c03c4d548383ba11fb29b9d7b273867a.ppt
- Количество слайдов: 27
 Contracting to Preserve Open Science: Lessons for a Microbial Research Commons Prof. Peter Lee UC Davis School of Law October 8, 2009 ptrlee@ucdavis. edu
Contracting to Preserve Open Science: Lessons for a Microbial Research Commons Prof. Peter Lee UC Davis School of Law October 8, 2009 ptrlee@ucdavis. edu
 Agenda • Present ongoing research on private ordering to promote “open science” • Apply lessons to designing a microbial research commons • Explore additional design principles and considerations
Agenda • Present ongoing research on private ordering to promote “open science” • Apply lessons to designing a microbial research commons • Explore additional design principles and considerations
 Context • Ongoing research on the role of “public institutions” in creating a noncommercial research commons in biomedicine Intellectual Property Physical Property Public Norms Research Commons Private ordering
Context • Ongoing research on the role of “public institutions” in creating a noncommercial research commons in biomedicine Intellectual Property Physical Property Public Norms Research Commons Private ordering
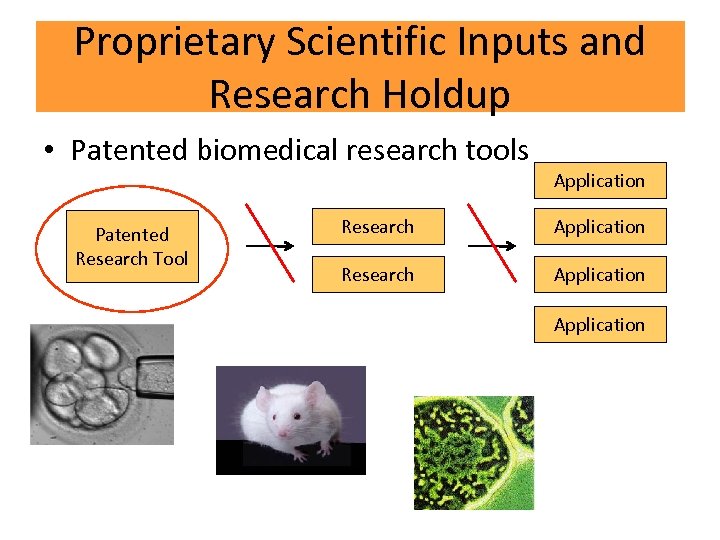 Proprietary Scientific Inputs and Research Holdup • Patented biomedical research tools Patented Research Tool Application Research Application
Proprietary Scientific Inputs and Research Holdup • Patented biomedical research tools Patented Research Tool Application Research Application
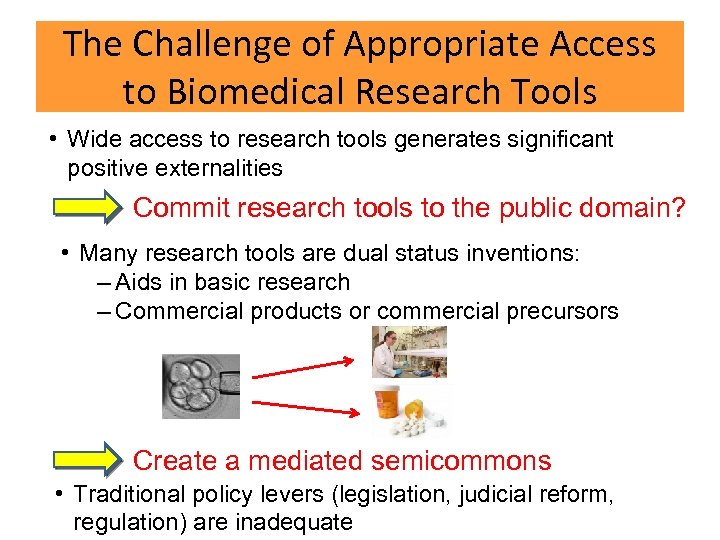 The Challenge of Appropriate Access to Biomedical Research Tools • Wide access to research tools generates significant positive externalities Commit research tools to the public domain? • Many research tools are dual status inventions: – Aids in basic research – Commercial products or commercial precursors Create a mediated semicommons • Traditional policy levers (legislation, judicial reform, regulation) are inadequate
The Challenge of Appropriate Access to Biomedical Research Tools • Wide access to research tools generates significant positive externalities Commit research tools to the public domain? • Many research tools are dual status inventions: – Aids in basic research – Commercial products or commercial precursors Create a mediated semicommons • Traditional policy levers (legislation, judicial reform, regulation) are inadequate
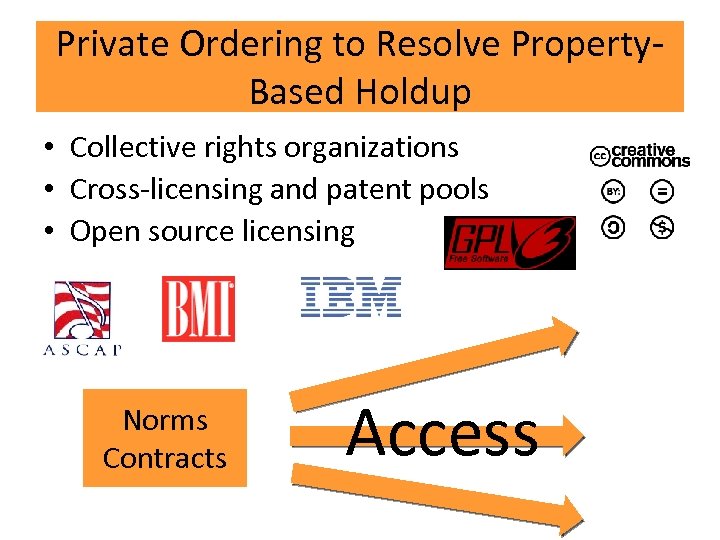 Private Ordering to Resolve Property. Based Holdup • Collective rights organizations • Cross-licensing and patent pools • Open source licensing Norms Contracts Access
Private Ordering to Resolve Property. Based Holdup • Collective rights organizations • Cross-licensing and patent pools • Open source licensing Norms Contracts Access
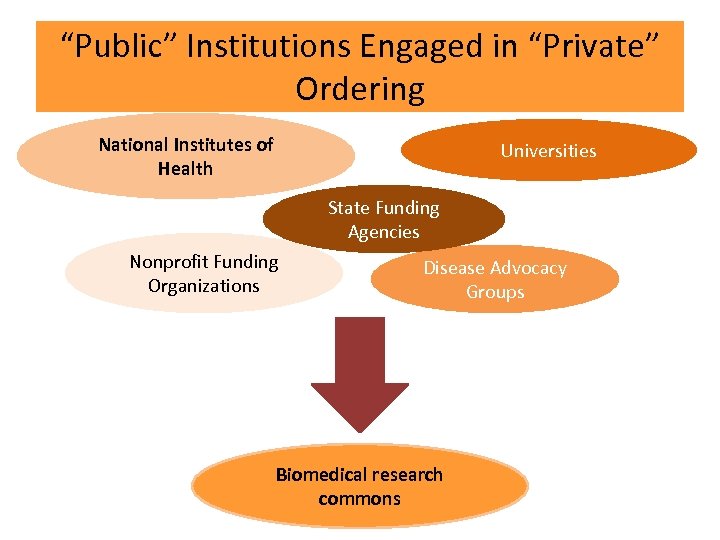 “Public” Institutions Engaged in “Private” Ordering National Institutes of Health Universities State Funding Agencies Nonprofit Funding Organizations Disease Advocacy Groups Biomedical research commons
“Public” Institutions Engaged in “Private” Ordering National Institutes of Health Universities State Funding Agencies Nonprofit Funding Organizations Disease Advocacy Groups Biomedical research commons
 The National Institutes of Health • $30 billion per year in research funds – Bayh-Dole Act – Principles and Guidelines (1999) • Advocate wide availability of patented research tools for noncommercial uses • Allow targeted exclusivity for commercial development – Compliance considered in awarding grants NIH Consideration Qualified Access Resource Developer
The National Institutes of Health • $30 billion per year in research funds – Bayh-Dole Act – Principles and Guidelines (1999) • Advocate wide availability of patented research tools for noncommercial uses • Allow targeted exclusivity for commercial development – Compliance considered in awarding grants NIH Consideration Qualified Access Resource Developer
 The California Institute for Regenerative Medicine • $3 billion over 10 years for stem cell research • Access requirements – “A Grantee Organization agrees to make its CIRMfunded patented inventions readily accessible on reasonable terms. . . to other Grantee Organizations for non-commercial purposes. ” • CAL. CODE REGS. tit. 17, § 100306(a) – Grantees must make materials described in a scientific publication widely available • CAL. CODE REGS. tit. 17, § 100304
The California Institute for Regenerative Medicine • $3 billion over 10 years for stem cell research • Access requirements – “A Grantee Organization agrees to make its CIRMfunded patented inventions readily accessible on reasonable terms. . . to other Grantee Organizations for non-commercial purposes. ” • CAL. CODE REGS. tit. 17, § 100306(a) – Grantees must make materials described in a scientific publication widely available • CAL. CODE REGS. tit. 17, § 100304
 The California Institute for Regenerative Medicine • Commercialization of CIRM-funded inventions is encouraged – CIRM collects “proportionate” royalties – If multiple funding sources contributed to an invention, the state is entitled to a share “proportionate to the support provided by CIRM for the discovery of the invention. ” • CAL. CODE REGS. tit. 17, § 100308(c)
The California Institute for Regenerative Medicine • Commercialization of CIRM-funded inventions is encouraged – CIRM collects “proportionate” royalties – If multiple funding sources contributed to an invention, the state is entitled to a share “proportionate to the support provided by CIRM for the discovery of the invention. ” • CAL. CODE REGS. tit. 17, § 100308(c)
 Universities • Major contributors to biomedical research • Promoting open science in technology transfer licenses – “Harvard will retain the right, for itself and other not-for-profit research organizations, to practice the subject matter of the patent rights for internal research, teaching and other educational purposes. ” • Licensing Harvard Patent Rights: A Guideline to the Essentials of Harvard’s License Agreements
Universities • Major contributors to biomedical research • Promoting open science in technology transfer licenses – “Harvard will retain the right, for itself and other not-for-profit research organizations, to practice the subject matter of the patent rights for internal research, teaching and other educational purposes. ” • Licensing Harvard Patent Rights: A Guideline to the Essentials of Harvard’s License Agreements
 Universities • Systematic overvaluation of assets – 1991 -2000: 238% increase in new patent applications – Median net licensing income for research institutions: $1. 13 million – Hit or miss (largely miss) • Normative plurality – Among universities – Within universities • Research faculties, individual scientists • Offices of technology transfer
Universities • Systematic overvaluation of assets – 1991 -2000: 238% increase in new patent applications – Median net licensing income for research institutions: $1. 13 million – Hit or miss (largely miss) • Normative plurality – Among universities – Within universities • Research faculties, individual scientists • Offices of technology transfer
 Non-Profit Funding Organizations • Significant sources of “scientific venture capital” • Howard Hughes Medical Institute – HHMI “expects all HHMI research tools to be made available to the scientific research community on reasonable terms and in a manner that enhances their widespread availability. ” • HHMI, Research Tools (SC-310), at 1 – Consistent with NIH Principles and Guidelines
Non-Profit Funding Organizations • Significant sources of “scientific venture capital” • Howard Hughes Medical Institute – HHMI “expects all HHMI research tools to be made available to the scientific research community on reasonable terms and in a manner that enhances their widespread availability. ” • HHMI, Research Tools (SC-310), at 1 – Consistent with NIH Principles and Guidelines
 Contracting to Preserve Open Science • Formalizing the informal norm of open science • Public institutions are engaged in private ordering • Leveraging upstream scientific capital to ensure access to proprietary research assets
Contracting to Preserve Open Science • Formalizing the informal norm of open science • Public institutions are engaged in private ordering • Leveraging upstream scientific capital to ensure access to proprietary research assets
 Agenda • Present ongoing research on private ordering to promote “open science” • Apply lessons to designing a microbial research commons • Explore additional design principles and considerations
Agenda • Present ongoing research on private ordering to promote “open science” • Apply lessons to designing a microbial research commons • Explore additional design principles and considerations
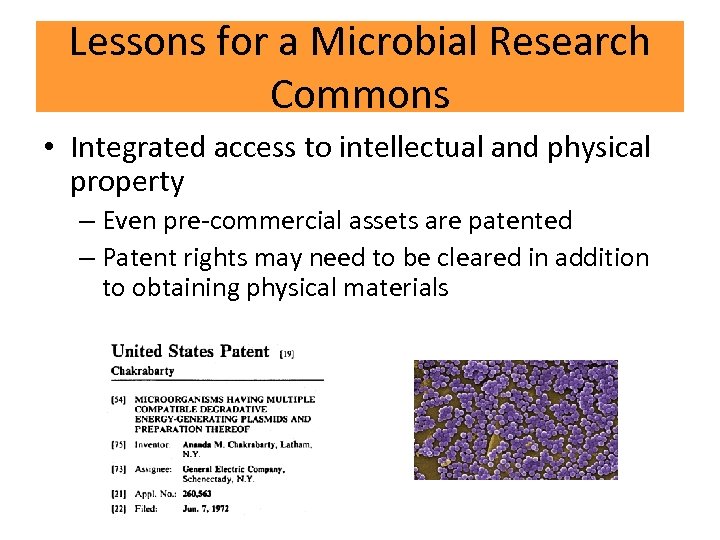 Lessons for a Microbial Research Commons • Integrated access to intellectual and physical property – Even pre-commercial assets are patented – Patent rights may need to be cleared in addition to obtaining physical materials
Lessons for a Microbial Research Commons • Integrated access to intellectual and physical property – Even pre-commercial assets are patented – Patent rights may need to be cleared in addition to obtaining physical materials
 Lessons for a Microbial Research Commons • Formalizing informal norms: authority and process – Who speaks for the scientific community? – How does a community arrive at normative consensus? – NIH Principles and Guidelines • Thorough consultative process • Notice and comment • Institutional buy-in – Normative plurality among and within institutions
Lessons for a Microbial Research Commons • Formalizing informal norms: authority and process – Who speaks for the scientific community? – How does a community arrive at normative consensus? – NIH Principles and Guidelines • Thorough consultative process • Notice and comment • Institutional buy-in – Normative plurality among and within institutions
 Lessons for a Microbial Research Commons • Encouraging participation – Systematic overvaluing of assets – Leverage upstream “public scientific capital” to mandate or encourage compliance with access objectives • Government agencies, universities, private foundations, scientific journals Consideration Public Institutions Downstream Owners Qualified Access
Lessons for a Microbial Research Commons • Encouraging participation – Systematic overvaluing of assets – Leverage upstream “public scientific capital” to mandate or encourage compliance with access objectives • Government agencies, universities, private foundations, scientific journals Consideration Public Institutions Downstream Owners Qualified Access
 Lessons for a Microbial Research Commons • Experience with dual-purpose assets – Noncommercial research use – Commercial application • Apportioning upstream/downstream contributions and calculating appropriate compensation C • CIRM’s proportionality principle A? B D
Lessons for a Microbial Research Commons • Experience with dual-purpose assets – Noncommercial research use – Commercial application • Apportioning upstream/downstream contributions and calculating appropriate compensation C • CIRM’s proportionality principle A? B D
 Lessons for a Microbial Research Commons • Institutional considerations – The need for centralized coordination and standardization • Lower information and transaction costs – The role of NIH or a similarly situated body – International challenges
Lessons for a Microbial Research Commons • Institutional considerations – The need for centralized coordination and standardization • Lower information and transaction costs – The role of NIH or a similarly situated body – International challenges
 Agenda • Present ongoing research on private ordering to promote “open science” • Apply lessons to designing a microbial research commons • Explore additional design principles and considerations
Agenda • Present ongoing research on private ordering to promote “open science” • Apply lessons to designing a microbial research commons • Explore additional design principles and considerations
 The Cooperative Research Goal • Incentives to share – Benefits: communal norms as well as self-interest • Patent pools in the software industry – Costs: education about the expected value of microbial assets • Distributing duplicates and derivatives – Open source/viral licenses for microbes
The Cooperative Research Goal • Incentives to share – Benefits: communal norms as well as self-interest • Patent pools in the software industry – Costs: education about the expected value of microbial assets • Distributing duplicates and derivatives – Open source/viral licenses for microbes
 Maintaining High Quality Standards • Enormous governance issues • Need for institutional leadership and community consensus – Define standards – Assign monitoring responsibility – Delineate sanctions
Maintaining High Quality Standards • Enormous governance issues • Need for institutional leadership and community consensus – Define standards – Assign monitoring responsibility – Delineate sanctions
 Promoting Reputational Benefits • Critical to the “normative economy” • A response to the incentives question – Participate to establish priority, increase citations, and obtain recognition • Carrots and sticks – A community of strangers – Cultivating trust and identifying bad actors – User-generated ratings • Distributed accreditation and “peer review”
Promoting Reputational Benefits • Critical to the “normative economy” • A response to the incentives question – Participate to establish priority, increase citations, and obtain recognition • Carrots and sticks – A community of strangers – Cultivating trust and identifying bad actors – User-generated ratings • Distributed accreditation and “peer review”
 Securing Equitable Compensation • Challenges of liability rule regimes – Valuation and apportionment – Who determines “equitable compensation”? – How is a reasonable royalty calculated? • Eliminating or mitigating the threat of exit – Ex ante commitments versus ex post profitability • Looking forward: commercialization and patenting – Structured rules for recipients to inform providers of patenting plans
Securing Equitable Compensation • Challenges of liability rule regimes – Valuation and apportionment – Who determines “equitable compensation”? – How is a reasonable royalty calculated? • Eliminating or mitigating the threat of exit – Ex ante commitments versus ex post profitability • Looking forward: commercialization and patenting – Structured rules for recipients to inform providers of patenting plans
 Summary • The microbial research commons is eminently feasible and can benefit from related experiences – Multiple purpose assets – Integrate intellectual, physical, and informational resources • Leveraging public norms and private ordering – Formalizing an informal ethos of sharing • Institutional challenges and opportunities – Standardization – Coordination – Determining equitable compensation
Summary • The microbial research commons is eminently feasible and can benefit from related experiences – Multiple purpose assets – Integrate intellectual, physical, and informational resources • Leveraging public norms and private ordering – Formalizing an informal ethos of sharing • Institutional challenges and opportunities – Standardization – Coordination – Determining equitable compensation
 Questions
Questions


