Limestone and Sandstone.ppt
- Количество слайдов: 26

 Content: o New words o Limestone o Sandstone o Question time o Conclusion
Content: o New words o Limestone o Sandstone o Question time o Conclusion
 New words: 1) 2) 3) 4) 5) 6) 7) Hardness – твердость Density – плотность Water absorbsion- вода впитность Porosity – пористость Compressive strength – Occurrence – происшествие Color - оттенок
New words: 1) 2) 3) 4) 5) 6) 7) Hardness – твердость Density – плотность Water absorbsion- вода впитность Porosity – пористость Compressive strength – Occurrence – происшествие Color - оттенок
 Ø Origin Ø Chemical properties Ø Physical properties Ø Using
Ø Origin Ø Chemical properties Ø Physical properties Ø Using
 Origin: Limestone – a rock consisting essentially of calcium carbonate (at least 50%). Most types of limestone are partly or wholly of organic origin and contain the hard parts of various organisms such as the shells of molluscs and the skeletons of corals. For example, chalk – a soft or greyish type of limestone. In its purest form it may contain as much as 99% of calcium carbonate.
Origin: Limestone – a rock consisting essentially of calcium carbonate (at least 50%). Most types of limestone are partly or wholly of organic origin and contain the hard parts of various organisms such as the shells of molluscs and the skeletons of corals. For example, chalk – a soft or greyish type of limestone. In its purest form it may contain as much as 99% of calcium carbonate.
 More about limestone • Limestone is dug up out of the ground in quarries • In Britain about 150 million tonnes of limestone are quarried each year • Limestone is the compound calcium carbonate • It’s formula is Ca. CO 3 • Limestone can be chemically changed into other things
More about limestone • Limestone is dug up out of the ground in quarries • In Britain about 150 million tonnes of limestone are quarried each year • Limestone is the compound calcium carbonate • It’s formula is Ca. CO 3 • Limestone can be chemically changed into other things
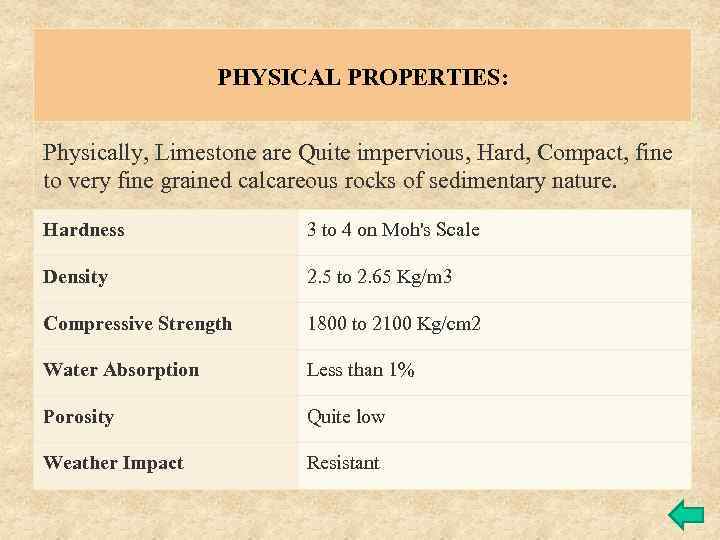 PHYSICAL PROPERTIES: Physically, Limestone are Quite impervious, Hard, Compact, fine to very fine grained calcareous rocks of sedimentary nature. Hardness 3 to 4 on Moh's Scale Density 2. 5 to 2. 65 Kg/m 3 Compressive Strength 1800 to 2100 Kg/cm 2 Water Absorption Less than 1% Porosity Quite low Weather Impact Resistant
PHYSICAL PROPERTIES: Physically, Limestone are Quite impervious, Hard, Compact, fine to very fine grained calcareous rocks of sedimentary nature. Hardness 3 to 4 on Moh's Scale Density 2. 5 to 2. 65 Kg/m 3 Compressive Strength 1800 to 2100 Kg/cm 2 Water Absorption Less than 1% Porosity Quite low Weather Impact Resistant
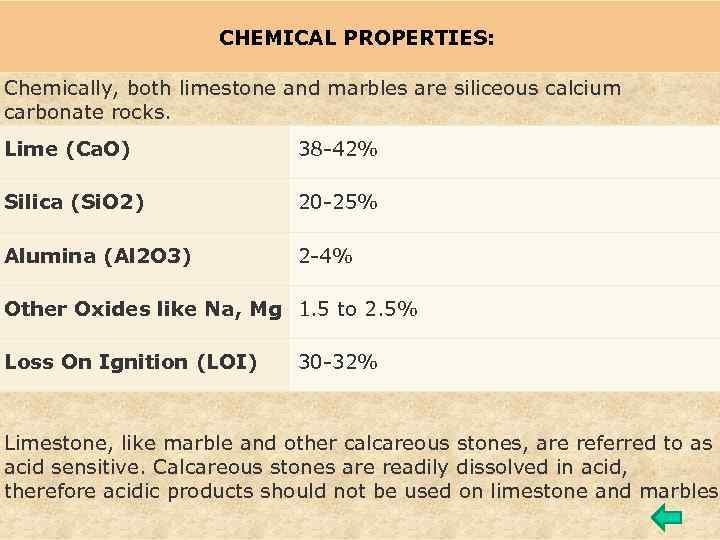 CHEMICAL PROPERTIES: Chemically, both limestone and marbles are siliceous calcium carbonate rocks. Lime (Ca. O) 38 -42% Silica (Si. O 2) 20 -25% Alumina (Al 2 O 3) 2 -4% Other Oxides like Na, Mg 1. 5 to 2. 5% Loss On Ignition (LOI) 30 -32% Limestone, like marble and other calcareous stones, are referred to as acid sensitive. Calcareous stones are readily dissolved in acid, therefore acidic products should not be used on limestone and marbles.
CHEMICAL PROPERTIES: Chemically, both limestone and marbles are siliceous calcium carbonate rocks. Lime (Ca. O) 38 -42% Silica (Si. O 2) 20 -25% Alumina (Al 2 O 3) 2 -4% Other Oxides like Na, Mg 1. 5 to 2. 5% Loss On Ignition (LOI) 30 -32% Limestone, like marble and other calcareous stones, are referred to as acid sensitive. Calcareous stones are readily dissolved in acid, therefore acidic products should not be used on limestone and marbles.
 Using: Limestone is an excellent building stone because it can be carved easily. It is most commonly used for flooring, wall cladding, vanity tops, furniture, and many times, ornate stonework. Apart from its application in the construction, it is also used in factories to clean waste gases and water, before releasing them into the environment. Limestone can also be used to make lime and to smelt iron ore. Слайд 2
Using: Limestone is an excellent building stone because it can be carved easily. It is most commonly used for flooring, wall cladding, vanity tops, furniture, and many times, ornate stonework. Apart from its application in the construction, it is also used in factories to clean waste gases and water, before releasing them into the environment. Limestone can also be used to make lime and to smelt iron ore. Слайд 2
 Sandstone: Ø Origin Ø Chemical properties Ø Physical properties Ø Using
Sandstone: Ø Origin Ø Chemical properties Ø Physical properties Ø Using
 Origin: Sandstones are sedimentary rocks composed of grains of sand a cementing material. The component grains of sandstone are chiefly quartz, but many other minerals occur, such as feldspar, mica, magnetite, etc. Size within the individual grains varies from coarse-grained to fine-grained, wellrounded. Sandstones exhibit a variety of colour, the various shades of grey, brown and red.
Origin: Sandstones are sedimentary rocks composed of grains of sand a cementing material. The component grains of sandstone are chiefly quartz, but many other minerals occur, such as feldspar, mica, magnetite, etc. Size within the individual grains varies from coarse-grained to fine-grained, wellrounded. Sandstones exhibit a variety of colour, the various shades of grey, brown and red.
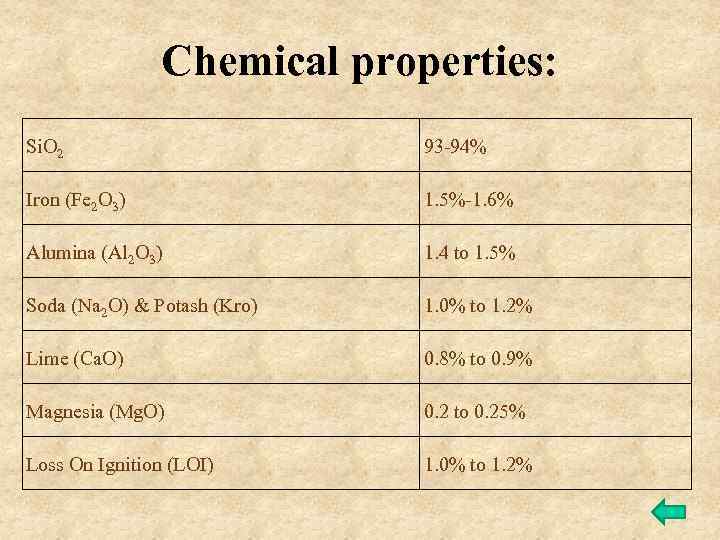 Chemical properties: Si. O 2 93 -94% Iron (Fe 2 O 3) 1. 5%-1. 6% Alumina (Al 2 O 3) 1. 4 to 1. 5% Soda (Na 2 O) & Potash (Kro) 1. 0% to 1. 2% Lime (Ca. O) 0. 8% to 0. 9% Magnesia (Mg. O) 0. 2 to 0. 25% Loss On Ignition (LOI) 1. 0% to 1. 2%
Chemical properties: Si. O 2 93 -94% Iron (Fe 2 O 3) 1. 5%-1. 6% Alumina (Al 2 O 3) 1. 4 to 1. 5% Soda (Na 2 O) & Potash (Kro) 1. 0% to 1. 2% Lime (Ca. O) 0. 8% to 0. 9% Magnesia (Mg. O) 0. 2 to 0. 25% Loss On Ignition (LOI) 1. 0% to 1. 2%
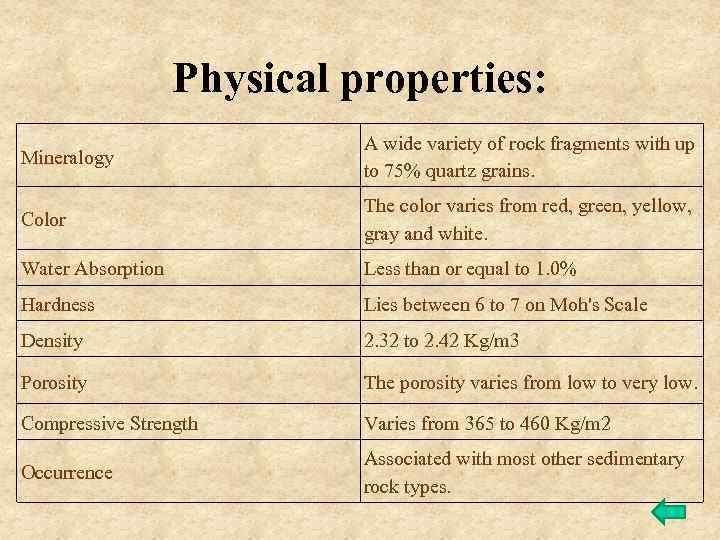 Physical properties: Mineralogy A wide variety of rock fragments with up to 75% quartz grains. Color The color varies from red, green, yellow, gray and white. Water Absorption Less than or equal to 1. 0% Hardness Lies between 6 to 7 on Moh's Scale Density 2. 32 to 2. 42 Kg/m 3 Porosity The porosity varies from low to very low. Compressive Strength Varies from 365 to 460 Kg/m 2 Occurrence Associated with most other sedimentary rock types.
Physical properties: Mineralogy A wide variety of rock fragments with up to 75% quartz grains. Color The color varies from red, green, yellow, gray and white. Water Absorption Less than or equal to 1. 0% Hardness Lies between 6 to 7 on Moh's Scale Density 2. 32 to 2. 42 Kg/m 3 Porosity The porosity varies from low to very low. Compressive Strength Varies from 365 to 460 Kg/m 2 Occurrence Associated with most other sedimentary rock types.
 Using: Sandstone has been used for domestic construction and housewares since prehistoric times, and continues to be used.
Using: Sandstone has been used for domestic construction and housewares since prehistoric times, and continues to be used.
 Continue… Sandstone was a popular building material from ancient times. It is relatively soft, making it easy to carve. It has been widely used around the world in constructing temples, homes, and other buildings. It has also been used for artistic purposes to create ornamental fountains and statues.
Continue… Sandstone was a popular building material from ancient times. It is relatively soft, making it easy to carve. It has been widely used around the world in constructing temples, homes, and other buildings. It has also been used for artistic purposes to create ornamental fountains and statues.
 17, 000 yr old sandstone oil lamp discovered at the caves of Lascaux, France.
17, 000 yr old sandstone oil lamp discovered at the caves of Lascaux, France.
 Sandstone statue Maria Immaculata by Fidelis Sporer, around 1770, in Freiburg, Germany.
Sandstone statue Maria Immaculata by Fidelis Sporer, around 1770, in Freiburg, Germany.
 Sandstone doorway in Heidelberg, German y.
Sandstone doorway in Heidelberg, German y.
 Question Time…
Question Time…
 A Question of Limestone 1 2 3 4 5 6 7 8 9
A Question of Limestone 1 2 3 4 5 6 7 8 9
 What is it? It is old sandstone oil lamp.
What is it? It is old sandstone oil lamp.
 Formula of the limestone is… Ca. CO 3
Formula of the limestone is… Ca. CO 3



 Conclusion….
Conclusion….


