2e2f75d31556669c6fac8401ae514f13.ppt
- Количество слайдов: 21

CORAL: COllaborative trial in Relapsed Aggressive Lymphoma R-ICE versus R-DHAP in relapsed patients with CD 20 diffuse large B-cell lymphoma (DLBCL) followed by autologous stem cell transplantation: CORAL study. C. Gisselbrecht, B. Glass, N. Mounier, D. Gill, D. C. Linch, M. Trneny, A. Bosly, O. Shpilberg, H. Hagberg, N. Ketterer, D. Ma, P. Gaulard, C. Moskowitz, and N. Schmitz.

WHAT TO DO IN RELAPSING DLBLCL Ø Standard of care in DLBCL, failing first line chemotherapy treatment, is salvage regimen followed in chemosensitive patients with autologous stem cell transplantation (ASCT). Ø In the Parma study the 7 yr event free survival rate was 41% for ASCT vs 13% for conventional arm. Ø Before rituximab era: different salvage regimens would provide a response rate between 50% to 68% with mobilization of hematopoetic peripheral stem cell in most situation. Ø In the rituximab era: Ø What is the best salvage regimen? No randomized comparison have been made previously Ø What is the place of rituximab after transplantation?
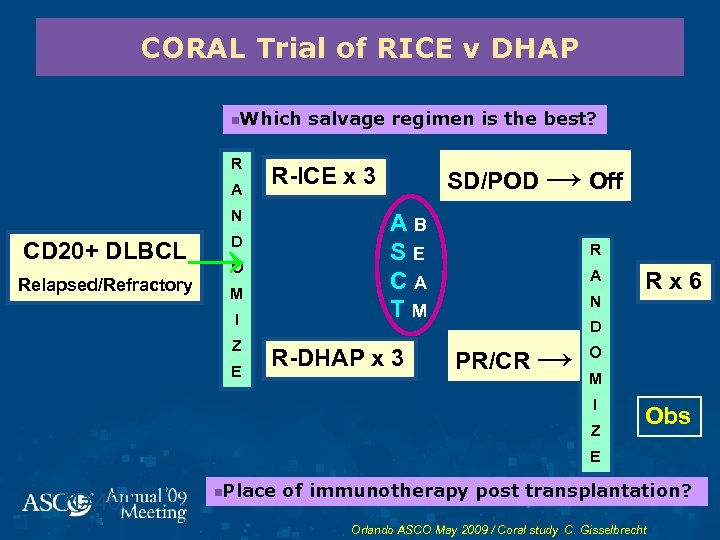
CORAL Trial of RICE v DHAP n. Which R A AB SE CA TM → Relapsed/Refractory D O M I Z E SD/POD → Off R-ICE x 3 N CD 20+ DLBCL salvage regimen is the best? R-DHAP x 3 R A N Rx 6 D PR/CR → O M I Z Obs E N=400 n. Place of immunotherapy post transplantation? Orlando ASCO May 2009 / Coral study C. Gisselbrecht
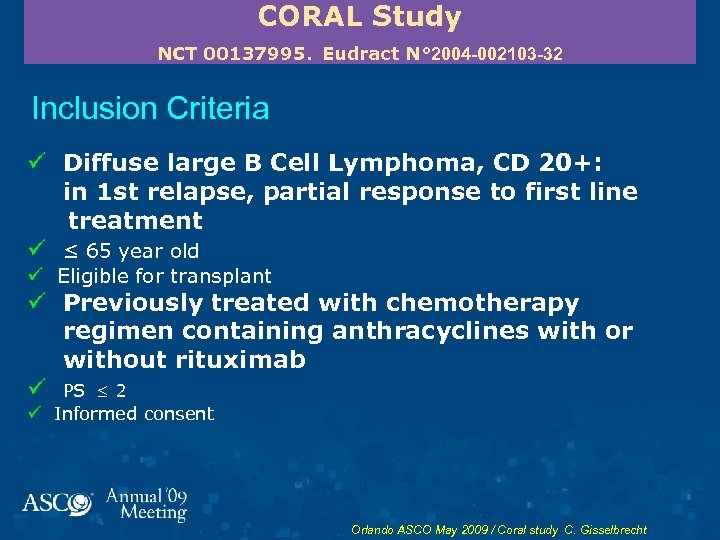
CORAL Study NCT 00137995. Eudract N° 2004 -002103 -32 Inclusion Criteria ü Diffuse large B Cell Lymphoma, CD 20+: in 1 st relapse, partial response to first line treatment ü ≤ 65 year old ü Eligible for transplant ü Previously treated with chemotherapy regimen containing anthracyclines with or without rituximab ü PS 2 ü Informed consent Orlando ASCO May 2009 / Coral study C. Gisselbrecht
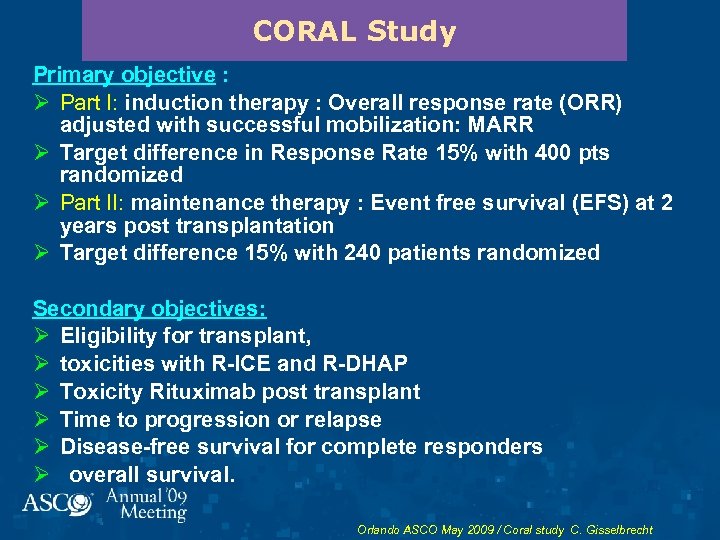
CORAL Study Primary objective : Ø Part I: induction therapy : Overall response rate (ORR) adjusted with successful mobilization: MARR Ø Target difference in Response Rate 15% with 400 pts randomized Ø Part II: maintenance therapy : Event free survival (EFS) at 2 years post transplantation Ø Target difference 15% with 240 patients randomized Secondary objectives: Ø Eligibility for transplant, Ø toxicities with R-ICE and R-DHAP Ø Toxicity Rituximab post transplant Ø Time to progression or relapse Ø Disease-free survival for complete responders Ø overall survival. Orlando ASCO May 2009 / Coral study C. Gisselbrecht
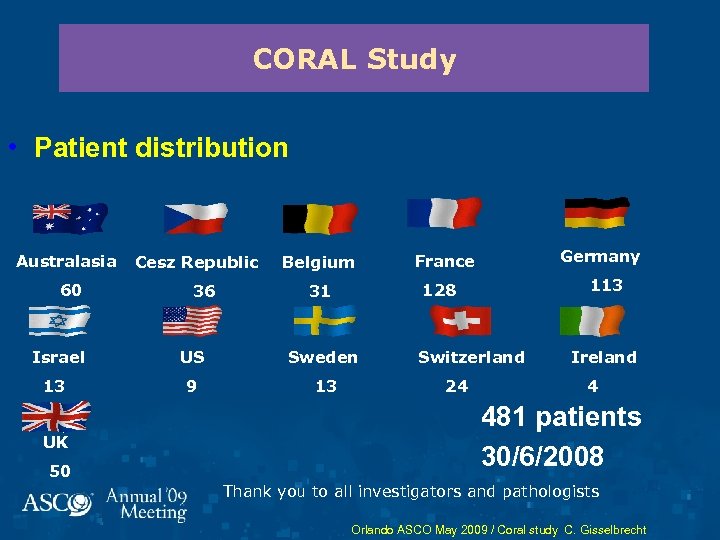
CORAL Study • Patient distribution Australasia 60 Cesz Republic 36 Belgium US Sweden 13 9 13 50 113 128 31 Israel UK Germany France Switzerland 24 Ireland 4 481 patients 30/6/2008 Thank you to all investigators and pathologists Orlando ASCO May 2009 / Coral study C. Gisselbrecht
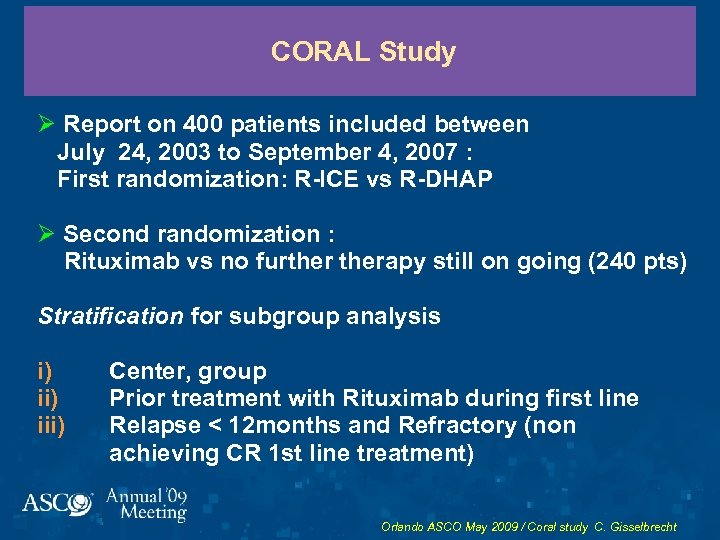
CORAL Study Ø Report on 400 patients included between July 24, 2003 to September 4, 2007 : First randomization: R-ICE vs R-DHAP Ø Second randomization : Rituximab vs no furtherapy still on going (240 pts) Stratification for subgroup analysis i) iii) Center, group Prior treatment with Rituximab during first line Relapse < 12 months and Refractory (non achieving CR 1 st line treatment) Orlando ASCO May 2009 / Coral study C. Gisselbrecht
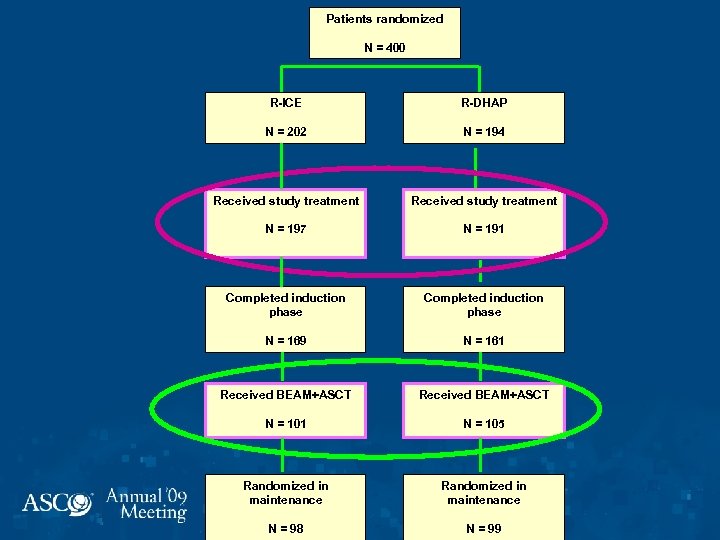
Patients randomized N = 400 R-ICE R-DHAP N = 202 N = 194 Received study treatment N = 197 N = 191 Completed induction phase N = 169 N = 161 Received BEAM+ASCT N = 101 N = 105 Randomized in maintenance N = 98 N = 99
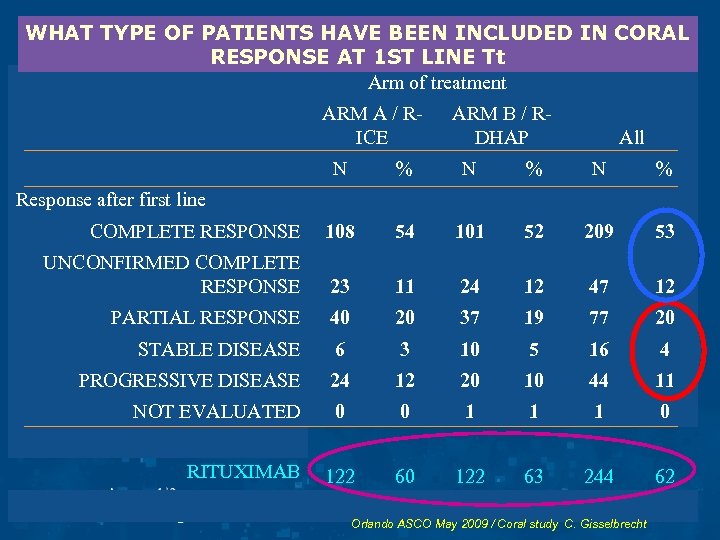
WHAT TYPE OF PATIENTS HAVE BEEN INCLUDED IN CORAL RESPONSE AT 1 ST LINE Tt Arm of treatment ARM A / RICE ARM B / RDHAP All N % N % COMPLETE RESPONSE 108 54 101 52 209 53 UNCONFIRMED COMPLETE RESPONSE 23 11 24 12 47 12 PARTIAL RESPONSE 40 20 37 19 77 20 STABLE DISEASE 6 3 10 5 16 4 PROGRESSIVE DISEASE 24 12 20 10 44 11 NOT EVALUATED 0 0 1 1 1 0 122 63 244 62 Response after first line RITUXIMAB Orlando ASCO May 2009 / Coral study C. Gisselbrecht
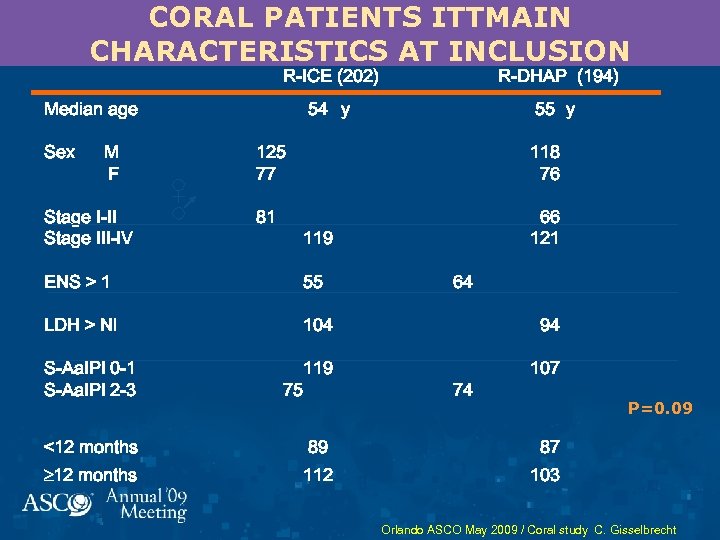
CORAL PATIENTS ITTMAIN CHARACTERISTICS AT INCLUSION R-ICE (202) R-DHAP (194) 54 y 55 y Median age Sex M F Stage I-II Stage III-IV 125 77 118 76 81 66 121 119 ENS > 1 55 64 LDH > Nl 104 94 S-Aa. IPI 0 -1 S-Aa. IPI 2 -3 119 75 107 <12 months 89 87 12 months 112 103 74 P=0. 09 Orlando ASCO May 2009 / Coral study C. Gisselbrecht
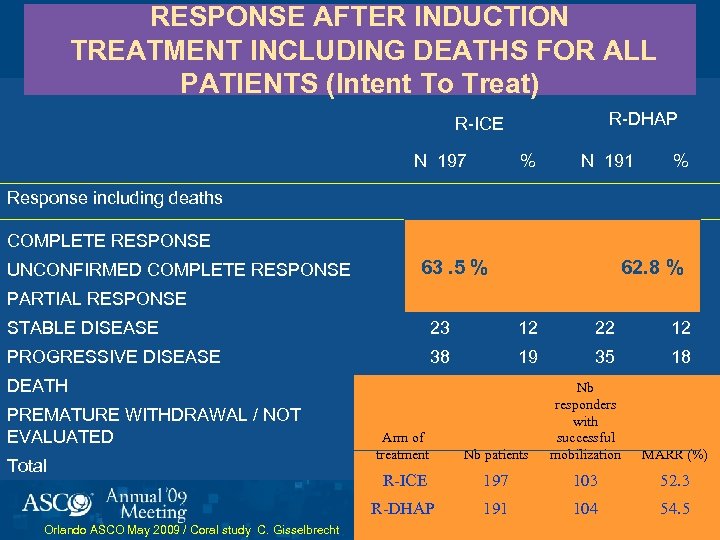
RESPONSE AFTER INDUCTION TREATMENT INCLUDING DEATHS FOR ALL PATIENTS (Intent To Treat) R-DHAP R-ICE N 197 % N 191 % 48 24 53 28 12 22 62. 8 % 12 Response including deaths COMPLETE RESPONSE UNCONFIRMED COMPLETE RESPONSE 63. 5 % 24 PARTIAL RESPONSE 53 27 45 24 STABLE DISEASE 23 12 22 12 PROGRESSIVE DISEASE 38 19 35 18 6 3 DEATH Total Orlando ASCO May 2009 / Coral study C. Gisselbrecht Nb patients MARR (%) R-ICE 197 103 52. 3 R-DHAP PREMATURE WITHDRAWAL / NOT EVALUATED Nb 10 responders with 4 successful mobilization 191 104 54. 5 Arm of treatment 4 197 2 100 191 5 2 100
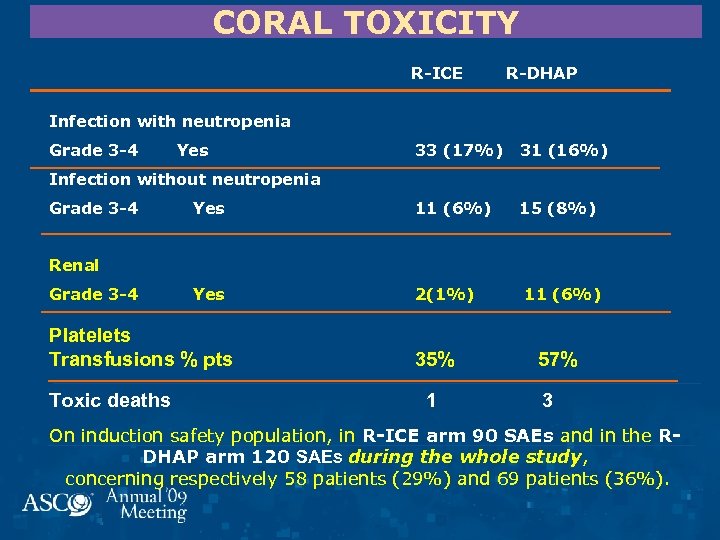
CORAL TOXICITY R-ICE R-DHAP Infection with neutropenia Grade 3 -4 Yes 33 (17%) 31 (16%) Yes 11 (6%) 15 (8%) Yes 2(1%) Infection without neutropenia Grade 3 -4 Renal Grade 3 -4 Platelets Transfusions % pts Toxic deaths 35% 1 11 (6%) 57% 3 On induction safety population, in R-ICE arm 90 SAEs and in the RDHAP arm 120 SAEs during the whole study, concerning respectively 58 patients (29%) and 69 patients (36%).
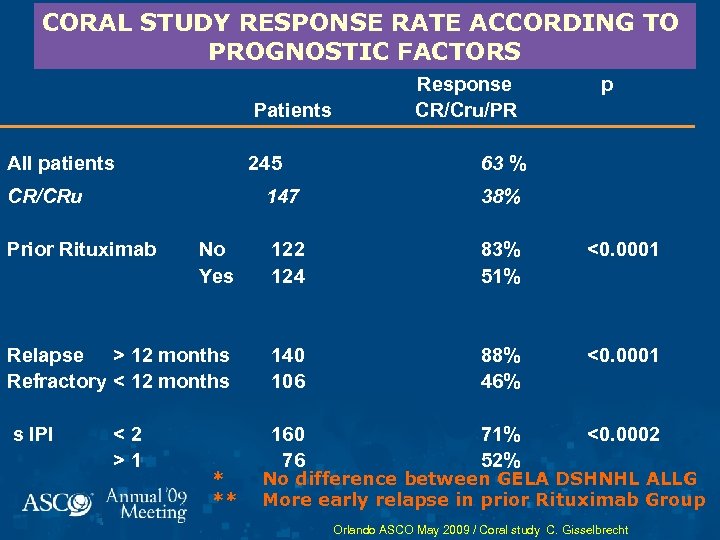
CORAL STUDY RESPONSE RATE ACCORDING TO PROGNOSTIC FACTORS Patients All patients 245 CR/CRu Response CR/Cru/PR p 63 % 147 38% No Yes 122 124 83% 51% <0. 0001 Relapse > 12 months Refractory < 12 months 140 106 88% 46% <0. 0001 s IPI 160 76 71% 52% <0. 0002 Prior Rituximab <2 >1 * ** No difference between GELA DSHNHL ALLG More early relapse in prior Rituximab Group Orlando ASCO May 2009 / Coral study C. Gisselbrecht
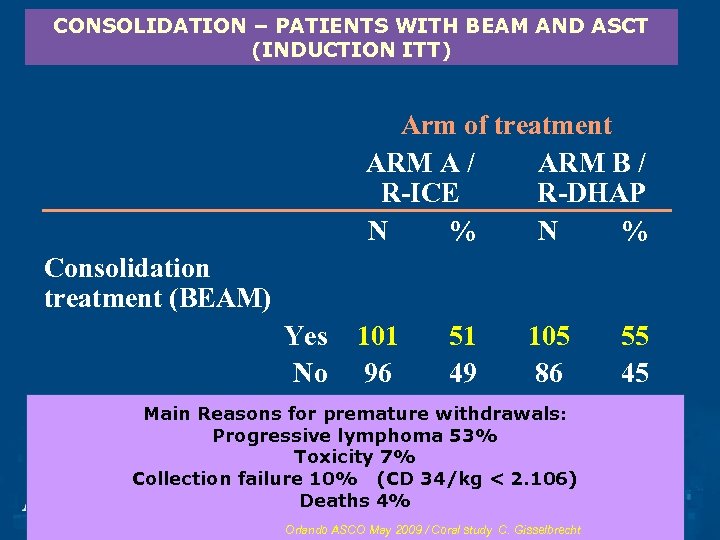
CONSOLIDATION – PATIENTS WITH BEAM AND ASCT (INDUCTION ITT) Arm of treatment ARM A / ARM B / R-ICE R-DHAP N % Consolidation treatment (BEAM) Yes No 101 51 105 96 49 86 Total Main Reasons for premature withdrawals: 197 100 191 Progressive lymphoma 53% Transplantation Toxicity 7% Collection failure 10% (CD 34/kg < 2. 106) Deaths 4% Orlando ASCO May 2009 / Coral study C. Gisselbrecht 55 45 100
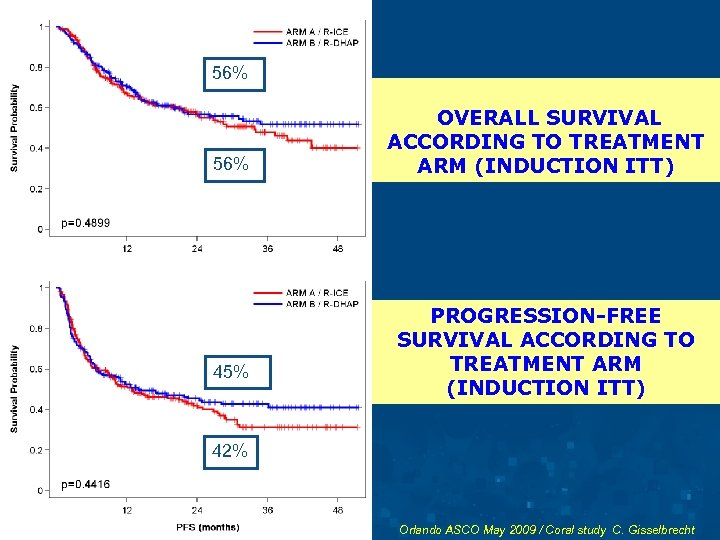
56% 45% OVERALL SURVIVAL ACCORDING TO TREATMENT ARM (INDUCTION ITT) PROGRESSION-FREE SURVIVAL ACCORDING TO TREATMENT ARM (INDUCTION ITT) 42% Orlando ASCO May 2009 / Coral study C. Gisselbrecht
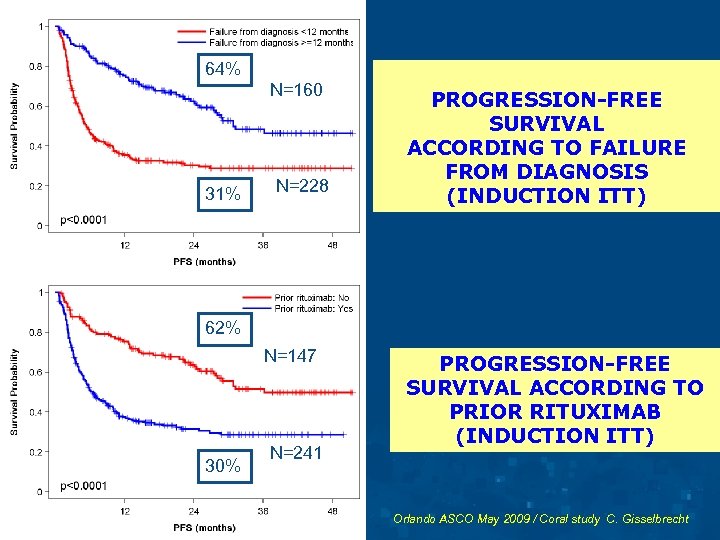
64% N=160 31% N=228 PROGRESSION-FREE SURVIVAL ACCORDING TO FAILURE FROM DIAGNOSIS (INDUCTION ITT) 62% N=147 30% N=241 PROGRESSION-FREE SURVIVAL ACCORDING TO PRIOR RITUXIMAB (INDUCTION ITT) Orlando ASCO May 2009 / Coral study C. Gisselbrecht
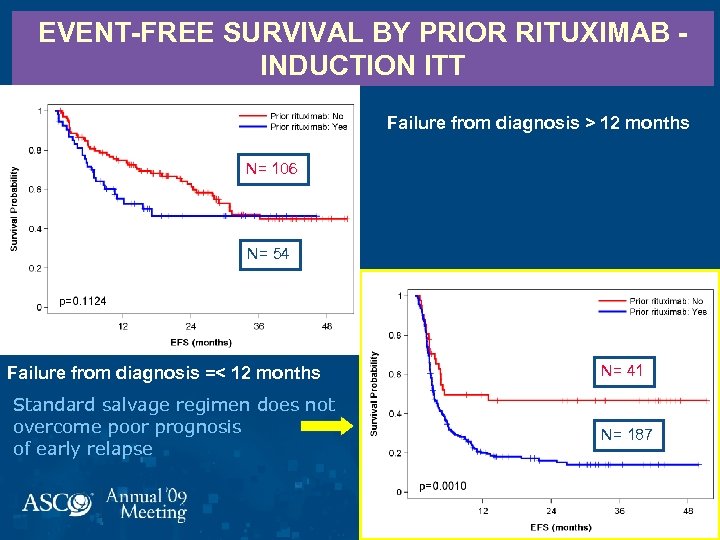
EVENT-FREE SURVIVAL BY PRIOR RITUXIMAB INDUCTION ITT Failure from diagnosis =>= 12 months Failure from diagnosis > 12 months N= 106 N= 54 Failure from diagnosis =< 12 months Standard salvage regimen does not overcome poor prognosis of early relapse N= 41 N= 187
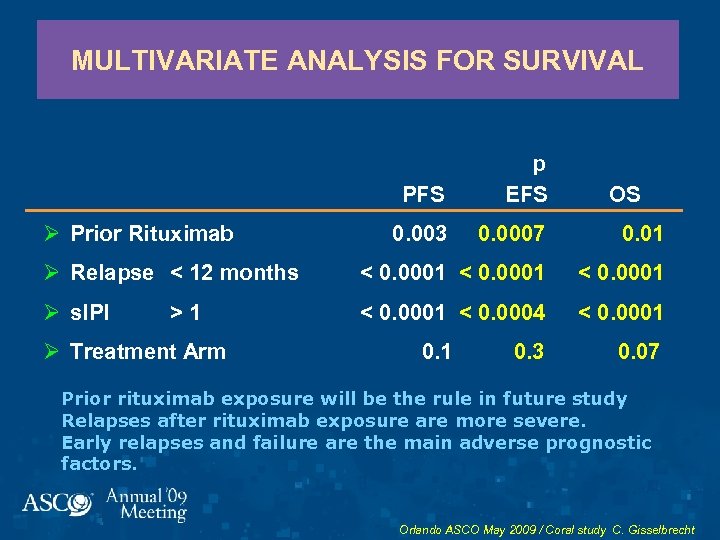
MULTIVARIATE ANALYSIS FOR SURVIVAL PFS p EFS 0. 003 0. 0007 0. 01 Ø Relapse < 12 months < 0. 0001 Ø s. IPI < 0. 0001 < 0. 0004 < 0. 0001 Ø Prior Rituximab >1 Ø Treatment Arm 0. 1 0. 3 OS 0. 07 Prior rituximab exposure will be the rule in future study Relapses after rituximab exposure are more severe. Early relapses and failure are the main adverse prognostic factors. Orlando ASCO May 2009 / Coral study C. Gisselbrecht
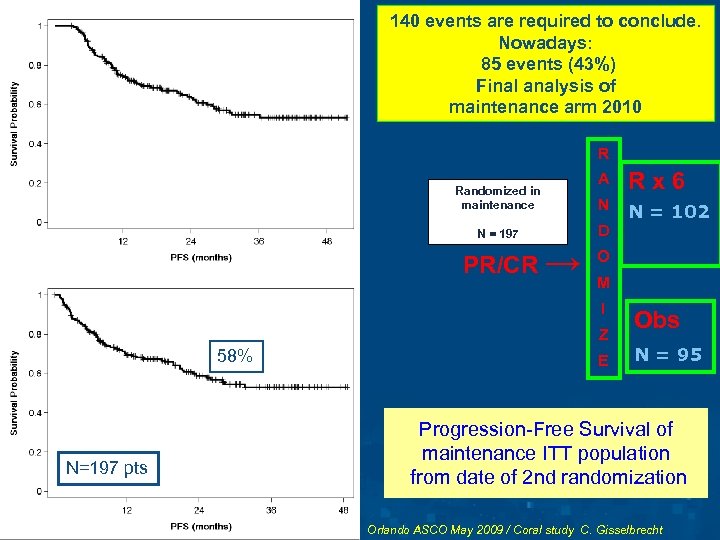
140 events are required to conclude. PROGRESSION-FREE Nowadays: SURVIVAL OF PATIENTS 85 events (43%) SUBMITTED TO ASCT from Final analysis of date of 1 st randomization maintenance arm 2010 (INDUCTION ITT) R Randomized in maintenance N = 197 PR/CR → A Rx 6 N N = 102 D O M I Z 58% N=197 pts E Obs N = 95 Progression-Free Survival of maintenance ITT population from date of 2 nd randomization Orlando ASCO May 2009 / Coral study C. Gisselbrecht
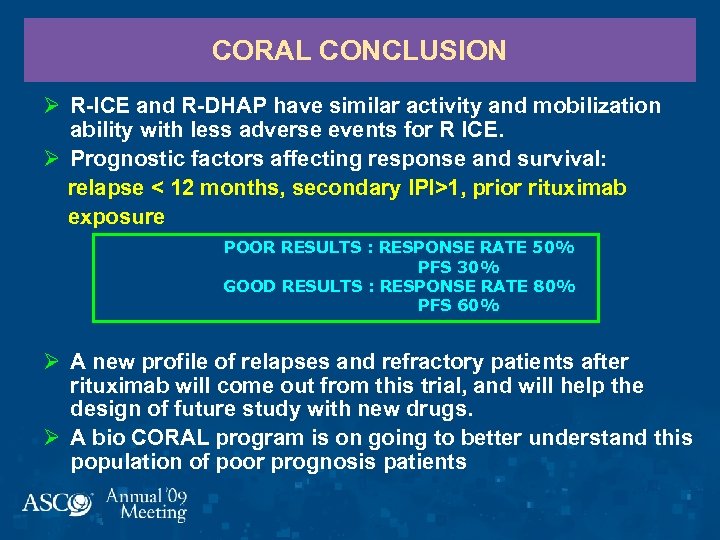
CORAL CONCLUSION Ø R-ICE and R-DHAP have similar activity and mobilization ability with less adverse events for R ICE. Ø Prognostic factors affecting response and survival: relapse < 12 months, secondary IPI>1, prior rituximab exposure POOR RESULTS : RESPONSE RATE 50% PFS 30% GOOD RESULTS : RESPONSE RATE 80% PFS 60% Ø A new profile of relapses and refractory patients after rituximab will come out from this trial, and will help the design of future study with new drugs. Ø A bio CORAL program is on going to better understand this population of poor prognosis patients

Many Thanks again investigators pathologists GELA RC M Fournier/N. Mounier statistics C. Pitrou/ G Chartier project leaders L. Gérard, Data management and all the project managers in the different countries Thank you for their continous support: Roche, Baxter, Chugai
2e2f75d31556669c6fac8401ae514f13.ppt