, larynx, esophagus, and pharynx Most prevalent forms in women, in addition to tobacco-related cancers are cervix, breast, and ovarian cancers Estimated prevalence and type of pain in India More than 1 million cancer patients suffer from severe pain every year http: //www. indiaenvironmentportal. org. in/files/file/PHFI_NCD_Report_Sep_2011. pdf http: //www. who. int/nmh/publications/ncd_report_full_en. pdf. 16 -03 -2018 3
a. What is the estimated prevalence and types of cancer in your country, mortality, and the prevalence of pain? Estimated prevalence and type of cancer in India incidence ~ 11 lakh per year, prevalence 27 -28 lakh per year, deaths about 5 lakh per year 2. 5 million cancer cases at any given point of time Age-standardized death for cancer is 78. 8 Most Prevalent forms in men are lung, oral, larynx, esophagus, and pharynx Most prevalent forms in women, in addition to tobacco-related cancers are cervix, breast, and ovarian cancers Estimated prevalence and type of pain in India More than 1 million cancer patients suffer from severe pain every year http: //www. indiaenvironmentportal. org. in/files/file/PHFI_NCD_Report_Sep_2011. pdf http: //www. who. int/nmh/publications/ncd_report_full_en. pdf. 16 -03 -2018 3
 b. Is there a national cancer control policy, plan, or program? If so, when did it start? What is the name of the office and person in charge? Are objectives for pain relief and palliative care included? Is availability of opioid analgesics specifically addressed? There is a National cancer control Policy. NCCP- started in 1975 -76. Office: Dr. R. K. Srivastava, DGHS, Ministry of Health and Family welfare, GOI NCCP got merged with NPCDCS with effect from July 2010. Its goals and objectives include pain relief and palliative care Availability of opioids are not specifically addressed http: //www. indg. in/india/sitemap-1/health/national_health_programmes/national-cancer-control-programme-current-status-strategies-in-india http: //www. who. int/cancer/nccp/en/ 16 -03 -2018 http: //india. gov. in/sectors/health_family/index. php? id=11 4
b. Is there a national cancer control policy, plan, or program? If so, when did it start? What is the name of the office and person in charge? Are objectives for pain relief and palliative care included? Is availability of opioid analgesics specifically addressed? There is a National cancer control Policy. NCCP- started in 1975 -76. Office: Dr. R. K. Srivastava, DGHS, Ministry of Health and Family welfare, GOI NCCP got merged with NPCDCS with effect from July 2010. Its goals and objectives include pain relief and palliative care Availability of opioids are not specifically addressed http: //www. indg. in/india/sitemap-1/health/national_health_programmes/national-cancer-control-programme-current-status-strategies-in-india http: //www. who. int/cancer/nccp/en/ 16 -03 -2018 http: //india. gov. in/sectors/health_family/index. php? id=11 4
 NPCDCS National Program for Prevention and Control of Cancer, Diabetes, CVD and Stroke NCD cell at State, Districts, CHC and Sub Centre level Palliative care, home based care as essential features of care Funding for awareness, training , personnel, equipment, NLEM Convergence with National Cancer Control Program National Rural Health Mission (NRHM) National Tobacco Control Programme (NTCP) National Programme for Health Care of Elderly (NPHCE) Other programs with similar theme 16 -03 -2018 5
NPCDCS National Program for Prevention and Control of Cancer, Diabetes, CVD and Stroke NCD cell at State, Districts, CHC and Sub Centre level Palliative care, home based care as essential features of care Funding for awareness, training , personnel, equipment, NLEM Convergence with National Cancer Control Program National Rural Health Mission (NRHM) National Tobacco Control Programme (NTCP) National Programme for Health Care of Elderly (NPHCE) Other programs with similar theme 16 -03 -2018 5
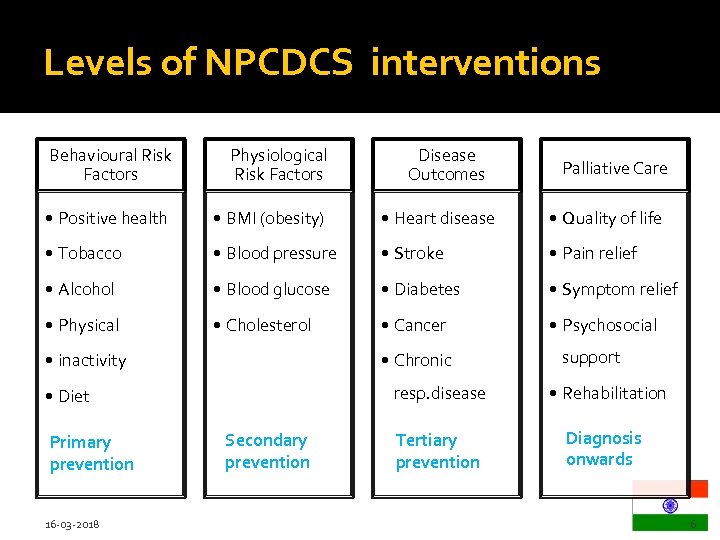 Levels of NPCDCS interventions Behavioural Risk Factors Physiological Risk Factors • Positive health • BMI (obesity) • Heart disease • Quality of life • Tobacco • Blood pressure • Stroke • Pain relief • Alcohol • Blood glucose • Diabetes • Symptom relief • Physical • Cholesterol • Cancer • Psychosocial • inactivity • Chronic 16 -03 -2018 Palliative Care support resp. disease • Diet Primary prevention Disease Outcomes Secondary prevention • Rehabilitation Tertiary prevention Diagnosis onwards 6
Levels of NPCDCS interventions Behavioural Risk Factors Physiological Risk Factors • Positive health • BMI (obesity) • Heart disease • Quality of life • Tobacco • Blood pressure • Stroke • Pain relief • Alcohol • Blood glucose • Diabetes • Symptom relief • Physical • Cholesterol • Cancer • Psychosocial • inactivity • Chronic 16 -03 -2018 Palliative Care support resp. disease • Diet Primary prevention Disease Outcomes Secondary prevention • Rehabilitation Tertiary prevention Diagnosis onwards 6
 c. Has the government endorsed the WHO method for relief of cancer pain? Has the government sponsored or endorsed training programs in cancer pain relief, palliative care and the medical use of opioid analgesics? Yes, Government has endorsed WHO method for relief of cancer pain In 1994 Government of India and WHOCC and PPSG conducted 2 workshops to understand simplify narcotic rules – resulted in recommendation of modified model NDPS rules for states Currently 13 states and 1 union territory have simplified rules The state of Kerala declared its Palliative Care policy in April 2008 It has “Aarogya Keralam” project under which there are 614 nurse led home care projects PC department in Government institutions e. g. AIIMS Delhi, PGI Chandigarh, SGPGI Lucknow, Few Regional Cancer Centres Palliative Medicine has recently got recognition as medical specialty - MD http: //www. whoindia. org/en/Section 1_1894. asp http: //www. painpolicy. wisc. edu/publicat/07 jpsm/india 07. pdf 16 -03 -2018 7
c. Has the government endorsed the WHO method for relief of cancer pain? Has the government sponsored or endorsed training programs in cancer pain relief, palliative care and the medical use of opioid analgesics? Yes, Government has endorsed WHO method for relief of cancer pain In 1994 Government of India and WHOCC and PPSG conducted 2 workshops to understand simplify narcotic rules – resulted in recommendation of modified model NDPS rules for states Currently 13 states and 1 union territory have simplified rules The state of Kerala declared its Palliative Care policy in April 2008 It has “Aarogya Keralam” project under which there are 614 nurse led home care projects PC department in Government institutions e. g. AIIMS Delhi, PGI Chandigarh, SGPGI Lucknow, Few Regional Cancer Centres Palliative Medicine has recently got recognition as medical specialty - MD http: //www. whoindia. org/en/Section 1_1894. asp http: //www. painpolicy. wisc. edu/publicat/07 jpsm/india 07. pdf 16 -03 -2018 7
 d. Describe in brief terms the availability of pain relief and palliative care services in the country and comment on the extent to which the needy population has access to such services, including children. Many of the cancer hospitals have opioid availability and the relevant license; but pain relief is unknown field to majority of oncologists There about 250 palliative care centres, majority run by NGOs with about 180 of them in the state of Kerala. These centres have either inpatient, out patient or home based facilities 16 out of India's 28 states and 7 union territories do not have any palliative care services at all Pain management is a poorly taught skill and 2 generations of doctors have graduated without training to understand pain nor any exposure to opioid usage for moderate to severe pains; including government run medical colleges How well is pediatric cancer pain treated? Do pediatric patients have access to opioid analgesics in the class of morphine? About 3 lakh children with various life limiting conditions need palliative care in India. The facilities are very poor for paediatric pain patients http: //www. palliativecare. in/ http: //www. jpalliativecare. com/temp/Indian. JPalliat. Care 174522108539_055125. pdf 16 -03 -2018 8
d. Describe in brief terms the availability of pain relief and palliative care services in the country and comment on the extent to which the needy population has access to such services, including children. Many of the cancer hospitals have opioid availability and the relevant license; but pain relief is unknown field to majority of oncologists There about 250 palliative care centres, majority run by NGOs with about 180 of them in the state of Kerala. These centres have either inpatient, out patient or home based facilities 16 out of India's 28 states and 7 union territories do not have any palliative care services at all Pain management is a poorly taught skill and 2 generations of doctors have graduated without training to understand pain nor any exposure to opioid usage for moderate to severe pains; including government run medical colleges How well is pediatric cancer pain treated? Do pediatric patients have access to opioid analgesics in the class of morphine? About 3 lakh children with various life limiting conditions need palliative care in India. The facilities are very poor for paediatric pain patients http: //www. palliativecare. in/ http: //www. jpalliativecare. com/temp/Indian. JPalliat. Care 174522108539_055125. pdf 16 -03 -2018 8
 e. Identify non-governmental organizations that have a focus on pain relief and palliative care and mention their relevant activities. Is there a national palliative care association? The Indian Association of Palliative care was formed in 1994 in consultation with World Health Organisation and Government of India Activities are aimed at the care of people with life limiting illness such as Cancer, AIDS and end-stage chronic medical diseases including access to pain relief, palliative care capacity building and advocacy. Children’s Palliative Care program was launched by IAPC in 2010, which focuses on pain relief in HIV positive children Pallium India – is an NGO and a WHOCC working on capacity building and opioid availability issues International Association of Study of Pain, Indian Chapter has 1525 members and 14 state chapters Cansupport, Cankids, Karunashraya trust, Cipla Palliative Care Centre and many other NGOs work through clinical service or supporting capacity building in their chosen population Departments of Palliative Medicine in private medical colleges and hospitals e. g. CMC Vellore, St John’s Medical College Hospital, HCG – BIO, Bangalore, Baptist Hospital Bangalore There also pain clinics in metros through efforts of anaesthesiologists, but mostly focused on regional nerve blocks – need to merge WHO analgesic ladder into services offered 16 -03 -2018 9
e. Identify non-governmental organizations that have a focus on pain relief and palliative care and mention their relevant activities. Is there a national palliative care association? The Indian Association of Palliative care was formed in 1994 in consultation with World Health Organisation and Government of India Activities are aimed at the care of people with life limiting illness such as Cancer, AIDS and end-stage chronic medical diseases including access to pain relief, palliative care capacity building and advocacy. Children’s Palliative Care program was launched by IAPC in 2010, which focuses on pain relief in HIV positive children Pallium India – is an NGO and a WHOCC working on capacity building and opioid availability issues International Association of Study of Pain, Indian Chapter has 1525 members and 14 state chapters Cansupport, Cankids, Karunashraya trust, Cipla Palliative Care Centre and many other NGOs work through clinical service or supporting capacity building in their chosen population Departments of Palliative Medicine in private medical colleges and hospitals e. g. CMC Vellore, St John’s Medical College Hospital, HCG – BIO, Bangalore, Baptist Hospital Bangalore There also pain clinics in metros through efforts of anaesthesiologists, but mostly focused on regional nerve blocks – need to merge WHO analgesic ladder into services offered 16 -03 -2018 9
 HIV/AIDS pain and palliative care 16 -03 -2018 10
HIV/AIDS pain and palliative care 16 -03 -2018 10
 What is the estimated prevalence of HIV/AIDS in your country, mortality and the prevalence of pain? http: //www. nacoonline. org Prevalence: The adult (15 -49 years) HIV prevalence in India is estimated at 0. 32% in 2008 and 0. 31% in 2009 with approximately 2. 4 million people living with HIV. High prevalence states - Manipur (1. 4%); followed by Andhra Pradesh (0. 90%), Mizoram (0. 81%), Nagaland (0. 78%), Karnataka (0. 63%) and Maharashtra (0. 55%). Delhi, Orissa, West Bengal, Chhattisgarh and Pondicherry have an estimated adult HIV prevalence of 0. 28 to 0. 30% whilst HIV prevalence in other states is less than 0. 28%. Mortality - Approximately 172, 000 people died of AIDS related causes in 2009 in India. 2008/2009 HIV estimates highlight the declining trend of annual AIDS deaths post 2004 Pain Prevalence: 66. 7% in admitted patients and 24. 5% out patients. Average 35. 5% Ref: IJPC Jan-June 2009, Vol 15 Issue 1 Approximately 1. 2 million HIV patients suffer from pain each year (30 -80% of HIV patient have pain) UNAID 16 -03 -2018 11
What is the estimated prevalence of HIV/AIDS in your country, mortality and the prevalence of pain? http: //www. nacoonline. org Prevalence: The adult (15 -49 years) HIV prevalence in India is estimated at 0. 32% in 2008 and 0. 31% in 2009 with approximately 2. 4 million people living with HIV. High prevalence states - Manipur (1. 4%); followed by Andhra Pradesh (0. 90%), Mizoram (0. 81%), Nagaland (0. 78%), Karnataka (0. 63%) and Maharashtra (0. 55%). Delhi, Orissa, West Bengal, Chhattisgarh and Pondicherry have an estimated adult HIV prevalence of 0. 28 to 0. 30% whilst HIV prevalence in other states is less than 0. 28%. Mortality - Approximately 172, 000 people died of AIDS related causes in 2009 in India. 2008/2009 HIV estimates highlight the declining trend of annual AIDS deaths post 2004 Pain Prevalence: 66. 7% in admitted patients and 24. 5% out patients. Average 35. 5% Ref: IJPC Jan-June 2009, Vol 15 Issue 1 Approximately 1. 2 million HIV patients suffer from pain each year (30 -80% of HIV patient have pain) UNAID 16 -03 -2018 11
 Is there a national AIDS policy, plan, or program? When did it start? What is the name of the office and person in charge? National AIDS Control Organisation is a division of the Ministry of Health and Family Welfare - 35 HIV/AIDS Prevention and Control Societies If so, when did it start? In 1986, following the detection of the first AIDS case in the country, the National AIDS Committee was constituted in the Ministry of Health and Family Welfare Office: National AIDS Control Organisation (NACO) was constituted in 1992 to implement the national program. Person In charge: Secretary & Director General NACO - Shri Sayan Chatterjee 16 -03 -2018 12
Is there a national AIDS policy, plan, or program? When did it start? What is the name of the office and person in charge? National AIDS Control Organisation is a division of the Ministry of Health and Family Welfare - 35 HIV/AIDS Prevention and Control Societies If so, when did it start? In 1986, following the detection of the first AIDS case in the country, the National AIDS Committee was constituted in the Ministry of Health and Family Welfare Office: National AIDS Control Organisation (NACO) was constituted in 1992 to implement the national program. Person In charge: Secretary & Director General NACO - Shri Sayan Chatterjee 16 -03 -2018 12
 Are objectives for pain relief and palliative care included? Is availability of opioid analgesics specifically addressed? NO Has the government endorsed the WHO method for relief of HIV/AIDS pain? Has the government sponsored or endorsed training programs in pain relief, palliative care and the medical use of opioid analgesics? NO Describe in brief terms the availability of pain relief and palliative care services in the country for HIV/AIDS patients and comment on the extent to which the needy population has access to such services, including children. How well is pediatric pain treated? Do pediatric patients have access to opioid analgesics in the class of morphine? NOT AVAILABLE 16 -03 -2018 13
Are objectives for pain relief and palliative care included? Is availability of opioid analgesics specifically addressed? NO Has the government endorsed the WHO method for relief of HIV/AIDS pain? Has the government sponsored or endorsed training programs in pain relief, palliative care and the medical use of opioid analgesics? NO Describe in brief terms the availability of pain relief and palliative care services in the country for HIV/AIDS patients and comment on the extent to which the needy population has access to such services, including children. How well is pediatric pain treated? Do pediatric patients have access to opioid analgesics in the class of morphine? NOT AVAILABLE 16 -03 -2018 13
 Opioid availability 16 -03 -2018 14
Opioid availability 16 -03 -2018 14
 Identify the office that is the National Competent Authority (NCA) for narcotics control for the country. Who is in charge of the office, and who is in charge of submitting the annual estimate of medical requirements for narcotic drugs, including morphine, to the International Narcotics Control Board? Is there a representative of this office at this meeting? Two National Competent Authorities for different aspects of narcotics control Narcotics Control Bureau (NCB) Ministry of Home Affairs Coordinating action among other drug law enforcement agencies Central Bureau of Narcotics (CBN) Department of Revenue, MO Finance Allots the estimates received from INCB as quotas to manufacturing companies in the country Collects consumption figures from such companies and arrives at ‘estimates’ and sends statistics to INCB through the NCB The mandate of CBN or NCB does not include aspects regarding medical use of opioids Officer in charge Director General of NCB and Narcotics Commissioner CBN Mr. Rajesh Nandan Srivastava, Director (Narcotics Control) is here. 16 -03 -2018 15
Identify the office that is the National Competent Authority (NCA) for narcotics control for the country. Who is in charge of the office, and who is in charge of submitting the annual estimate of medical requirements for narcotic drugs, including morphine, to the International Narcotics Control Board? Is there a representative of this office at this meeting? Two National Competent Authorities for different aspects of narcotics control Narcotics Control Bureau (NCB) Ministry of Home Affairs Coordinating action among other drug law enforcement agencies Central Bureau of Narcotics (CBN) Department of Revenue, MO Finance Allots the estimates received from INCB as quotas to manufacturing companies in the country Collects consumption figures from such companies and arrives at ‘estimates’ and sends statistics to INCB through the NCB The mandate of CBN or NCB does not include aspects regarding medical use of opioids Officer in charge Director General of NCB and Narcotics Commissioner CBN Mr. Rajesh Nandan Srivastava, Director (Narcotics Control) is here. 16 -03 -2018 15
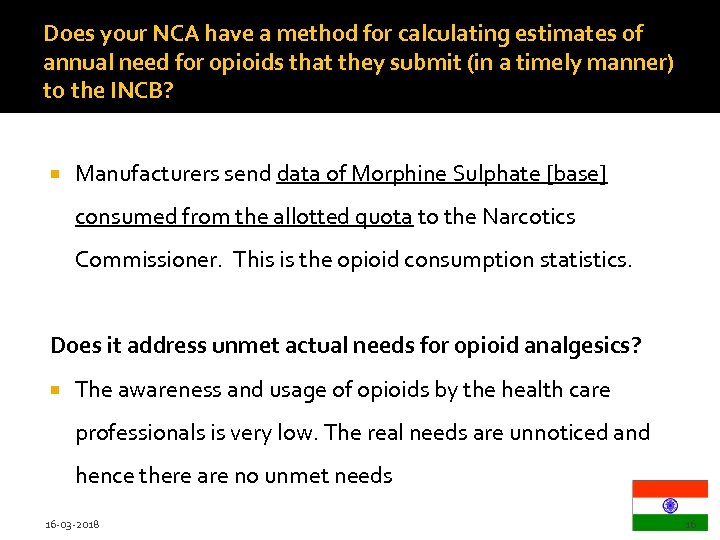 Does your NCA have a method for calculating estimates of annual need for opioids that they submit (in a timely manner) to the INCB? Manufacturers send data of Morphine Sulphate [base] consumed from the allotted quota to the Narcotics Commissioner. This is the opioid consumption statistics. Does it address unmet actual needs for opioid analgesics? The awareness and usage of opioids by the health care professionals is very low. The real needs are unnoticed and hence there are no unmet needs 16 -03 -2018 16
Does your NCA have a method for calculating estimates of annual need for opioids that they submit (in a timely manner) to the INCB? Manufacturers send data of Morphine Sulphate [base] consumed from the allotted quota to the Narcotics Commissioner. This is the opioid consumption statistics. Does it address unmet actual needs for opioid analgesics? The awareness and usage of opioids by the health care professionals is very low. The real needs are unnoticed and hence there are no unmet needs 16 -03 -2018 16
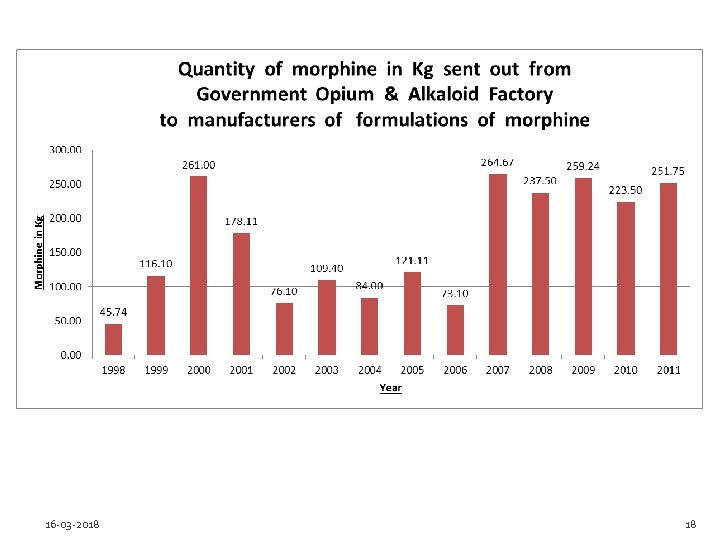
 Does your NCA report annual statistics on the consumption of opioid analgesics to the INCB? Yes, however the country has experienced difficulties in reporting the statistics of ‘consumption’ as defined in the 1961 Convention. It was with the DCGI. Possibilities for error exists e. g. small quantities are exported to Nepal. There is certainty that all the morphine has not really reached retail levels as significant quantities may be idling with manufacturers. Since 2010, the responsibility of collection of consumption statistics has been given to the Narcotics Commissioner. 16 -03 -2018 17
Does your NCA report annual statistics on the consumption of opioid analgesics to the INCB? Yes, however the country has experienced difficulties in reporting the statistics of ‘consumption’ as defined in the 1961 Convention. It was with the DCGI. Possibilities for error exists e. g. small quantities are exported to Nepal. There is certainty that all the morphine has not really reached retail levels as significant quantities may be idling with manufacturers. Since 2010, the responsibility of collection of consumption statistics has been given to the Narcotics Commissioner. 16 -03 -2018 17
 16 -03 -2018 18
16 -03 -2018 18
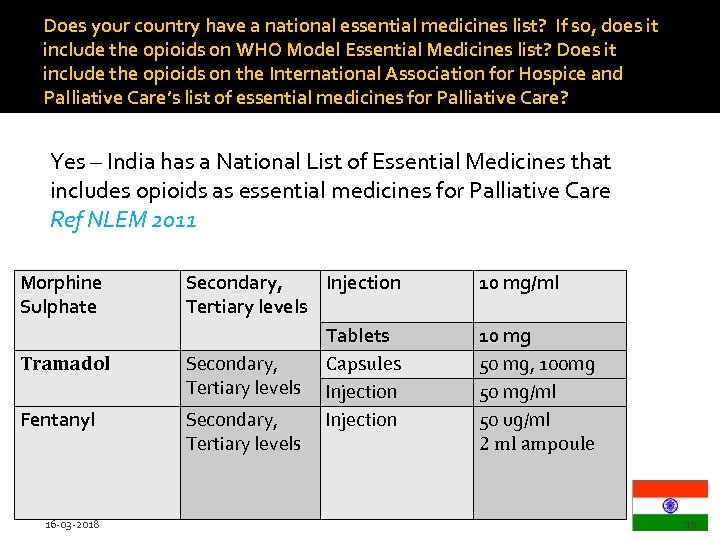 Does your country have a national essential medicines list? If so, does it include the opioids on WHO Model Essential Medicines list? Does it include the opioids on the International Association for Hospice and Palliative Care’s list of essential medicines for Palliative Care? Yes – India has a National List of Essential Medicines that includes opioids as essential medicines for Palliative Care Ref NLEM 2011 Morphine Sulphate Tramadol Fentanyl 16 -03 -2018 Secondary, Injection Tertiary levels Tablets Secondary, Capsules Tertiary levels Injection Secondary, Injection Tertiary levels 10 mg/ml 10 mg 50 mg, 100 mg 50 mg/ml 50 ug/ml 2 ml ampoule 19
Does your country have a national essential medicines list? If so, does it include the opioids on WHO Model Essential Medicines list? Does it include the opioids on the International Association for Hospice and Palliative Care’s list of essential medicines for Palliative Care? Yes – India has a National List of Essential Medicines that includes opioids as essential medicines for Palliative Care Ref NLEM 2011 Morphine Sulphate Tramadol Fentanyl 16 -03 -2018 Secondary, Injection Tertiary levels Tablets Secondary, Capsules Tertiary levels Injection Secondary, Injection Tertiary levels 10 mg/ml 10 mg 50 mg, 100 mg 50 mg/ml 50 ug/ml 2 ml ampoule 19
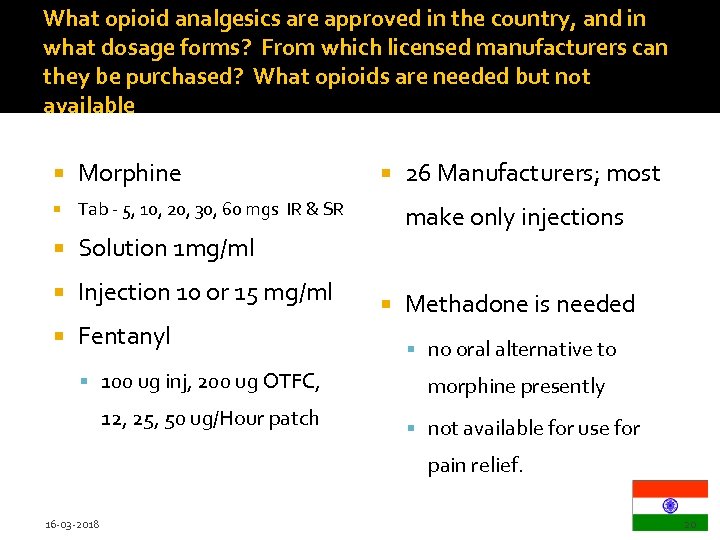 What opioid analgesics are approved in the country, and in what dosage forms? From which licensed manufacturers can they be purchased? What opioids are needed but not available Morphine Tab - 5, 10, 20, 30, 60 mgs IR & SR Solution 1 mg/ml Injection 10 or 15 mg/ml Fentanyl 100 ug inj, 200 ug OTFC, 12, 25, 50 ug/Hour patch 26 Manufacturers; most make only injections Methadone is needed no oral alternative to morphine presently not available for use for pain relief. 16 -03 -2018 20
What opioid analgesics are approved in the country, and in what dosage forms? From which licensed manufacturers can they be purchased? What opioids are needed but not available Morphine Tab - 5, 10, 20, 30, 60 mgs IR & SR Solution 1 mg/ml Injection 10 or 15 mg/ml Fentanyl 100 ug inj, 200 ug OTFC, 12, 25, 50 ug/Hour patch 26 Manufacturers; most make only injections Methadone is needed no oral alternative to morphine presently not available for use for pain relief. 16 -03 -2018 20
 For those opioids that are available, do you think they are sufficiently available in the places where cancer patients are treated in the country, i. e. , all hospitals with cancer units, hospices, pain clinics, palliative care programs, etc. ? No. These drugs are not readily available and accessible at the retail level for professionals or patients to access even with valid prescriptions Are there shortages or “stock-outs” so that prescriptions cannot be dispensed? Yes, stock outs do happen 16 -03 -2018 21
For those opioids that are available, do you think they are sufficiently available in the places where cancer patients are treated in the country, i. e. , all hospitals with cancer units, hospices, pain clinics, palliative care programs, etc. ? No. These drugs are not readily available and accessible at the retail level for professionals or patients to access even with valid prescriptions Are there shortages or “stock-outs” so that prescriptions cannot be dispensed? Yes, stock outs do happen 16 -03 -2018 21
 What are the basic requirements for a physician to prescribe an opioid such as morphine? What licenses or authorizations are required by the government and medical institutions? There are 2 situations. In 13 states with modified rules, State Drug Controller is a single contact authorisation nodus (at least in theory) for inspecting and granting an institution a status of “Recognised Medical Institution” which allows transaction on opioids through an approved medical practitioner who has undergone hands-on training in the use of opioids is recommended In other states, there is need for institutions to have individual licenses for possession, import, export and transport from excise department, Collector. Each of them have validity periods. Even states that adopt model rules have problems as differences in regulations still exist across state lines. Ideally, uniform rules are needed 16 -03 -2018 22
What are the basic requirements for a physician to prescribe an opioid such as morphine? What licenses or authorizations are required by the government and medical institutions? There are 2 situations. In 13 states with modified rules, State Drug Controller is a single contact authorisation nodus (at least in theory) for inspecting and granting an institution a status of “Recognised Medical Institution” which allows transaction on opioids through an approved medical practitioner who has undergone hands-on training in the use of opioids is recommended In other states, there is need for institutions to have individual licenses for possession, import, export and transport from excise department, Collector. Each of them have validity periods. Even states that adopt model rules have problems as differences in regulations still exist across state lines. Ideally, uniform rules are needed 16 -03 -2018 22
 What are the basic requirements for a physician to prescribe an opioid such as morphine? Are special prescription forms required? Are they easy to complete, and are they easily accessible? No. The drug, dosage, format and duration have to be specified along with details of the patient and signature and name of the approved medical practitioner. Model rule recommends duplicate prescription (one stays with the institution, the other with the patient) Is special training required for opioid prescribing? Any approved medical doctor, dentist or veterinary doctor may prescribe opioid for their patients. Model rules stipulate 10 days of training in pain management to stock the medicines Is prescribing limited to only certain types of doctors? NOT according Act. But the model rules recommend 10 days of training. Are (specially trained) nurses authorized to prescribe? NO 16 -03 -2018 23
What are the basic requirements for a physician to prescribe an opioid such as morphine? Are special prescription forms required? Are they easy to complete, and are they easily accessible? No. The drug, dosage, format and duration have to be specified along with details of the patient and signature and name of the approved medical practitioner. Model rule recommends duplicate prescription (one stays with the institution, the other with the patient) Is special training required for opioid prescribing? Any approved medical doctor, dentist or veterinary doctor may prescribe opioid for their patients. Model rules stipulate 10 days of training in pain management to stock the medicines Is prescribing limited to only certain types of doctors? NOT according Act. But the model rules recommend 10 days of training. Are (specially trained) nurses authorized to prescribe? NO 16 -03 -2018 23
 What are the other requirements for writing a prescription for an opioid such as morphine? Is there a maximum amount that can be prescribed at one time, for example a limitation on the number of dosage units or number of days? No Is there a maximum length of time that a patient can receive opioids? No What is the period of time that a prescription for an opioid such as morphine is valid? Once pain relief is achieved and is stable on a dosage, a prescription may be given for 100 dosages, which usually lasts for over 2 weeks. Do prescribing regulations exclude patient populations or diagnoses? No there are no strict exclusions. Although not stipulated as such, opioids are used mostly for cancer patients. Some states restrict it to cancer in the new model rules, however 16 -03 -2018 24
What are the other requirements for writing a prescription for an opioid such as morphine? Is there a maximum amount that can be prescribed at one time, for example a limitation on the number of dosage units or number of days? No Is there a maximum length of time that a patient can receive opioids? No What is the period of time that a prescription for an opioid such as morphine is valid? Once pain relief is achieved and is stable on a dosage, a prescription may be given for 100 dosages, which usually lasts for over 2 weeks. Do prescribing regulations exclude patient populations or diagnoses? No there are no strict exclusions. Although not stipulated as such, opioids are used mostly for cancer patients. Some states restrict it to cancer in the new model rules, however 16 -03 -2018 24
 What are the other requirements for writing a prescription for an opioid such as morphine? Are there different legal requirements for prescribing, dispensing or purchasing different dosage forms of the same opioid, i. e. , oral, transdermal, injectable? If an institution is licensed for a certain formulation of a certain drug (e. g. inj morphine), one will need additional permissions to get Tab Morphine / solution etc. What is the minimum and maximum penalty for a physician or pharmacist who violates the prescribing laws or regulations? 6 months / Rs. 10, 000 for possession of quantity < 5 gms up to 10 years of rigorous imprisonment for quantity > 250 gms physicians or pharmacists are not specifically mentioned in the law Does the national law or regulation require reporting names of patients who receive opioid prescriptions to the government? No 16 -03 -2018 25
What are the other requirements for writing a prescription for an opioid such as morphine? Are there different legal requirements for prescribing, dispensing or purchasing different dosage forms of the same opioid, i. e. , oral, transdermal, injectable? If an institution is licensed for a certain formulation of a certain drug (e. g. inj morphine), one will need additional permissions to get Tab Morphine / solution etc. What is the minimum and maximum penalty for a physician or pharmacist who violates the prescribing laws or regulations? 6 months / Rs. 10, 000 for possession of quantity < 5 gms up to 10 years of rigorous imprisonment for quantity > 250 gms physicians or pharmacists are not specifically mentioned in the law Does the national law or regulation require reporting names of patients who receive opioid prescriptions to the government? No 16 -03 -2018 25
 What, if any, changes have been made in laws, regulations or commercialization to improve the medical use and availability of opioid analgesics? 1998 – Central Government developed Model rules to modify state rules Allowed a single licensing entity of State Drug Controller that would help ensure that “registered medical institutions” can access opioids through a streamlined process. This has helped to a limited extent 13 states have modified rules Arunachal Pradesh, Jammu and Kashmir, Kerala, Mizoram, Sikkim, Andhra Pradesh, Goa, Karnataka, Madhya Pradesh, Orissa, Tamilnadu, Maharashtra, Delhi, Dadra-Nagarhaveli and Tripura. Workshops ongoing July 2012 –formation of Technical Resource Group [MOH, DOR and HC professionals] 16 -03 -2018 26
What, if any, changes have been made in laws, regulations or commercialization to improve the medical use and availability of opioid analgesics? 1998 – Central Government developed Model rules to modify state rules Allowed a single licensing entity of State Drug Controller that would help ensure that “registered medical institutions” can access opioids through a streamlined process. This has helped to a limited extent 13 states have modified rules Arunachal Pradesh, Jammu and Kashmir, Kerala, Mizoram, Sikkim, Andhra Pradesh, Goa, Karnataka, Madhya Pradesh, Orissa, Tamilnadu, Maharashtra, Delhi, Dadra-Nagarhaveli and Tripura. Workshops ongoing July 2012 –formation of Technical Resource Group [MOH, DOR and HC professionals] 16 -03 -2018 26
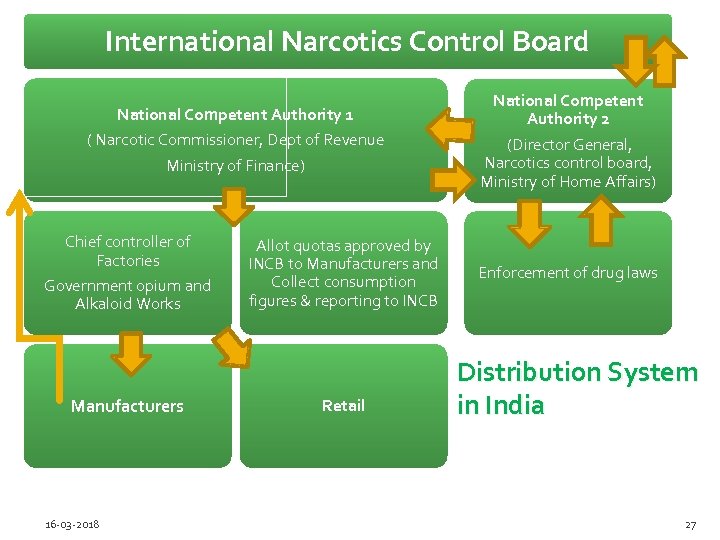 International Narcotics Control Board National Competent Authority 1 ( Narcotic Commissioner, Dept of Revenue Ministry of Finance) Chief controller of Factories Government opium and Alkaloid Works Manufacturers 16 -03 -2018 Allot quotas approved by INCB to Manufacturers and Collect consumption figures & reporting to INCB Retail National Competent Authority 2 (Director General, Narcotics control board, Ministry of Home Affairs) Enforcement of drug laws Distribution System in India 27
International Narcotics Control Board National Competent Authority 1 ( Narcotic Commissioner, Dept of Revenue Ministry of Finance) Chief controller of Factories Government opium and Alkaloid Works Manufacturers 16 -03 -2018 Allot quotas approved by INCB to Manufacturers and Collect consumption figures & reporting to INCB Retail National Competent Authority 2 (Director General, Narcotics control board, Ministry of Home Affairs) Enforcement of drug laws Distribution System in India 27
 Is the cost of medications or health insurance coverage a barrier to patient accessibility to opioid analgesics? Explain. Cost is a factor although medicines are available at different cost ranges. E. g. 10 mg if bought as a strip is Rs 5/tab and as a tin is Rs 1/tab. Cost also varies between different manufacturers Also, many palliative care initiatives and cancer support organisations make the medicines available free. Also, two RCCs produce solutions directly from Morphine powder that is very cheap [e. g. one time registration of Rs 75/-] Many of the national insurance policies [ECHS, CGHS, ESIS] do not cover the cost of supportive care medicines or pain relief medicines. Marketing and research is cost driven e. g. Transdermal fentanyl patches are very expensive and well marketed 16 -03 -2018 28
Is the cost of medications or health insurance coverage a barrier to patient accessibility to opioid analgesics? Explain. Cost is a factor although medicines are available at different cost ranges. E. g. 10 mg if bought as a strip is Rs 5/tab and as a tin is Rs 1/tab. Cost also varies between different manufacturers Also, many palliative care initiatives and cancer support organisations make the medicines available free. Also, two RCCs produce solutions directly from Morphine powder that is very cheap [e. g. one time registration of Rs 75/-] Many of the national insurance policies [ECHS, CGHS, ESIS] do not cover the cost of supportive care medicines or pain relief medicines. Marketing and research is cost driven e. g. Transdermal fentanyl patches are very expensive and well marketed 16 -03 -2018 28
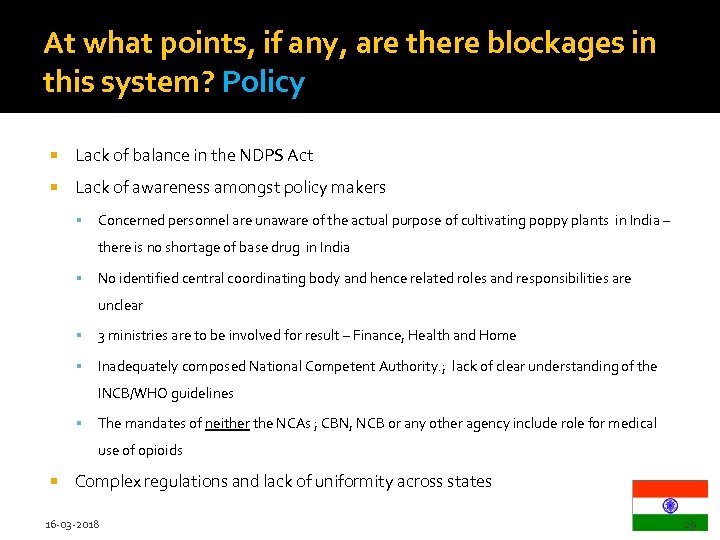 At what points, if any, are there blockages in this system? Policy Lack of balance in the NDPS Act Lack of awareness amongst policy makers Concerned personnel are unaware of the actual purpose of cultivating poppy plants in India – there is no shortage of base drug in India No identified central coordinating body and hence related roles and responsibilities are unclear 3 ministries are to be involved for result – Finance, Health and Home Inadequately composed National Competent Authority. ; lack of clear understanding of the INCB/WHO guidelines The mandates of neither the NCAs ; CBN, NCB or any other agency include role for medical use of opioids Complex regulations and lack of uniformity across states 16 -03 -2018 29
At what points, if any, are there blockages in this system? Policy Lack of balance in the NDPS Act Lack of awareness amongst policy makers Concerned personnel are unaware of the actual purpose of cultivating poppy plants in India – there is no shortage of base drug in India No identified central coordinating body and hence related roles and responsibilities are unclear 3 ministries are to be involved for result – Finance, Health and Home Inadequately composed National Competent Authority. ; lack of clear understanding of the INCB/WHO guidelines The mandates of neither the NCAs ; CBN, NCB or any other agency include role for medical use of opioids Complex regulations and lack of uniformity across states 16 -03 -2018 29
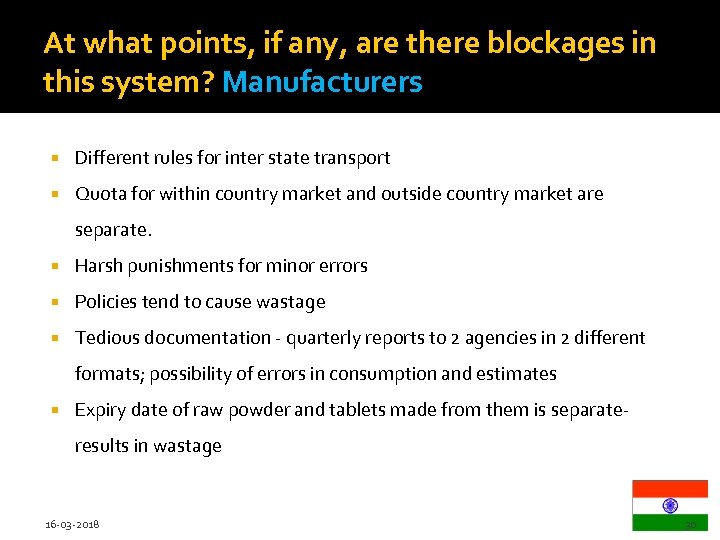 At what points, if any, are there blockages in this system? Manufacturers Different rules for inter state transport Quota for within country market and outside country market are separate. Harsh punishments for minor errors Policies tend to cause wastage Tedious documentation - quarterly reports to 2 agencies in 2 different formats; possibility of errors in consumption and estimates Expiry date of raw powder and tablets made from them is separateresults in wastage 16 -03 -2018 30
At what points, if any, are there blockages in this system? Manufacturers Different rules for inter state transport Quota for within country market and outside country market are separate. Harsh punishments for minor errors Policies tend to cause wastage Tedious documentation - quarterly reports to 2 agencies in 2 different formats; possibility of errors in consumption and estimates Expiry date of raw powder and tablets made from them is separateresults in wastage 16 -03 -2018 30
 At what points, if any, are there blockages in this system? End users Poor healthcare delivery systems. Public: Private : : 20: 80 Poor awareness regarding usage and misconceptions regarding addiction amongst professionals Lack of knowledge regarding WHO ladder drugs Even in states with “Model Rules “ availability continues to be poor. Even in institutions where opioids are available, acknowledging , assessing pain and prescribing practices poor, leading to untreated pain Poor awareness and myths amongst needy patients leading to low demand Cost > 80% health related expenses in India are Out Of Pocket Pharma companies do not market the cheaper alternatives nor support them entering market e. g. Methodone availability for medical usage is delayed due to lack of will to complete a 200 patient survey 16 -03 -2018 31
At what points, if any, are there blockages in this system? End users Poor healthcare delivery systems. Public: Private : : 20: 80 Poor awareness regarding usage and misconceptions regarding addiction amongst professionals Lack of knowledge regarding WHO ladder drugs Even in states with “Model Rules “ availability continues to be poor. Even in institutions where opioids are available, acknowledging , assessing pain and prescribing practices poor, leading to untreated pain Poor awareness and myths amongst needy patients leading to low demand Cost > 80% health related expenses in India are Out Of Pocket Pharma companies do not market the cheaper alternatives nor support them entering market e. g. Methodone availability for medical usage is delayed due to lack of will to complete a 200 patient survey 16 -03 -2018 31
 Thank You aanandini@gmail. com 32
Thank You aanandini@gmail. com 32


