Llecture_5_on_Physics_1.pptx
- Количество слайдов: 21
 Contemporary Physics: Part 1 Lecture № 5 Hydrodynamics The theory for ideal gases makes the following assumptions: 1. The gas consists of very small particles, all with non-zero mass 2. The number of molecules is large such that statistical treatment can be applied. 3. These molecules are in constant, random motion. The rapidly moving particles constantly collide with the walls of the container. 4. The collisions of gas particles with the walls of the container holding them are perfectly elastic. 5. Except during collisions the interactions among molecules are negligible (they exert no forces on one another). 6. The total volume of the individual gas molecules added up is negligible compared to the volume of the container. This is equivalent to stating that the average distance separating the gas particles is large compared to their size. 7. The molecules are perfectly spherical in shape, and elastic in nature. 8. The average kinetic energy of the gas particles depends only on the temperature of the system. 9. Relativistic effects are negligible. 10. Quantum –mechanical effects are negligible. This means the molecules are treated as classical objects.
Contemporary Physics: Part 1 Lecture № 5 Hydrodynamics The theory for ideal gases makes the following assumptions: 1. The gas consists of very small particles, all with non-zero mass 2. The number of molecules is large such that statistical treatment can be applied. 3. These molecules are in constant, random motion. The rapidly moving particles constantly collide with the walls of the container. 4. The collisions of gas particles with the walls of the container holding them are perfectly elastic. 5. Except during collisions the interactions among molecules are negligible (they exert no forces on one another). 6. The total volume of the individual gas molecules added up is negligible compared to the volume of the container. This is equivalent to stating that the average distance separating the gas particles is large compared to their size. 7. The molecules are perfectly spherical in shape, and elastic in nature. 8. The average kinetic energy of the gas particles depends only on the temperature of the system. 9. Relativistic effects are negligible. 10. Quantum –mechanical effects are negligible. This means the molecules are treated as classical objects.
 Hydrodynamics An elastic collision is an encounter between two bodies in which the total kinetic energy of the two bodies after the encounter is equal to their total kinetic energy before the encounter. Elastic collisions occur only if there is no net conversion of kinetic energy into other forms.
Hydrodynamics An elastic collision is an encounter between two bodies in which the total kinetic energy of the two bodies after the encounter is equal to their total kinetic energy before the encounter. Elastic collisions occur only if there is no net conversion of kinetic energy into other forms.
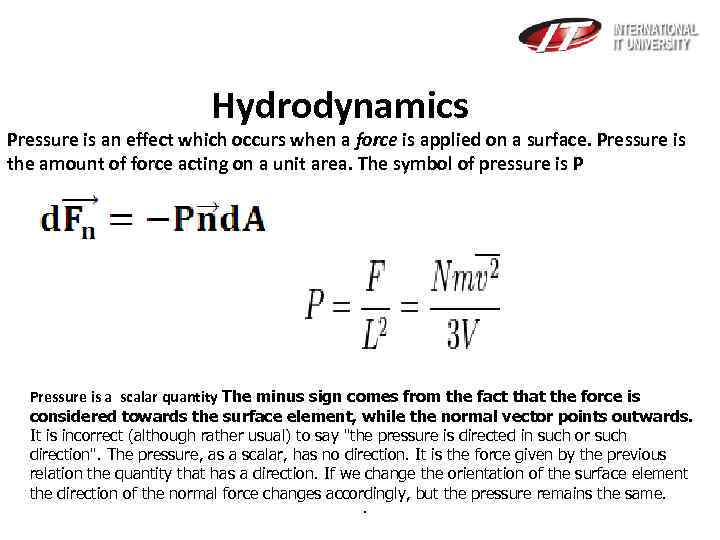 Hydrodynamics Pressure is an effect which occurs when a force is applied on a surface. Pressure is the amount of force acting on a unit area. The symbol of pressure is P Pressure is a scalar quantity The minus sign comes from the fact that the force is considered towards the surface element, while the normal vector points outwards. It is incorrect (although rather usual) to say "the pressure is directed in such or such direction". The pressure, as a scalar, has no direction. It is the force given by the previous relation the quantity that has a direction. If we change the orientation of the surface element the direction of the normal force changes accordingly, but the pressure remains the same. .
Hydrodynamics Pressure is an effect which occurs when a force is applied on a surface. Pressure is the amount of force acting on a unit area. The symbol of pressure is P Pressure is a scalar quantity The minus sign comes from the fact that the force is considered towards the surface element, while the normal vector points outwards. It is incorrect (although rather usual) to say "the pressure is directed in such or such direction". The pressure, as a scalar, has no direction. It is the force given by the previous relation the quantity that has a direction. If we change the orientation of the surface element the direction of the normal force changes accordingly, but the pressure remains the same. .
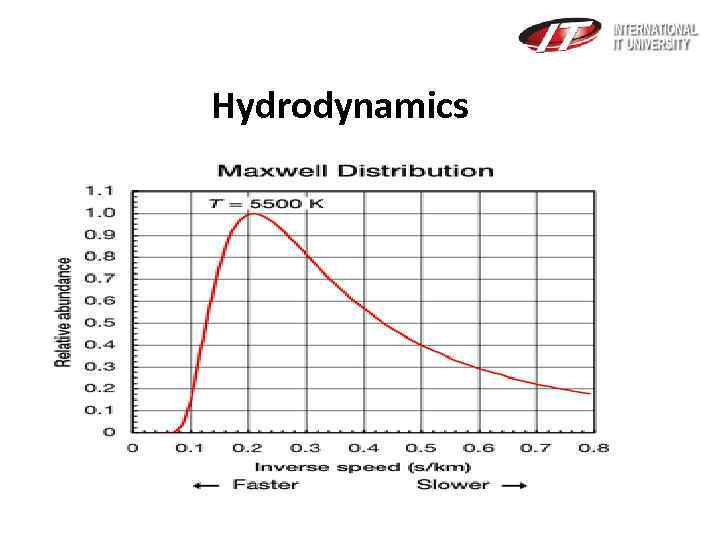 Hydrodynamics
Hydrodynamics
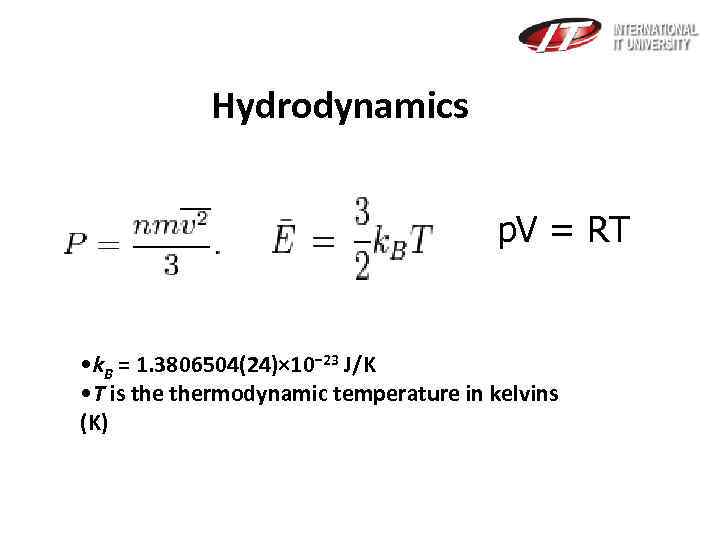 Hydrodynamics p. V = RT • k. B = 1. 3806504(24)× 10− 23 J/K • T is thermodynamic temperature in kelvins (K)
Hydrodynamics p. V = RT • k. B = 1. 3806504(24)× 10− 23 J/K • T is thermodynamic temperature in kelvins (K)
 Hydrodynamics Let’s introduce such a function distribution which shows the expected number of molecules within the physical volume surrounding the point with the velocities in the range
Hydrodynamics Let’s introduce such a function distribution which shows the expected number of molecules within the physical volume surrounding the point with the velocities in the range
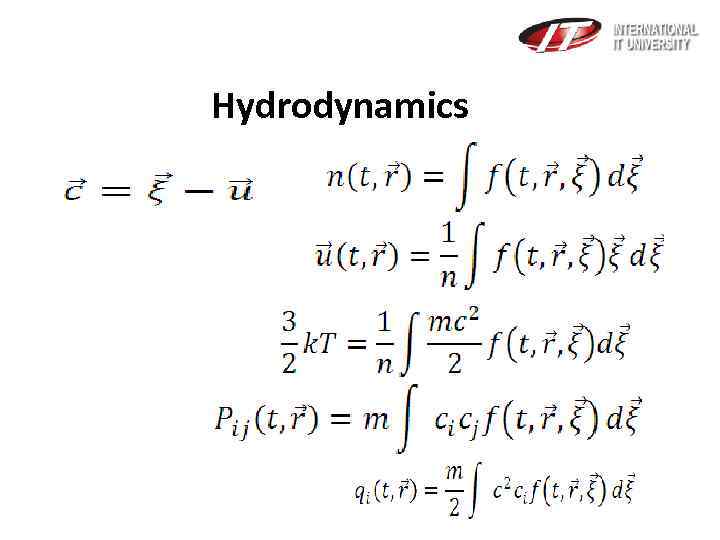 Hydrodynamics
Hydrodynamics
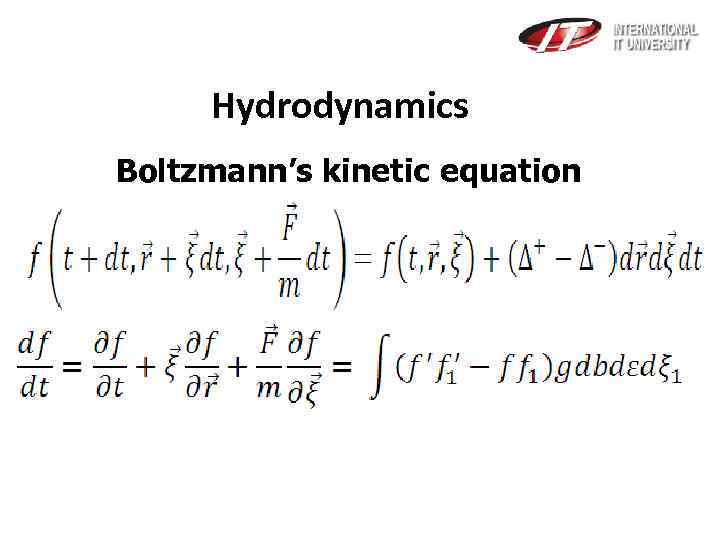 Hydrodynamics Boltzmann’s kinetic equation
Hydrodynamics Boltzmann’s kinetic equation
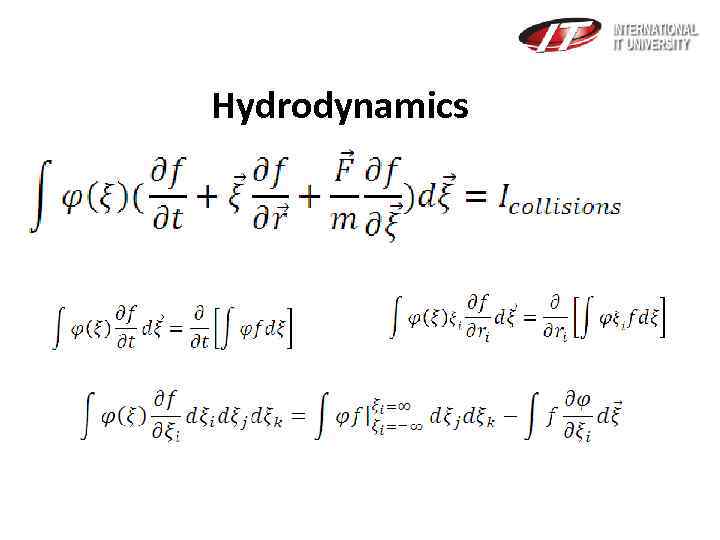 Hydrodynamics
Hydrodynamics
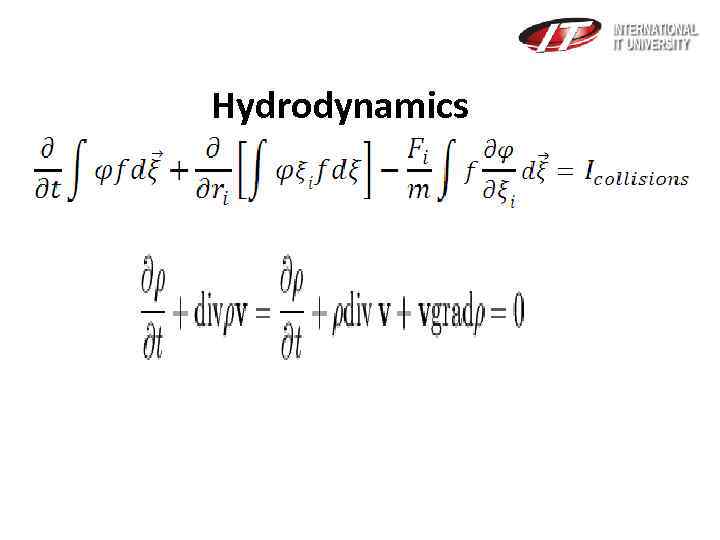 Hydrodynamics
Hydrodynamics
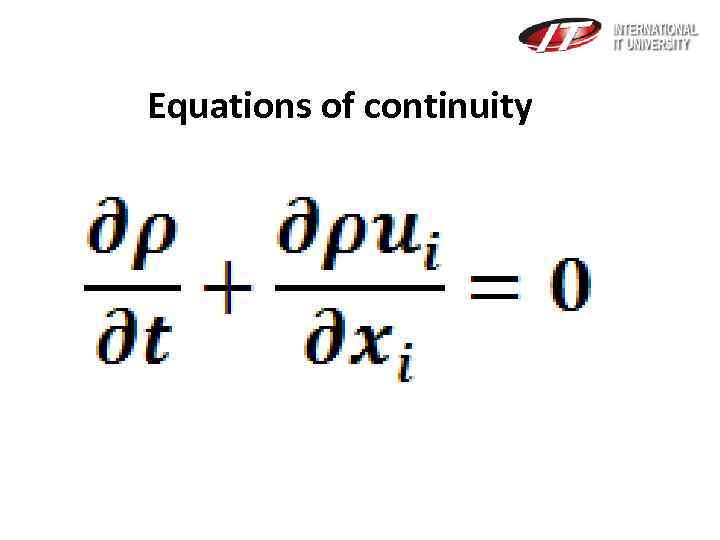 Equations of continuity
Equations of continuity
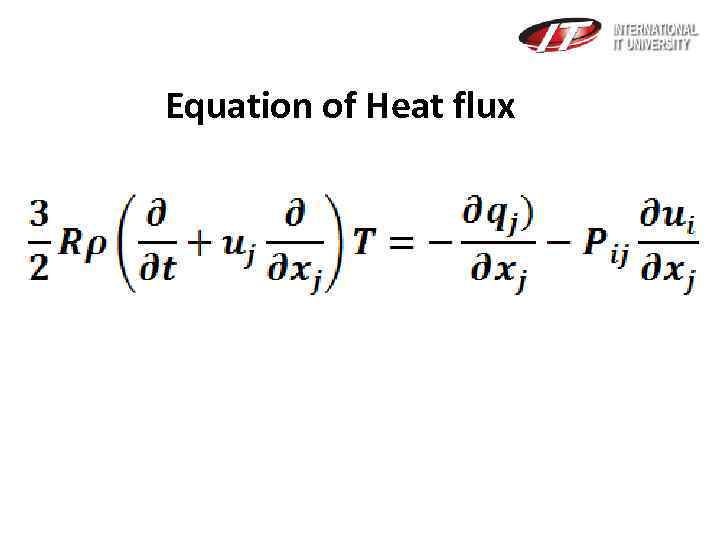 Equation of Heat flux
Equation of Heat flux
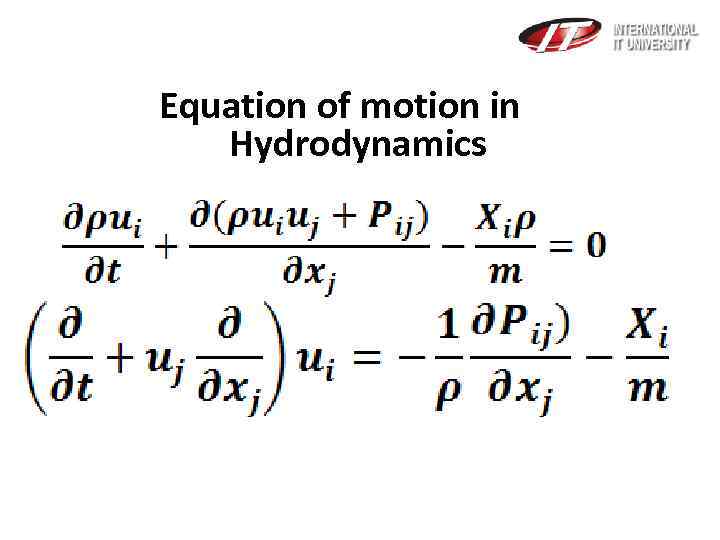 Equation of motion in Hydrodynamics
Equation of motion in Hydrodynamics
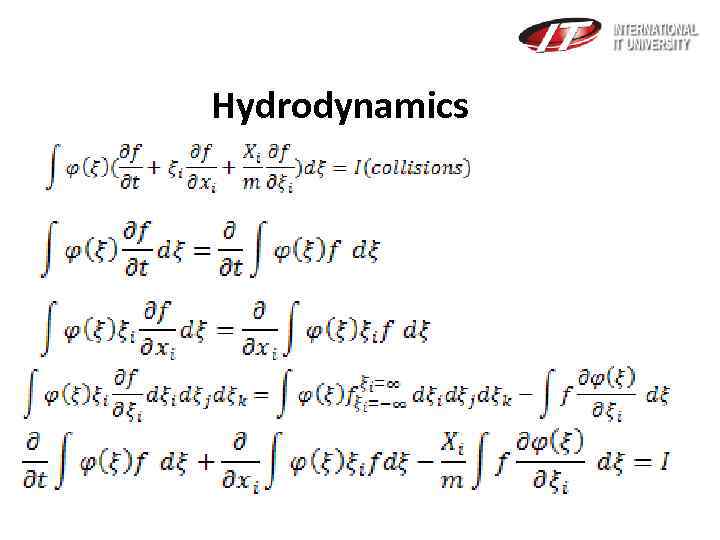 Hydrodynamics
Hydrodynamics
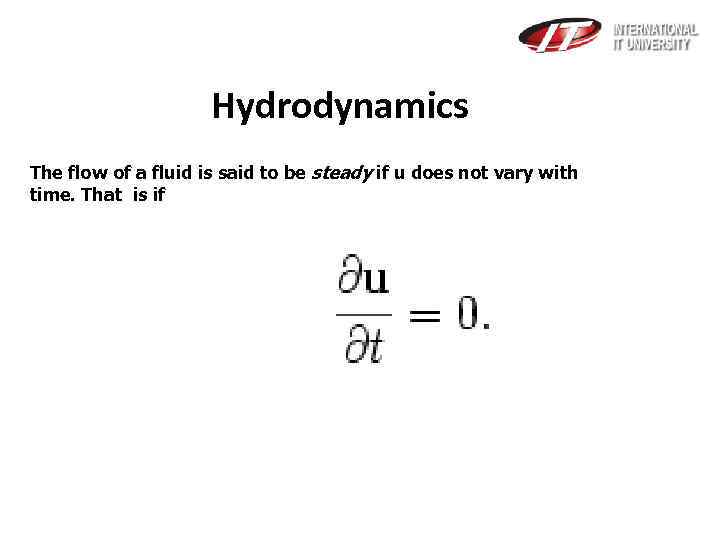 Hydrodynamics The flow of a fluid is said to be steady if u does not vary with time. That is if
Hydrodynamics The flow of a fluid is said to be steady if u does not vary with time. That is if
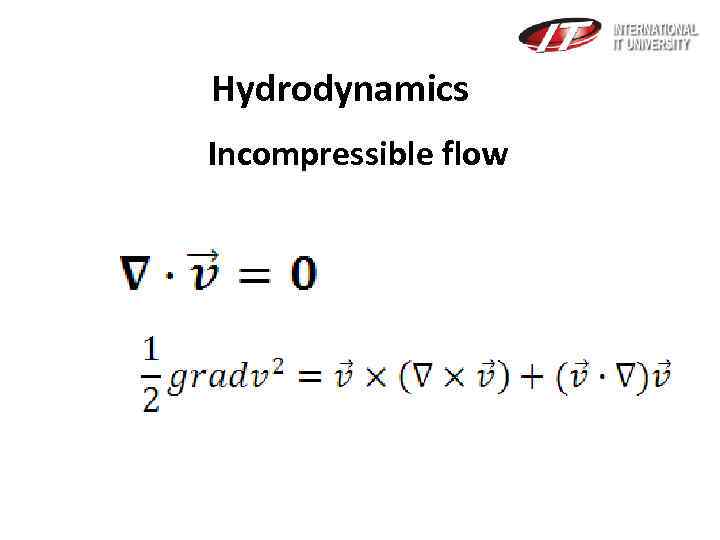 Hydrodynamics Incompressible flow
Hydrodynamics Incompressible flow
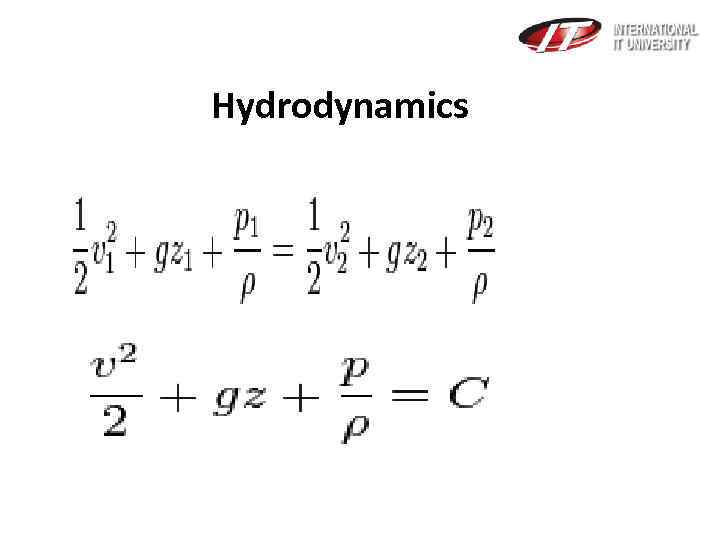 Hydrodynamics
Hydrodynamics
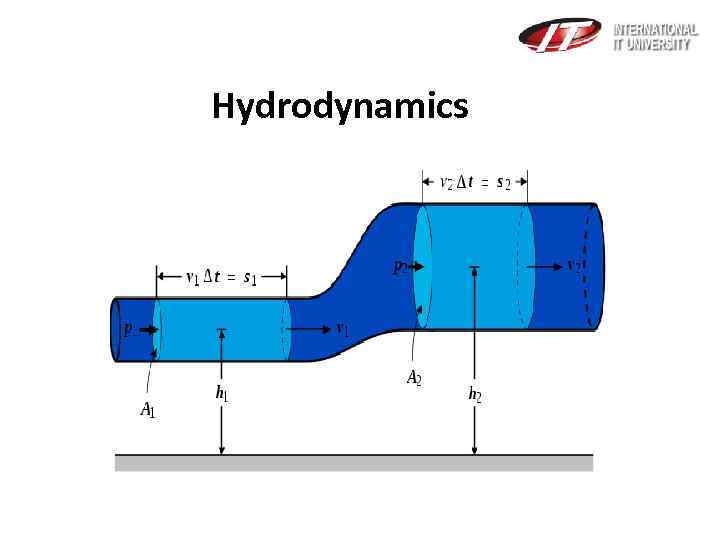
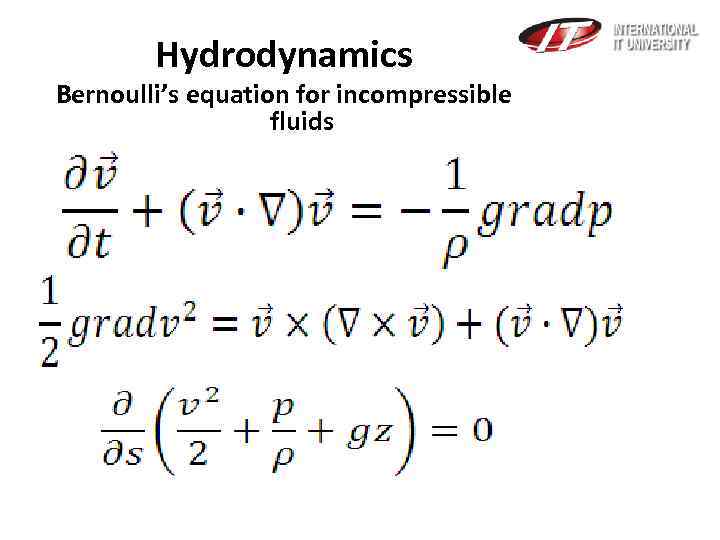 Hydrodynamics Bernoulli’s equation for incompressible fluids
Hydrodynamics Bernoulli’s equation for incompressible fluids
 Hydrodynamics
Hydrodynamics
 Hydrodynamics
Hydrodynamics
 The mole(unit) The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities (e. g. , atoms, molecules, ions, electrons) as there atoms in 12 grams of pure carbon-12 (12 C), the isotope of carbon with atomic weight 12
The mole(unit) The mole is a unit of measurement used in chemistry to express amounts of a chemical substance, defined as an amount of a substance that contains as many elementary entities (e. g. , atoms, molecules, ions, electrons) as there atoms in 12 grams of pure carbon-12 (12 C), the isotope of carbon with atomic weight 12


