05afb01b464d093ec61f6ed8bb48e8a0.ppt
- Количество слайдов: 27
 Correspondence to ISO 9001 Dr W. Huisman Cairo, november 18 th 2012 1
Correspondence to ISO 9001 Dr W. Huisman Cairo, november 18 th 2012 1
 • A presentation by David Burnett formed the base for this one 2
• A presentation by David Burnett formed the base for this one 2
 What is a standard? standard document, established by consensus and approved by a recognized body, that provides, for common and repeated use, rules, guidelines or characteristics for activities or their results, aimed at the achievement of the optimum degree of order in a given context ISO/IEC Guide 2 Standardization and related activities - General terms and their definitions 3
What is a standard? standard document, established by consensus and approved by a recognized body, that provides, for common and repeated use, rules, guidelines or characteristics for activities or their results, aimed at the achievement of the optimum degree of order in a given context ISO/IEC Guide 2 Standardization and related activities - General terms and their definitions 3
 • Written by medical laboratory professionals BS EN ISO 15189: 2007 (2 nd Edition) • Responsibility of ISO/TC 212 WG 1 • Requirements for quality and competence • It has its origins in two ISO Standards …ISO 9001 and ISO 17025 4
• Written by medical laboratory professionals BS EN ISO 15189: 2007 (2 nd Edition) • Responsibility of ISO/TC 212 WG 1 • Requirements for quality and competence • It has its origins in two ISO Standards …ISO 9001 and ISO 17025 4
 It has its origins in two ISO Standards… • ISO 9001: 2008 Quality management systems – Requirements (ISO/TC 176) • ISO/IEC 17025: 2005 General requirements for the competence of testing and calibration laboratories (ISO/CASCO) 5
It has its origins in two ISO Standards… • ISO 9001: 2008 Quality management systems – Requirements (ISO/TC 176) • ISO/IEC 17025: 2005 General requirements for the competence of testing and calibration laboratories (ISO/CASCO) 5
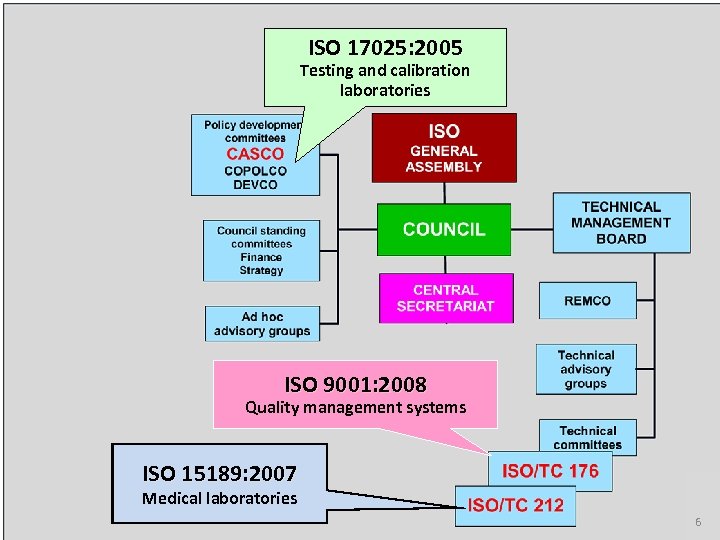 ISO 17025: 2005 Testing and calibration laboratories ISO 9001: 2008 Quality management systems ISO 15189: 2007 Medical laboratories 6
ISO 17025: 2005 Testing and calibration laboratories ISO 9001: 2008 Quality management systems ISO 15189: 2007 Medical laboratories 6
 ISO 15189 - What is its purpose? • Primarily it is for use by medical laboratories in developing their systems for managing quality and in assessing their competence… • Secondarily…it may be used by Accreditation Bodies in confirming or recognising the competence of medical laboratories 7
ISO 15189 - What is its purpose? • Primarily it is for use by medical laboratories in developing their systems for managing quality and in assessing their competence… • Secondarily…it may be used by Accreditation Bodies in confirming or recognising the competence of medical laboratories 7
 ISO 15189: 2007 – its requirements 8
ISO 15189: 2007 – its requirements 8
 ISO 15189 contrasted to ISO 17025 focuses on the patient outcome without downgrading the need for accuracy of measurements emphasizes not only the quality of the measurement but of the total service of a medical laboratory (consultation, turn around time, cost effectiveness etc. ) uses a language and terms that are familiar in the profession highlights important features of pre and post investigational (examination) issues addresses ethics and information needs of the medical laboratory. modified from ISO Bulletin February 2002 ‘Specifying quality and competence requirements for medical laboratories ’ 9
ISO 15189 contrasted to ISO 17025 focuses on the patient outcome without downgrading the need for accuracy of measurements emphasizes not only the quality of the measurement but of the total service of a medical laboratory (consultation, turn around time, cost effectiveness etc. ) uses a language and terms that are familiar in the profession highlights important features of pre and post investigational (examination) issues addresses ethics and information needs of the medical laboratory. modified from ISO Bulletin February 2002 ‘Specifying quality and competence requirements for medical laboratories ’ 9
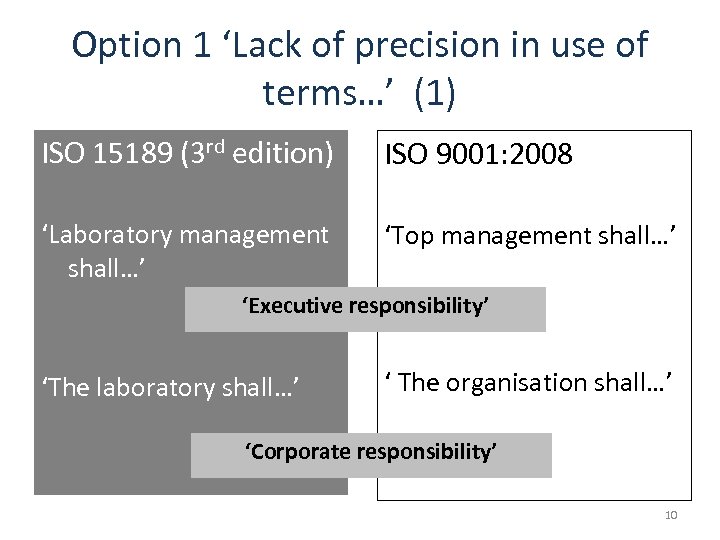 Option 1 ‘Lack of precision in use of terms…’ (1) ISO 15189 (3 rd edition) ISO 9001: 2008 ‘Laboratory management shall…’ ‘Top management shall…’ ‘Executive responsibility’ ‘The laboratory shall…’ ‘ The organisation shall…’ ‘Corporate responsibility’ 10
Option 1 ‘Lack of precision in use of terms…’ (1) ISO 15189 (3 rd edition) ISO 9001: 2008 ‘Laboratory management shall…’ ‘Top management shall…’ ‘Executive responsibility’ ‘The laboratory shall…’ ‘ The organisation shall…’ ‘Corporate responsibility’ 10
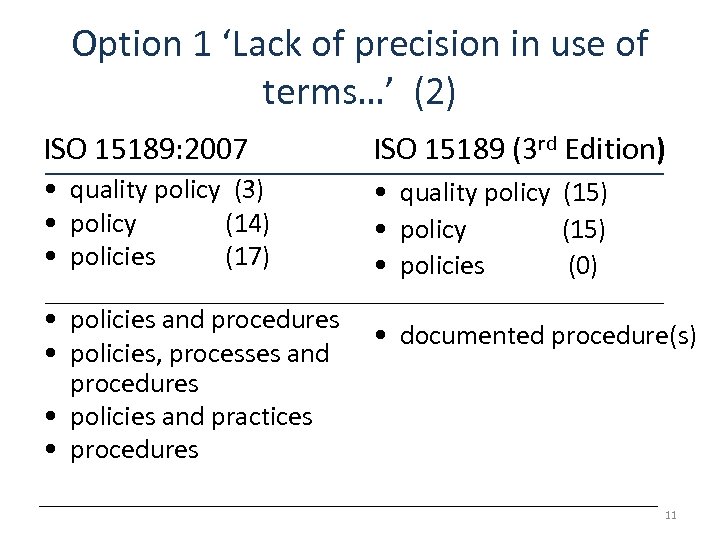 Option 1 ‘Lack of precision in use of terms…’ (2) ISO 15189: 2007 ISO 15189 (3 rd Edition) • quality policy (3) • policy (14) • policies (17) • quality policy (15) • policy (15) • policies (0) • policies and procedures • policies, processes and procedures • policies and practices • procedures • documented procedure(s) 11
Option 1 ‘Lack of precision in use of terms…’ (2) ISO 15189: 2007 ISO 15189 (3 rd Edition) • quality policy (3) • policy (14) • policies (17) • quality policy (15) • policy (15) • policies (0) • policies and procedures • policies, processes and procedures • policies and practices • procedures • documented procedure(s) 11
 ‘Documented procedure(s)’ NOTE 1 Where the term ‘documented procedure’ appears within this International Standard, this means that the procedure is established, documented, implemented and maintained. A single document may address the requirements for more than one procedure or alternately the requirement for a documented procedure may be covered by more than one document. 12
‘Documented procedure(s)’ NOTE 1 Where the term ‘documented procedure’ appears within this International Standard, this means that the procedure is established, documented, implemented and maintained. A single document may address the requirements for more than one procedure or alternately the requirement for a documented procedure may be covered by more than one document. 12
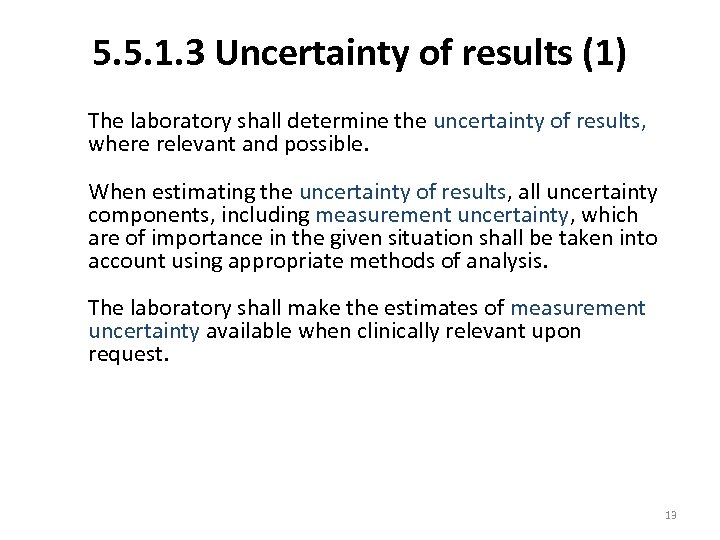 5. 5. 1. 3 Uncertainty of results (1) The laboratory shall determine the uncertainty of results, where relevant and possible. When estimating the uncertainty of results, all uncertainty components, including measurement uncertainty, which are of importance in the given situation shall be taken into account using appropriate methods of analysis. The laboratory shall make the estimates of measurement uncertainty available when clinically relevant upon request. 13
5. 5. 1. 3 Uncertainty of results (1) The laboratory shall determine the uncertainty of results, where relevant and possible. When estimating the uncertainty of results, all uncertainty components, including measurement uncertainty, which are of importance in the given situation shall be taken into account using appropriate methods of analysis. The laboratory shall make the estimates of measurement uncertainty available when clinically relevant upon request. 13
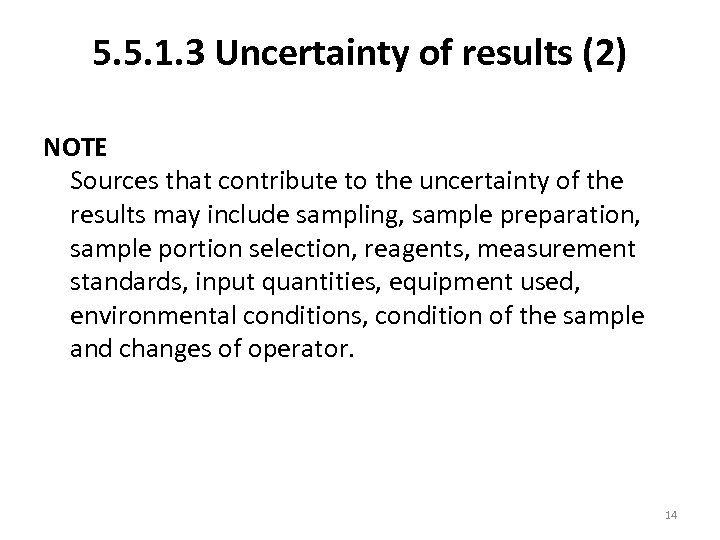 5. 5. 1. 3 Uncertainty of results (2) NOTE Sources that contribute to the uncertainty of the results may include sampling, sample preparation, sample portion selection, reagents, measurement standards, input quantities, equipment used, environmental conditions, condition of the sample and changes of operator. 14
5. 5. 1. 3 Uncertainty of results (2) NOTE Sources that contribute to the uncertainty of the results may include sampling, sample preparation, sample portion selection, reagents, measurement standards, input quantities, equipment used, environmental conditions, condition of the sample and changes of operator. 14
 To ISO 15189 (3 rd Edition) ‘ 5. 5 Examination processes’ … to the content being contained in titled clauses… 5. 5. 1 Selection, validation and verification of examination procedures 5. 5. 1. 1 Validation of examinations procedures 5. 5. 1. 2 Verification of examination procedures 5. 5. 1. 3 Uncertainty of results 5. 5. 2 Biological reference intervals 5. 5. 3 Documentation of examination procedures 15
To ISO 15189 (3 rd Edition) ‘ 5. 5 Examination processes’ … to the content being contained in titled clauses… 5. 5. 1 Selection, validation and verification of examination procedures 5. 5. 1. 1 Validation of examinations procedures 5. 5. 1. 2 Verification of examination procedures 5. 5. 1. 3 Uncertainty of results 5. 5. 2 Biological reference intervals 5. 5. 3 Documentation of examination procedures 15
 From ISO 15189: 2007 - Untitled clauses… ‘ 5. 6 Assuring the quality of examination procedures’ …from the content being in 7 untitled sub clauses… 16
From ISO 15189: 2007 - Untitled clauses… ‘ 5. 6 Assuring the quality of examination procedures’ …from the content being in 7 untitled sub clauses… 16
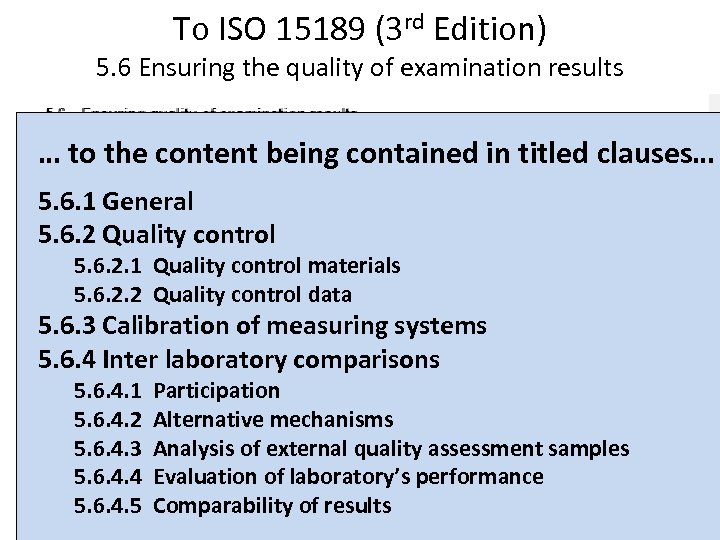 To ISO 15189 (3 rd Edition) 5. 6 Ensuring the quality of examination results … to the content being contained in titled clauses… 5. 6. 1 General 5. 6. 2 Quality control 5. 6. 2. 1 Quality control materials 5. 6. 2. 2 Quality control data 5. 6. 3 Calibration of measuring systems 5. 6. 4 Inter laboratory comparisons 5. 6. 4. 1 5. 6. 4. 2 5. 6. 4. 3 5. 6. 4. 4 5. 6. 4. 5 Participation Alternative mechanisms Analysis of external quality assessment samples Evaluation of laboratory’s performance Comparability of results 17
To ISO 15189 (3 rd Edition) 5. 6 Ensuring the quality of examination results … to the content being contained in titled clauses… 5. 6. 1 General 5. 6. 2 Quality control 5. 6. 2. 1 Quality control materials 5. 6. 2. 2 Quality control data 5. 6. 3 Calibration of measuring systems 5. 6. 4 Inter laboratory comparisons 5. 6. 4. 1 5. 6. 4. 2 5. 6. 4. 3 5. 6. 4. 4 5. 6. 4. 5 Participation Alternative mechanisms Analysis of external quality assessment samples Evaluation of laboratory’s performance Comparability of results 17
 The lost opportunity… ? • Problems with the fundamental structure of ISO 15189… • Restructure the Standard into a ‘process and outcome model’ • How – use the synergy that can be created from using ISO 15189 with ISO 9001 18
The lost opportunity… ? • Problems with the fundamental structure of ISO 15189… • Restructure the Standard into a ‘process and outcome model’ • How – use the synergy that can be created from using ISO 15189 with ISO 9001 18
 Problems with the fundamental structure of ISO 15189? • Quality management system requirements (4 Management requirements) are arbitrarily separated from the competence requirements (5 Technical requirements)… • …resulting in a limited understanding of the role of quality management in securing the improvement of all aspects of laboratory work 19
Problems with the fundamental structure of ISO 15189? • Quality management system requirements (4 Management requirements) are arbitrarily separated from the competence requirements (5 Technical requirements)… • …resulting in a limited understanding of the role of quality management in securing the improvement of all aspects of laboratory work 19
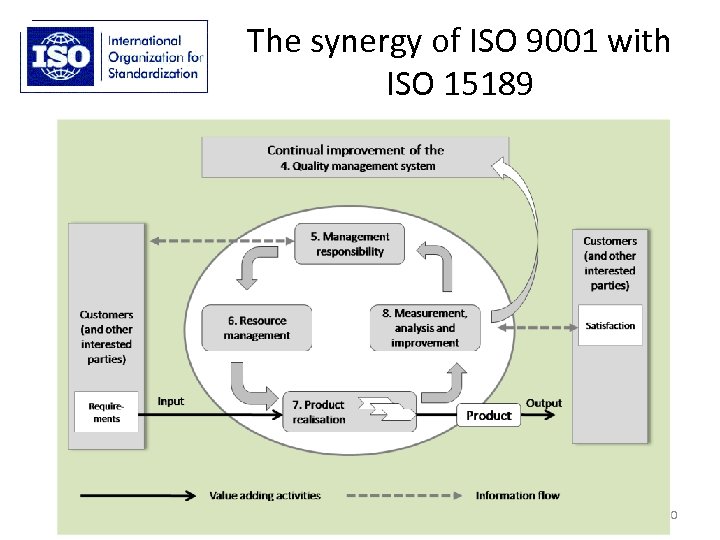 The synergy of ISO 9001 with ISO 15189 20
The synergy of ISO 9001 with ISO 15189 20
 ISO 15189: 2007 – its requirements From linear lists with no clear interrelationships… 21
ISO 15189: 2007 – its requirements From linear lists with no clear interrelationships… 21
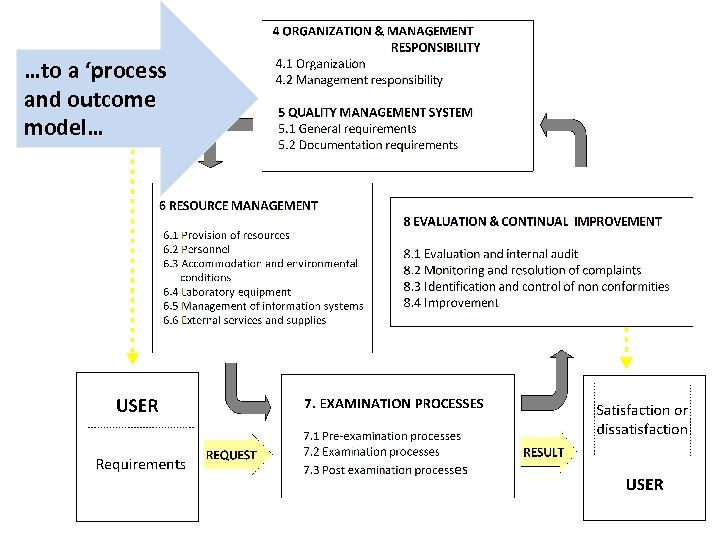 …to a ‘process and outcome model… 22
…to a ‘process and outcome model… 22
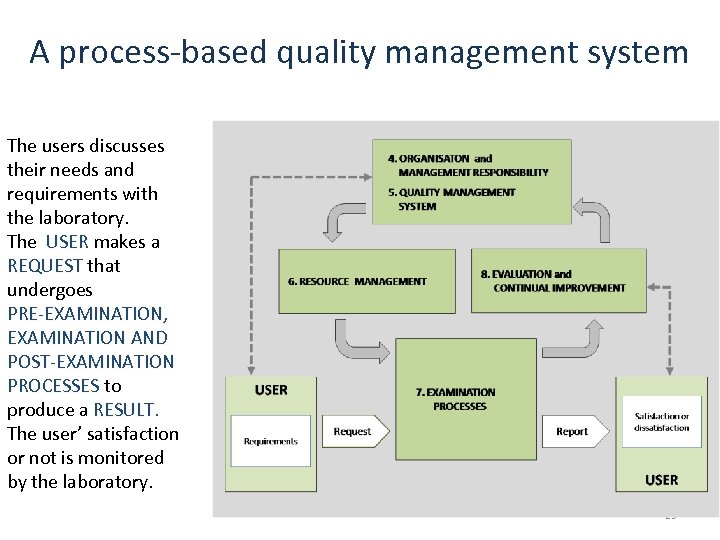 A process-based quality management system The users discusses their needs and requirements with the laboratory. The USER makes a REQUEST that undergoes PRE-EXAMINATION, EXAMINATION AND POST-EXAMINATION PROCESSES to PROCESSES produce a RESULT. The user’ satisfaction or not is monitored by the laboratory. 23
A process-based quality management system The users discusses their needs and requirements with the laboratory. The USER makes a REQUEST that undergoes PRE-EXAMINATION, EXAMINATION AND POST-EXAMINATION PROCESSES to PROCESSES produce a RESULT. The user’ satisfaction or not is monitored by the laboratory. 23
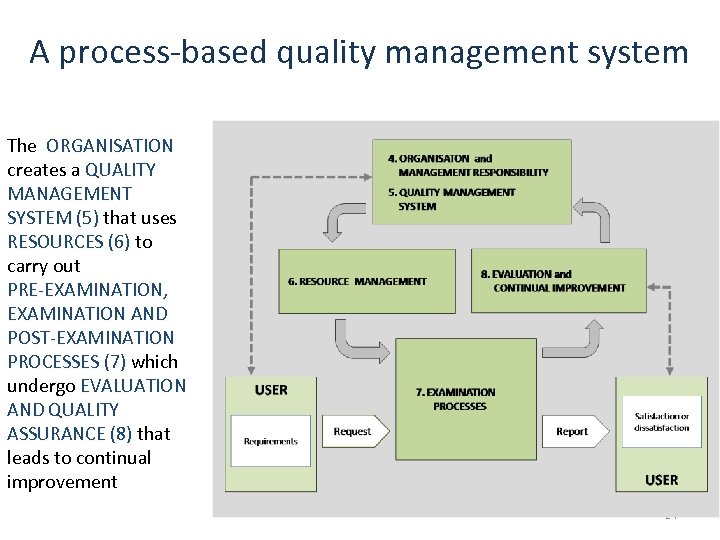 A process-based quality management system The ORGANISATION creates a QUALITY MANAGEMENT SYSTEM (5) that uses SYSTEM (5) RESOURCES (6) to RESOURCES (6) carry out PRE-EXAMINATION, EXAMINATION AND POST-EXAMINATION PROCESSES (7) which PROCESSES (7) undergo EVALUATION AND QUALITY ASSURANCE (8) that ASSURANCE (8) leads to continual improvement 24
A process-based quality management system The ORGANISATION creates a QUALITY MANAGEMENT SYSTEM (5) that uses SYSTEM (5) RESOURCES (6) to RESOURCES (6) carry out PRE-EXAMINATION, EXAMINATION AND POST-EXAMINATION PROCESSES (7) which PROCESSES (7) undergo EVALUATION AND QUALITY ASSURANCE (8) that ASSURANCE (8) leads to continual improvement 24
 Annex A of ISO 15189: 2007 • Correlation with ISO 9001: 2000 in Table A. 1 • You can find this in your ISO standard • It indicates the different items and shows the correspondence 25
Annex A of ISO 15189: 2007 • Correlation with ISO 9001: 2000 in Table A. 1 • You can find this in your ISO standard • It indicates the different items and shows the correspondence 25
 Annex A of ISO/FDIS 15189; 2012 • Correspondence with ISO 9001: 2008 • You can find this in the FDIS • Better corresponce 26
Annex A of ISO/FDIS 15189; 2012 • Correspondence with ISO 9001: 2008 • You can find this in the FDIS • Better corresponce 26
 ‘A medical laboratory’s fulfilment of the requirements of ISO 15189: 2007 means the laboratory meets both the technical competence requirements and the management system requirements that are necessary for it to consistently deliver technically valid results. The management system requirements in ISO 15189 (Section 4) are written in a language relevant to a medical laboratories operations and meet the principles of ISO 9001: 2008 Quality management systems- Requirements and are aligned with its pertinent requirements’ Joint IAF-ILAC-ISO Communiqué (2009) 27
‘A medical laboratory’s fulfilment of the requirements of ISO 15189: 2007 means the laboratory meets both the technical competence requirements and the management system requirements that are necessary for it to consistently deliver technically valid results. The management system requirements in ISO 15189 (Section 4) are written in a language relevant to a medical laboratories operations and meet the principles of ISO 9001: 2008 Quality management systems- Requirements and are aligned with its pertinent requirements’ Joint IAF-ILAC-ISO Communiqué (2009) 27


