10e3857f7ce471d42ad984d8ca9b2d1c.ppt
- Количество слайдов: 18

Corrosion and Scale Control in Subsea Production Systems Marion Seiersten Institute for Energy Technology
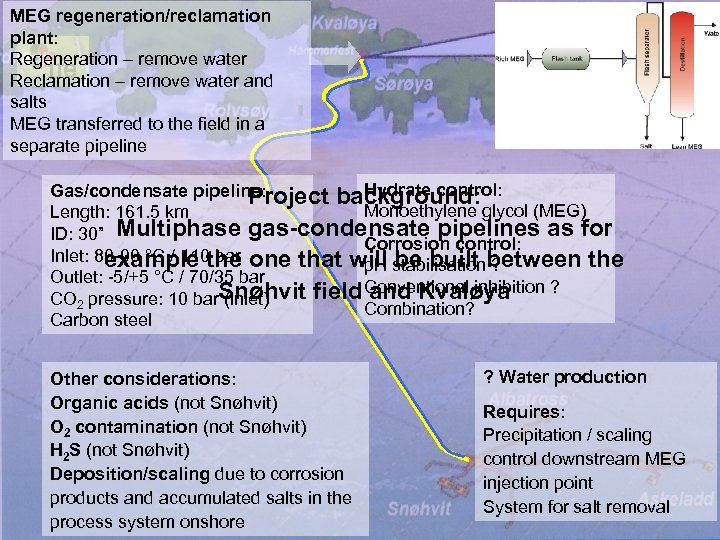
MEG regeneration/reclamation plant: Regeneration – remove water Reclamation – remove water and salts MEG transferred to the field in a separate pipeline Hydrate control: Gas/condensate pipeline: Project background: Monoethylene glycol (MEG) Length: 161. 5 km ID: 30” Multiphase gas-condensate pipelines as for Corrosion control: Inlet: 80 -90 °C / 110 the one that will be built between the bar example p. H stabilisation ? Outlet: -5/+5 °C / 70/35 bar Conventional inhibition ? CO 2 pressure: 10 bar. Snøhvit field and Kvaløya (inlet) Combination? Carbon steel Other considerations: Organic acids (not Snøhvit) O 2 contamination (not Snøhvit) H 2 S (not Snøhvit) Deposition/scaling due to corrosion products and accumulated salts in the process system onshore ? Water production Requires: Precipitation / scaling control downstream MEG injection point System for salt removal

All this requires that the chemistry of MEG-water systems is known: • Solubility of salts and gases as function of temperature, pressure and solvent composition • Precipitation / scaling rates • Corrosion / scale interaction That is what our project has been about
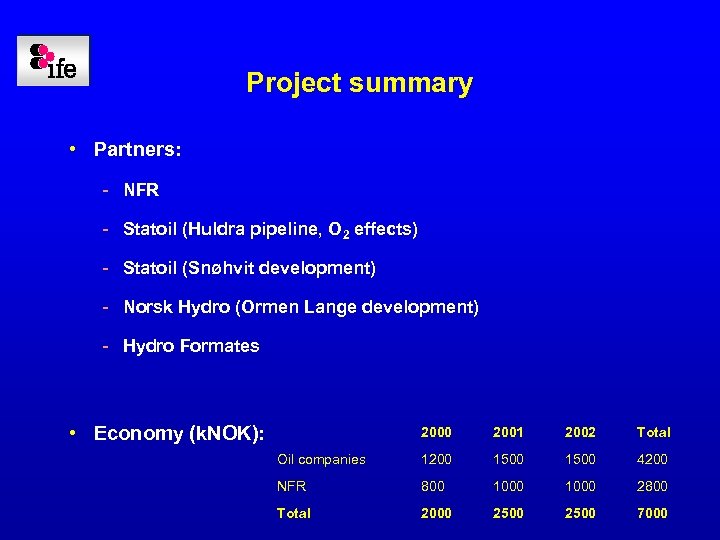
Project summary • Partners: - NFR - Statoil (Huldra pipeline, O 2 effects) - Statoil (Snøhvit development) - Norsk Hydro (Ormen Lange development) - Hydro Formates • Economy (k. NOK): 2000 2001 2002 Total Oil companies 1200 1500 4200 NFR 800 1000 2800 Total 2000 2500 7000

Objectives • To generate data that make it possible to predict the risk of precipitation and scaling in gas-condensate pipelines • To develop a model for MEG – water solutions that is able to - evaluate the p. H stabilisation technique; conditions for the formation of a stable Fe. CO 3 film - predict precipitation and dissolution of particles and scale that may result from mixing formation water and MEG with increased alkalinity (p. H adjusted or stabilised) - determine acetic acid / acetate build up in regenerated MEG - determine O 2 solubility in MEG water - look into the effect that O 2 has on precipitation and dissolution of corrosion products and scales
State of the art at the start of the project • Data on the solubility of gases and salts in mixed solvents (MEG-water) were sparse and scattered. A large part of the data that existed had been generated by IFE. Existing data at the start of the project: - CO 2 solubility: IFE had covered whole temperature and composition range, literature data at 20 -30 °C. - Dissociation constants for H 2 CO 3: Only part of the temperature and composition range was covered - Solubility of Fe. CO 3: Inaccurate data and only part of the ranges covered • There was no model that could be used to predict the solubilities • Need for data in the temperature range – 25 – +140°C and 0 -90 wt% MEG: - H 2 CO 3 dissociation constants, Fe. CO 3, Ca. CO 3, Na. Cl, Na. HCO 3, Na 2 CO 3, HAc, H 2 S, and others.

Working method • Solubility model: - At least 20 -30 experiments are needed to cover the whole temperature and composition range. More experiments are needed if ionic strength variations shall be covered. - Model: Based on regression of stoichiometric solubility functions to measured data; equilibrium constants that are functions of temperature, pressure, solvent composition and ionic strength. - By running the model development in parallel with experiments, the amount of experiments could be kept at a minimum. • Corrosion experiments: - Experiments to evaluate the effect of trace amounts of oxygen on pipeline corrosion

Solubility experiments • Simple experimental set-up • Extensive analysis of dissolved ions • Solubility of the following compounds have been measured in water - MEG: - Ca. CO 3 and Mg. CO 3 - Na. Cl - Na. HCO 3 - Na 2 CO 3 - Acetic acid - CO 2 (formate solutions) and H 2 S (partly)
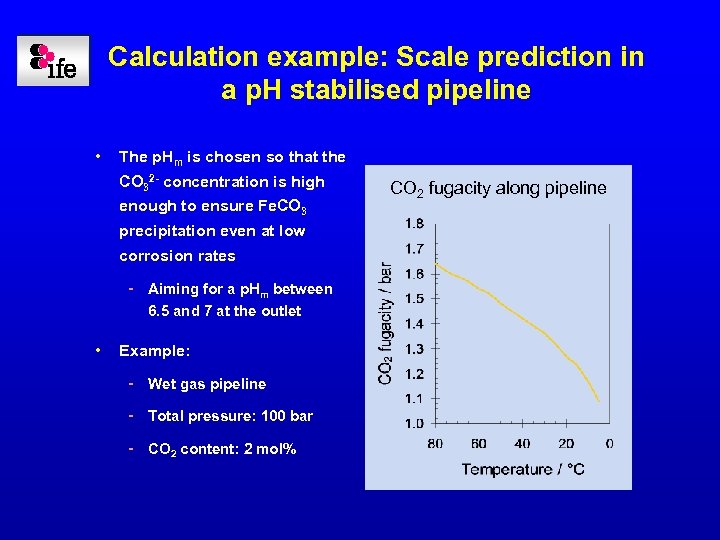
Calculation example: Scale prediction in a p. H stabilised pipeline • The p. Hm is chosen so that the CO 32 - concentration is high enough to ensure Fe. CO 3 precipitation even at low corrosion rates - Aiming for a p. Hm between 6. 5 and 7 at the outlet • Example: - Wet gas pipeline - Total pressure: 100 bar - CO 2 content: 2 mol% CO 2 fugacity along pipeline
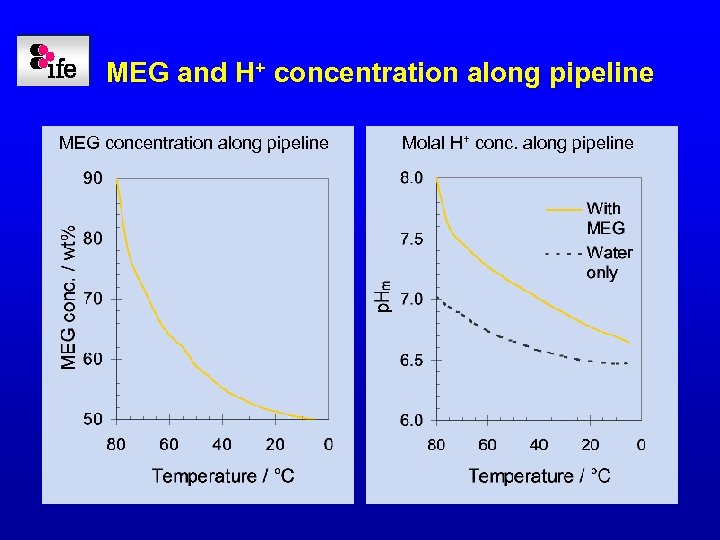
MEG and H+ concentration along pipeline MEG concentration along pipeline Molal H+ conc. along pipeline
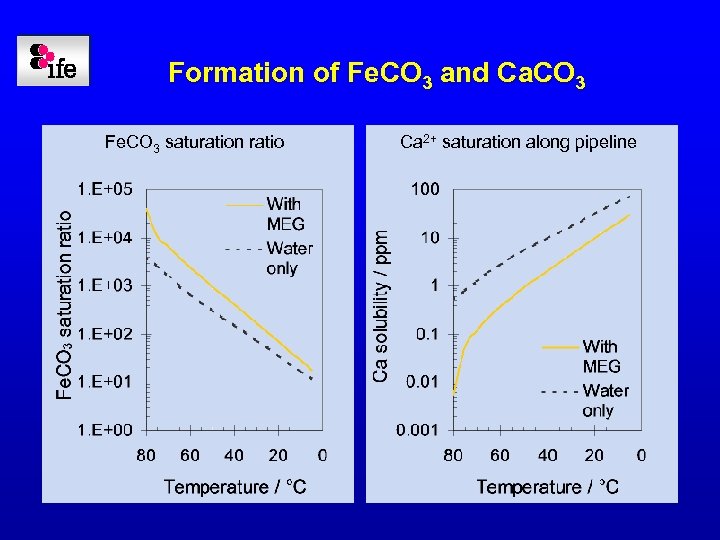
Formation of Fe. CO 3 and Ca. CO 3 Fe. CO 3 saturation ratio Ca 2+ saturation along pipeline
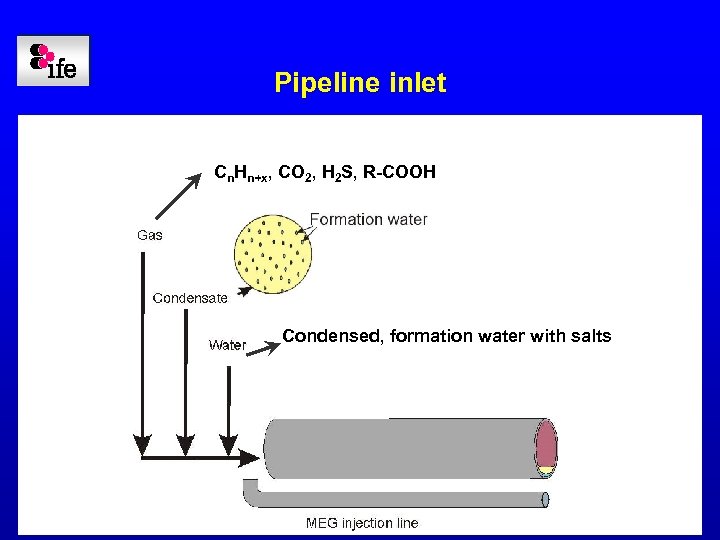
Pipeline inlet Cn. Hn+x, CO 2, H 2 S, R-COOH Condensed, formation water with salts
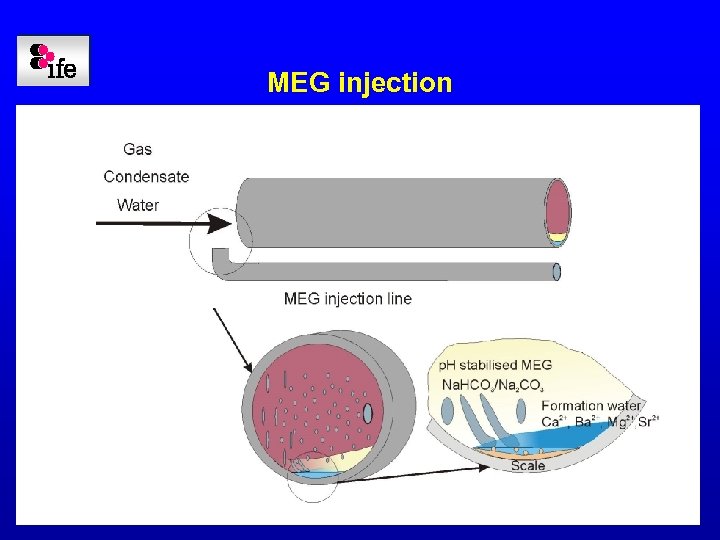
MEG injection
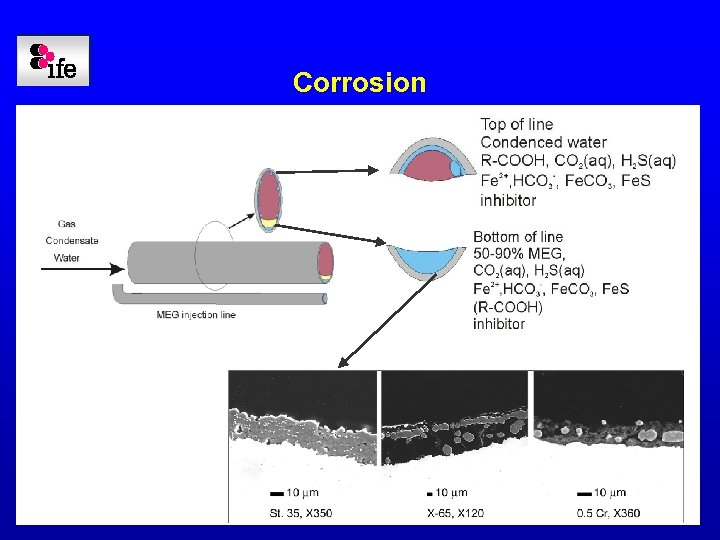
Corrosion
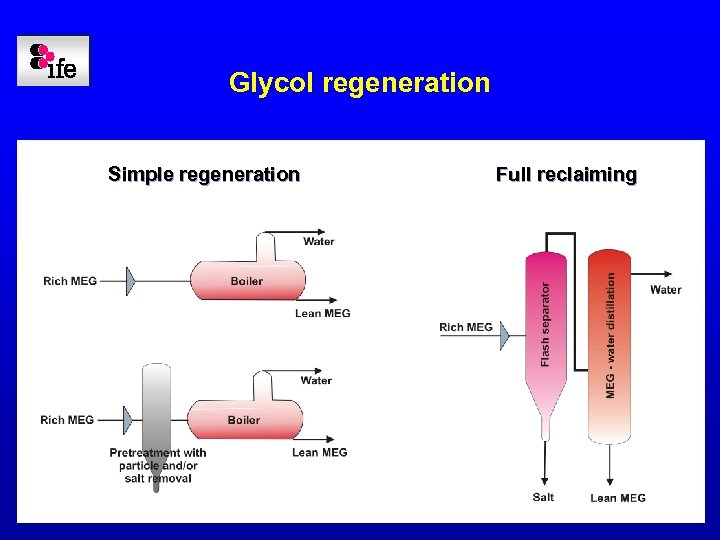
Glycol regeneration Simple regeneration Full reclaiming
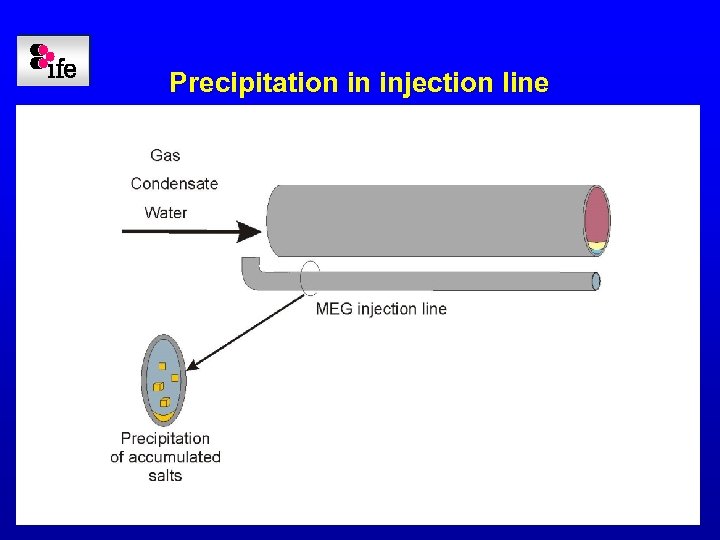
Precipitation in injection line

Exploitation of the results • In-house model at IFE for equilibrium chemistry calculations in MEG / water • A simplified model for p. H calculations delivered to Statoil • Data delivered to Hydro for implementation in their own simulation tool • Co-operation with Statoil, Hydro, NTNU and IFE to develop MEGScale – a commercial equilibrium model for MEG solutions, first version planned by the end of 2004 • The IFE in-house model has been used for the evaluation of p. H stabilisation or MEG regeneration/reclaiming units all over the World (Åsgard (Statoil), Snøhvit (Statoil), Brodgar (Conoco Phillips), South Pars (Several), Shah Deniz (BP), Nnwara Doro (AKT))

Conclusions • A successful project because it has: - Met the objectives (revised objectives) and enhanced a good relationship between the project partners - Upgraded the partners competence and contributed to • Optimised developments (increased robustness, better safety, reduced environmental impact, new solutions) • Increased amount of projects within the field at IFE - Formed a fundament for further model development through better understanding of MEG - water systems • MEGScale – to develop software which implements the results (started) • Glycol loops – integrated calculation tools that combine thermodynamic models for MEG and chemicals with a multiphase flow pipeline model and process models for the MEG regeneration system (co-operation with Aker Kværner, not started)
10e3857f7ce471d42ad984d8ca9b2d1c.ppt