c6d5bea17b858d082d0b9fb0af409ad3.ppt
- Количество слайдов: 126
 Contact Lens Care & Maintenance Lecture 5 L 1 Version: 2012. May. 10
Contact Lens Care & Maintenance Lecture 5 L 1 Version: 2012. May. 10
 COPYRIGHT NOTICE The IACLE Contact Lens Course (all formats) is the sole property of the International Association of Contact Lens Educators (IACLE) and is protected, without limitations, by copyright. By accessing this material, you agree to the following terms and conditions: You may only access and use the IACLE Contact Lens Course for personal or educational purposes. Any dissemination or sale of the IACLE Contact Lens Course, either in whole or in part, or use of the materials for other than educational and personal purposes, is strictly prohibited without the express written consent of IACLE. Except as declared below, you may not reproduce, republish, post, transmit, or distribute any material included in the IACLE Contact Lens Course. You may print materials for personal or educational purposes only. All copyright information, including the IACLE logo, must remain on the material. Appropriate reference must be provided to any use of the content of the IACLE Contact Lens Course, including text, images, &/or illustrations.
COPYRIGHT NOTICE The IACLE Contact Lens Course (all formats) is the sole property of the International Association of Contact Lens Educators (IACLE) and is protected, without limitations, by copyright. By accessing this material, you agree to the following terms and conditions: You may only access and use the IACLE Contact Lens Course for personal or educational purposes. Any dissemination or sale of the IACLE Contact Lens Course, either in whole or in part, or use of the materials for other than educational and personal purposes, is strictly prohibited without the express written consent of IACLE. Except as declared below, you may not reproduce, republish, post, transmit, or distribute any material included in the IACLE Contact Lens Course. You may print materials for personal or educational purposes only. All copyright information, including the IACLE logo, must remain on the material. Appropriate reference must be provided to any use of the content of the IACLE Contact Lens Course, including text, images, &/or illustrations.
 SPONSORS Development and delivery of contact lens education by IACLE is supported through educational grants and in-kind contributions Industry Supporters Major In-Kind Supporters
SPONSORS Development and delivery of contact lens education by IACLE is supported through educational grants and in-kind contributions Industry Supporters Major In-Kind Supporters
 Published in Australia by The International Association of Contact Lens Educators Revised Edition 2011 The International Association of Contact Lens Educators 2000 -2011 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission, in writing, of: The International Association of Contact Lens Educators IACLE Secretariat, PO Box 656 KENSINGTON NSW 1465 Australia Email: iacle@iacle. org
Published in Australia by The International Association of Contact Lens Educators Revised Edition 2011 The International Association of Contact Lens Educators 2000 -2011 All rights reserved. No part of this publication may be reproduced, stored in a retrieval system, or transmitted, in any form or by any means, without the prior permission, in writing, of: The International Association of Contact Lens Educators IACLE Secretariat, PO Box 656 KENSINGTON NSW 1465 Australia Email: iacle@iacle. org
 CONTRIBUTORS Introduction to Contact Lens Care: Lakshman Subbaraman, Ph. D, BSOptom, MSc, FAAO Lewis Williams, AQIT (Optom), MOptom, Ph. D For a complete list of acknowledgements please see our website: www. iacle. org
CONTRIBUTORS Introduction to Contact Lens Care: Lakshman Subbaraman, Ph. D, BSOptom, MSc, FAAO Lewis Williams, AQIT (Optom), MOptom, Ph. D For a complete list of acknowledgements please see our website: www. iacle. org
 WHAT IS CONTACT LENS CARE? GP CONTACT LENSES 5 L 1 -6
WHAT IS CONTACT LENS CARE? GP CONTACT LENSES 5 L 1 -6
 WHAT IS CONTACT LENS CARE? SOFT CONTACT LENSES 5 L 1 -7
WHAT IS CONTACT LENS CARE? SOFT CONTACT LENSES 5 L 1 -7
 WHAT IS CONTACT LENS CARE? SILICONE HYDROGEL CONTACT LENSES 5 L 1 -8
WHAT IS CONTACT LENS CARE? SILICONE HYDROGEL CONTACT LENSES 5 L 1 -8
 WHAT IS CONTACT LENS CARE? GP LENS CASES 5 L 1 -9
WHAT IS CONTACT LENS CARE? GP LENS CASES 5 L 1 -9
 WHAT IS CONTACT LENS CARE? LENS CASES: SCL 5 L 1 -10
WHAT IS CONTACT LENS CARE? LENS CASES: SCL 5 L 1 -10
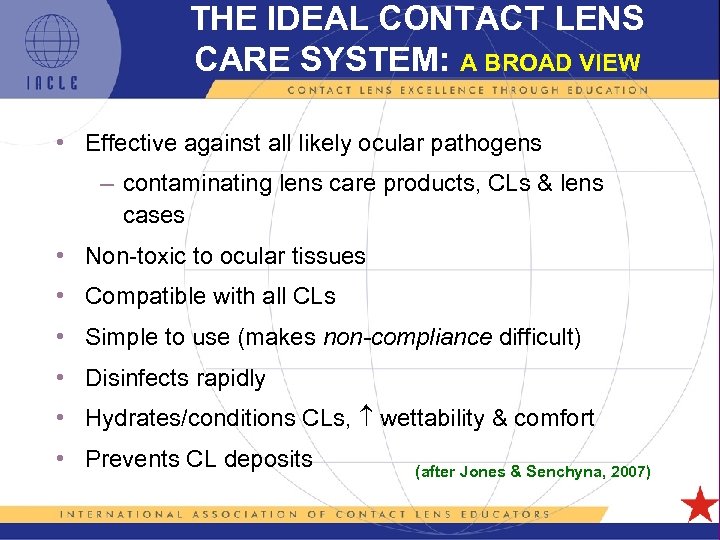 THE IDEAL CONTACT LENS CARE SYSTEM: A BROAD VIEW • Effective against all likely ocular pathogens – contaminating lens care products, CLs & lens cases • Non-toxic to ocular tissues • Compatible with all CLs • Simple to use (makes non-compliance difficult) • Disinfects rapidly • Hydrates/conditions CLs, wettability & comfort • Prevents CL deposits (after Jones & Senchyna, 2007) 5 L 1 -11
THE IDEAL CONTACT LENS CARE SYSTEM: A BROAD VIEW • Effective against all likely ocular pathogens – contaminating lens care products, CLs & lens cases • Non-toxic to ocular tissues • Compatible with all CLs • Simple to use (makes non-compliance difficult) • Disinfects rapidly • Hydrates/conditions CLs, wettability & comfort • Prevents CL deposits (after Jones & Senchyna, 2007) 5 L 1 -11
 THE IDEAL CONTACT LENS CARE SYSTEM: A NARROWER VIEW • Kills ALL viable organisms in: – lens case – solutions – & on CLs • Removes ALL: – dead organisms – skin lipids (from eyelids) – tear, finger & air-borne contaminants – cosmetic residues – LCP residues & by-products – any other contaminants • Hydrates & conditions lenses ready-to-use • Non-toxic • Suitable for all CLs • Quick acting (to allow disinfection between brief uses) 5 L 1 -12
THE IDEAL CONTACT LENS CARE SYSTEM: A NARROWER VIEW • Kills ALL viable organisms in: – lens case – solutions – & on CLs • Removes ALL: – dead organisms – skin lipids (from eyelids) – tear, finger & air-borne contaminants – cosmetic residues – LCP residues & by-products – any other contaminants • Hydrates & conditions lenses ready-to-use • Non-toxic • Suitable for all CLs • Quick acting (to allow disinfection between brief uses) 5 L 1 -12
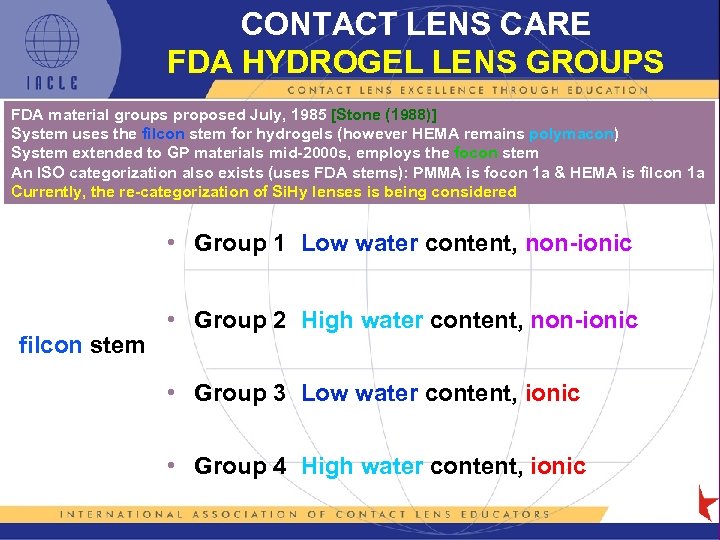 CONTACT LENS CARE FDA HYDROGEL LENS GROUPS FDA material groups proposed July, 1985 [Stone (1988)] System uses the filcon stem for hydrogels (however HEMA remains polymacon) System extended to GP materials mid-2000 s, employs the focon stem An ISO categorization also exists (uses FDA stems): PMMA is focon 1 a & HEMA is filcon 1 a Currently, the re-categorization of Si. Hy lenses is being considered • Group 1 Low water content, non-ionic filcon stem • Group 2 High water content, non-ionic • Group 3 Low water content, ionic • Group 4 High water content, ionic 5 L 1 -13
CONTACT LENS CARE FDA HYDROGEL LENS GROUPS FDA material groups proposed July, 1985 [Stone (1988)] System uses the filcon stem for hydrogels (however HEMA remains polymacon) System extended to GP materials mid-2000 s, employs the focon stem An ISO categorization also exists (uses FDA stems): PMMA is focon 1 a & HEMA is filcon 1 a Currently, the re-categorization of Si. Hy lenses is being considered • Group 1 Low water content, non-ionic filcon stem • Group 2 High water content, non-ionic • Group 3 Low water content, ionic • Group 4 High water content, ionic 5 L 1 -13
![CONTACT LENS CARE FDA HYDROPHOBIC LENS GROUPS (Stone [2007]) • Group 1 No silicon CONTACT LENS CARE FDA HYDROPHOBIC LENS GROUPS (Stone [2007]) • Group 1 No silicon](https://present5.com/presentation/c6d5bea17b858d082d0b9fb0af409ad3/image-14.jpg) CONTACT LENS CARE FDA HYDROPHOBIC LENS GROUPS (Stone [2007]) • Group 1 No silicon or fluorine content • Group 2 Contains silicon but no fluorine focon stem • Group 3 Contains silicon & fluorine • Group 4 Contains fluorine but no silicon 5 L 1 -14
CONTACT LENS CARE FDA HYDROPHOBIC LENS GROUPS (Stone [2007]) • Group 1 No silicon or fluorine content • Group 2 Contains silicon but no fluorine focon stem • Group 3 Contains silicon & fluorine • Group 4 Contains fluorine but no silicon 5 L 1 -14
 THE IDEAL CONTACT LENS CARE SYSTEM • Restores lenses to factory-original condition • Maintains lenses in this condition for as long as required (or at least until their next use) Unfortunately, these ideals are seldom realized in the real world Once on the eye, the factory-original condition of a new CL cannot be restored 5 L 1 -15
THE IDEAL CONTACT LENS CARE SYSTEM • Restores lenses to factory-original condition • Maintains lenses in this condition for as long as required (or at least until their next use) Unfortunately, these ideals are seldom realized in the real world Once on the eye, the factory-original condition of a new CL cannot be restored 5 L 1 -15
 FACTORS ALTERING PERCEPTIONS OF LENS CARE • >20 years of disposable CLs • >15 years of daily disposable CLs (requiring no lens care) • Claims of lower complication rates from disposable & regularreplacement CLs • The advent of ‘convenient’ LCPs – multi-purpose solutions (MPSs) – the availability of single-purpose products – the in the number & variety of LCPs marketed – the in the number of manufacturers of LCPs • The promotion of NO RUB & NO RUB products • Relatively few problems reported 5 L 1 -16
FACTORS ALTERING PERCEPTIONS OF LENS CARE • >20 years of disposable CLs • >15 years of daily disposable CLs (requiring no lens care) • Claims of lower complication rates from disposable & regularreplacement CLs • The advent of ‘convenient’ LCPs – multi-purpose solutions (MPSs) – the availability of single-purpose products – the in the number & variety of LCPs marketed – the in the number of manufacturers of LCPs • The promotion of NO RUB & NO RUB products • Relatively few problems reported 5 L 1 -16
 DEFINITION OF CL DEPOSITS Any surface coating or contaminant, or any formation within the CL matrix, that is not flushed or wiped from the lens by the combined actions of the tears & blinking Realistic addendum: …and that is not removed by routine CL care procedures following CL wear 5 L 1 -17
DEFINITION OF CL DEPOSITS Any surface coating or contaminant, or any formation within the CL matrix, that is not flushed or wiped from the lens by the combined actions of the tears & blinking Realistic addendum: …and that is not removed by routine CL care procedures following CL wear 5 L 1 -17
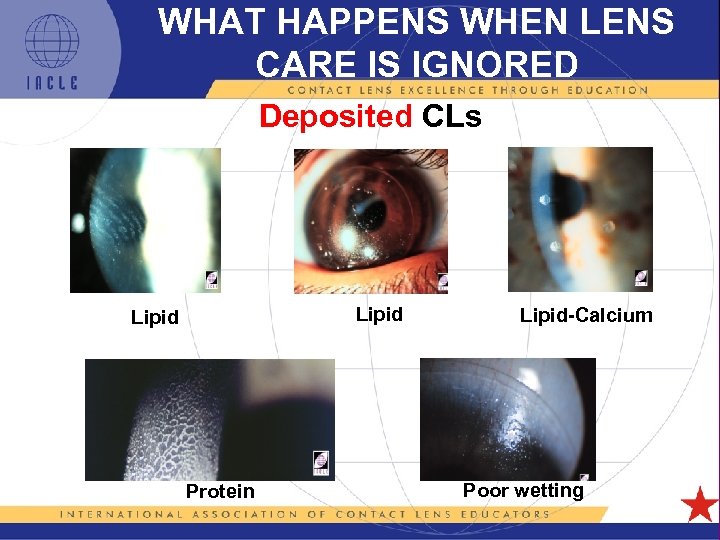 WHAT HAPPENS WHEN LENS CARE IS IGNORED Deposited CLs Lipid-Calcium Poor wetting Protein 5 L 1 -18
WHAT HAPPENS WHEN LENS CARE IS IGNORED Deposited CLs Lipid-Calcium Poor wetting Protein 5 L 1 -18
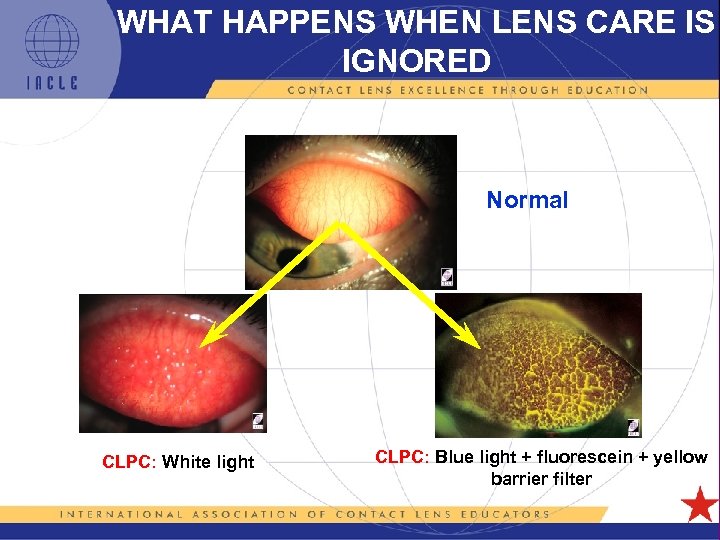 WHAT HAPPENS WHEN LENS CARE IS IGNORED Normal CLPC: White light CLPC: Blue light + fluorescein + yellow barrier filter 5 L 1 -19
WHAT HAPPENS WHEN LENS CARE IS IGNORED Normal CLPC: White light CLPC: Blue light + fluorescein + yellow barrier filter 5 L 1 -19
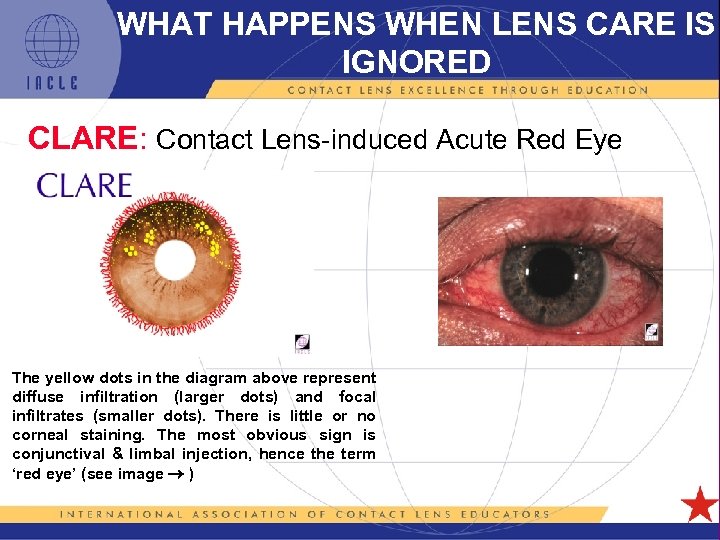 WHAT HAPPENS WHEN LENS CARE IS IGNORED CLARE: Contact Lens-induced Acute Red Eye The yellow dots in the diagram above represent diffuse infiltration (larger dots) and focal infiltrates (smaller dots). There is little or no corneal staining. The most obvious sign is conjunctival & limbal injection, hence the term ‘red eye’ (see image ) 5 L 1 -20
WHAT HAPPENS WHEN LENS CARE IS IGNORED CLARE: Contact Lens-induced Acute Red Eye The yellow dots in the diagram above represent diffuse infiltration (larger dots) and focal infiltrates (smaller dots). There is little or no corneal staining. The most obvious sign is conjunctival & limbal injection, hence the term ‘red eye’ (see image ) 5 L 1 -20
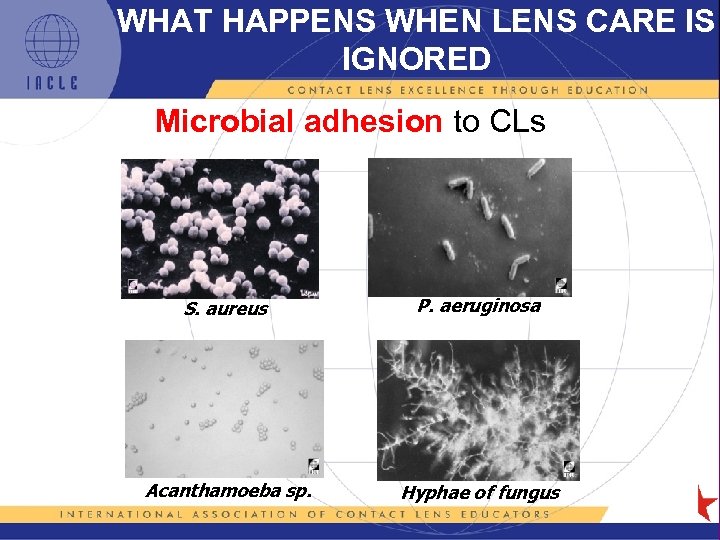 WHAT HAPPENS WHEN LENS CARE IS IGNORED Microbial adhesion to CLs S. aureus P. aeruginosa Acanthamoeba sp. Hyphae of fungus 5 L 1 -21
WHAT HAPPENS WHEN LENS CARE IS IGNORED Microbial adhesion to CLs S. aureus P. aeruginosa Acanthamoeba sp. Hyphae of fungus 5 L 1 -21
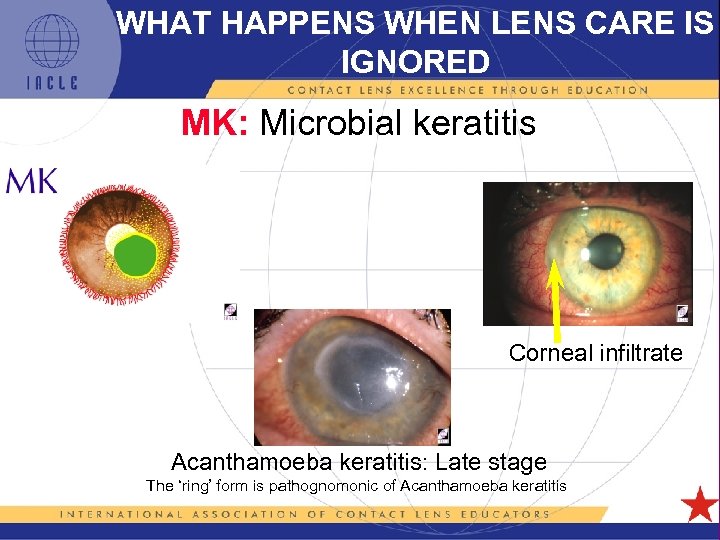 WHAT HAPPENS WHEN LENS CARE IS IGNORED MK: Microbial keratitis Corneal infiltrate Acanthamoeba keratitis: Late stage The ‘ring’ form is pathognomonic of Acanthamoeba keratitis 5 L 1 -22
WHAT HAPPENS WHEN LENS CARE IS IGNORED MK: Microbial keratitis Corneal infiltrate Acanthamoeba keratitis: Late stage The ‘ring’ form is pathognomonic of Acanthamoeba keratitis 5 L 1 -22
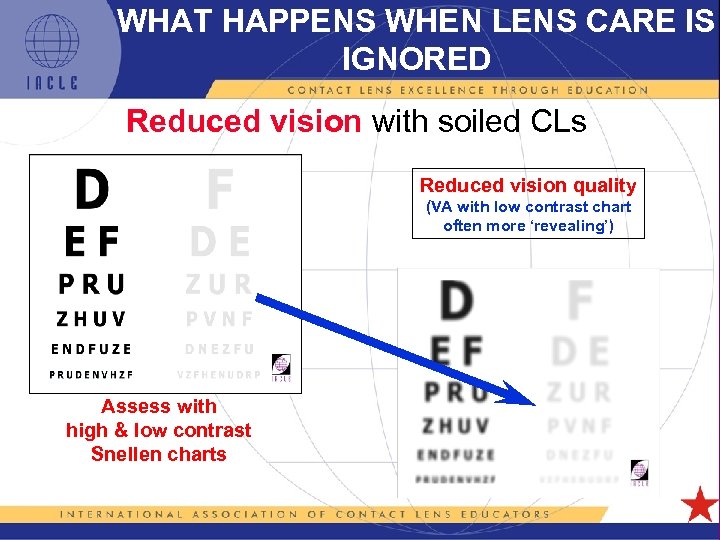 WHAT HAPPENS WHEN LENS CARE IS IGNORED Reduced vision with soiled CLs Reduced vision quality (VA with low contrast chart often more ‘revealing’) Assess with high & low contrast Snellen charts 5 L 1 -23
WHAT HAPPENS WHEN LENS CARE IS IGNORED Reduced vision with soiled CLs Reduced vision quality (VA with low contrast chart often more ‘revealing’) Assess with high & low contrast Snellen charts 5 L 1 -23
 CONTACT LENS CARE THE CURRENT SITUATION • The Convenience Factor – 1 -step peroxide systems – MPSs (>1 function in a single product) – DD CLs (usually, no lens care) • But at what cost? 5 L 1 -24
CONTACT LENS CARE THE CURRENT SITUATION • The Convenience Factor – 1 -step peroxide systems – MPSs (>1 function in a single product) – DD CLs (usually, no lens care) • But at what cost? 5 L 1 -24
 CONTACT LENS CARE THE COST • Fusarium spp. infections • Acanthamoeba spp. infections • Lost vision, penetrating keratoplasties • LCP recalls • FDA & other regulators forced to investigate care system performance, esp. against fungi & Acanthamoeba spp. 5 L 1 -25
CONTACT LENS CARE THE COST • Fusarium spp. infections • Acanthamoeba spp. infections • Lost vision, penetrating keratoplasties • LCP recalls • FDA & other regulators forced to investigate care system performance, esp. against fungi & Acanthamoeba spp. 5 L 1 -25
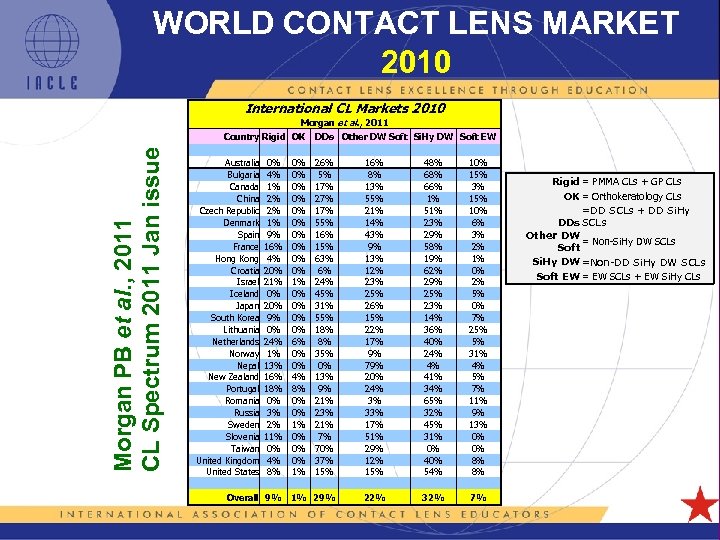 WORLD CONTACT LENS MARKET 2010 International CL Markets 2010 Morgan et al. , 2011 Morgan PB et al. , 2011 CL Spectrum 2011 Jan issue Country Rigid OK Australia Bulgaria Canada China Czech Republic Denmark Spain France Hong Kong Croatia Israel Iceland Japan South Korea Lithuania Netherlands Norway Nepal New Zealand Portugal Romania Russia Sweden Slovenia Taiwan United Kingdom United States 0% 4% 1% 2% 2% 1% 9% 16% 4% 20% 21% 0% 20% 9% 0% 24% 1% 13% 16% 18% 0% 3% 2% 11% 0% 4% 8% 0% 0% 0% 1% 0% 0% 6% 0% 0% 4% 8% 0% 0% 1% 0% 0% 0% 1% DDs Other DW Soft Si. Hy DW Soft EW 26% 5% 17% 27% 17% 55% 16% 15% 63% 6% 24% 45% 31% 55% 18% 8% 35% 0% 13% 9% 21% 23% 21% 7% 70% 37% 15% 16% 8% 13% 55% 21% 14% 43% 9% 13% 12% 23% 25% 26% 15% 22% 17% 9% 79% 20% 24% 3% 33% 17% 51% 29% 12% 15% 48% 66% 1% 51% 23% 29% 58% 19% 62% 29% 25% 23% 14% 36% 40% 24% 4% 41% 34% 65% 32% 45% 31% 0% 40% 54% 10% 15% 3% 15% 10% 6% 3% 2% 1% 0% 2% 5% 0% 7% 25% 5% 31% 4% 5% 7% 11% 9% 13% 0% 0% 8% 8% Overall 9% 1% 29% 22% 32% 7% 5 L 1 -26 Rigid = PMMA CLs + GP CLs OK = Orthokeratology CLs =DD SCLs + DD Si. Hy DDs SCLs Other DW = Non-Si. Hy DW SCLs Soft Si. Hy DW =Non-DD Si. Hy DW SCLs Soft EW = EW SCLs + EW Si. Hy CLs
WORLD CONTACT LENS MARKET 2010 International CL Markets 2010 Morgan et al. , 2011 Morgan PB et al. , 2011 CL Spectrum 2011 Jan issue Country Rigid OK Australia Bulgaria Canada China Czech Republic Denmark Spain France Hong Kong Croatia Israel Iceland Japan South Korea Lithuania Netherlands Norway Nepal New Zealand Portugal Romania Russia Sweden Slovenia Taiwan United Kingdom United States 0% 4% 1% 2% 2% 1% 9% 16% 4% 20% 21% 0% 20% 9% 0% 24% 1% 13% 16% 18% 0% 3% 2% 11% 0% 4% 8% 0% 0% 0% 1% 0% 0% 6% 0% 0% 4% 8% 0% 0% 1% 0% 0% 0% 1% DDs Other DW Soft Si. Hy DW Soft EW 26% 5% 17% 27% 17% 55% 16% 15% 63% 6% 24% 45% 31% 55% 18% 8% 35% 0% 13% 9% 21% 23% 21% 7% 70% 37% 15% 16% 8% 13% 55% 21% 14% 43% 9% 13% 12% 23% 25% 26% 15% 22% 17% 9% 79% 20% 24% 3% 33% 17% 51% 29% 12% 15% 48% 66% 1% 51% 23% 29% 58% 19% 62% 29% 25% 23% 14% 36% 40% 24% 4% 41% 34% 65% 32% 45% 31% 0% 40% 54% 10% 15% 3% 15% 10% 6% 3% 2% 1% 0% 2% 5% 0% 7% 25% 5% 31% 4% 5% 7% 11% 9% 13% 0% 0% 8% 8% Overall 9% 1% 29% 22% 32% 7% 5 L 1 -26 Rigid = PMMA CLs + GP CLs OK = Orthokeratology CLs =DD SCLs + DD Si. Hy DDs SCLs Other DW = Non-Si. Hy DW SCLs Soft Si. Hy DW =Non-DD Si. Hy DW SCLs Soft EW = EW SCLs + EW Si. Hy CLs
 WORLD CONTACT LENS MARKET THE FUTURE • dominance of disposable CLs • Rx range already available • range of BOZRs possible • range of TDs less likely • Evolution of CL materials 5 L 1 -27
WORLD CONTACT LENS MARKET THE FUTURE • dominance of disposable CLs • Rx range already available • range of BOZRs possible • range of TDs less likely • Evolution of CL materials 5 L 1 -27
 WORLD CONTACT LENS MARKET THE FUTURE What follows Si. Hy CLs? • DD Si. Hy to their market share • DDs in conventional hydrogels to • parameter range likely to remain limited • trade-off between inventory size required & parameter range stocked 5 L 1 -28
WORLD CONTACT LENS MARKET THE FUTURE What follows Si. Hy CLs? • DD Si. Hy to their market share • DDs in conventional hydrogels to • parameter range likely to remain limited • trade-off between inventory size required & parameter range stocked 5 L 1 -28
 WORLD CONTACT LENS MARKET THE FUTURE • Hybrid CLs to have an impact as technology evolves & parameter range & number of indications – care required when selecting lens care • Conventional lenses to remain available – special lenses – custom Rxs – extreme Rxs – trial lenses for all of these 5 L 1 -29
WORLD CONTACT LENS MARKET THE FUTURE • Hybrid CLs to have an impact as technology evolves & parameter range & number of indications – care required when selecting lens care • Conventional lenses to remain available – special lenses – custom Rxs – extreme Rxs – trial lenses for all of these 5 L 1 -29
 WORLD CONTACT LENS MARKET THE FUTURE • MPSs domination to continue – single-purpose products - small market share only • Market & products for protein removal will further as disposable lenses achieve even greater market penetration 5 L 1 -30
WORLD CONTACT LENS MARKET THE FUTURE • MPSs domination to continue – single-purpose products - small market share only • Market & products for protein removal will further as disposable lenses achieve even greater market penetration 5 L 1 -30
 WORLD CONTACT LENS MARKET THE FUTURE • Peroxide seen as problem solver • Rivalry between polihexanide (PHX) & polyquaternium (PQ-1) disinfectants to as products containing BOTH are released – so-called dual-action products • Opportunities for novel disinfectants exist – but small number of manufacturers an issue • Material compatibility to remain an issue 5 L 1 -31
WORLD CONTACT LENS MARKET THE FUTURE • Peroxide seen as problem solver • Rivalry between polihexanide (PHX) & polyquaternium (PQ-1) disinfectants to as products containing BOTH are released – so-called dual-action products • Opportunities for novel disinfectants exist – but small number of manufacturers an issue • Material compatibility to remain an issue 5 L 1 -31
 CONTACT LENS CARE DEPOSITS • CLs still deposit-prone • Deposit type & deposition rate depend on CL material & patient characteristics • Simply, disposability only cuts short the deposition process – clinical significance, usually to subclinical levels – obviates the need for ‘deep’ cleaning – or avoids the need for protein removal • DD may be the ultimate answer 5 L 1 -32
CONTACT LENS CARE DEPOSITS • CLs still deposit-prone • Deposit type & deposition rate depend on CL material & patient characteristics • Simply, disposability only cuts short the deposition process – clinical significance, usually to subclinical levels – obviates the need for ‘deep’ cleaning – or avoids the need for protein removal • DD may be the ultimate answer 5 L 1 -32
 CONTACT LENS CARE IN THE DISPOSABLE LENS ERA SUMMARY • The need for lens care remains unchanged • Disposable CLs do not need protein removal – if protein deposits prove to be a problem, review frequency of disposal • DDs = the answer for many but not all wearers • LCP performance likely to • The perceptions surrounding the importance of lens care FALSE 5 L 1 -33
CONTACT LENS CARE IN THE DISPOSABLE LENS ERA SUMMARY • The need for lens care remains unchanged • Disposable CLs do not need protein removal – if protein deposits prove to be a problem, review frequency of disposal • DDs = the answer for many but not all wearers • LCP performance likely to • The perceptions surrounding the importance of lens care FALSE 5 L 1 -33
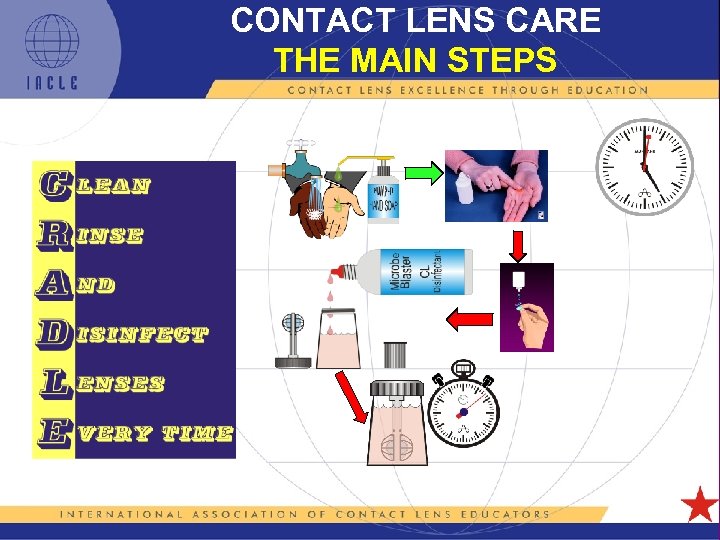 CONTACT LENS CARE THE MAIN STEPS 5 L 1 -34
CONTACT LENS CARE THE MAIN STEPS 5 L 1 -34
 CONTACT LENS CARE HAND CLEANING • Cleaning hands before CL handling is THE starting point for sound & efficacious lens care – use potable water & select ‘plain’ soap (fragrance-free, little/no colouring agents, etc. ) – include palms, between fingers, under fingernails – lather & rub - minimum of 15 seconds – rinse soap off thoroughly (10 -15 sec) – dry hands with low-lint or lint-free towel — paper towel is unsuitable — use towel to turn tap off – once clean, take care with what the hands touch 5 L 1 -35
CONTACT LENS CARE HAND CLEANING • Cleaning hands before CL handling is THE starting point for sound & efficacious lens care – use potable water & select ‘plain’ soap (fragrance-free, little/no colouring agents, etc. ) – include palms, between fingers, under fingernails – lather & rub - minimum of 15 seconds – rinse soap off thoroughly (10 -15 sec) – dry hands with low-lint or lint-free towel — paper towel is unsuitable — use towel to turn tap off – once clean, take care with what the hands touch 5 L 1 -35
 CONTACT LENS CARE LENS CLEANING • Cleaning CLs is part of the disinfection process – cleaning significant in microbial load (Houlsby et al. , 1984, Shih et al. 1985, 1991) – rubbing & rinsing corneal staining (at least with the combination of balafilcon-A Si. Hy CLs & a PHX-based MPS) (Peterson et al. , 2010) 5 L 1 -36
CONTACT LENS CARE LENS CLEANING • Cleaning CLs is part of the disinfection process – cleaning significant in microbial load (Houlsby et al. , 1984, Shih et al. 1985, 1991) – rubbing & rinsing corneal staining (at least with the combination of balafilcon-A Si. Hy CLs & a PHX-based MPS) (Peterson et al. , 2010) 5 L 1 -36
 CONTACT LENS CLEANING RUB or NO RUB? NO RUB Why? • Rinsing alone does not detach adherent/penetrating fungi • Micro-organisms are not the only ‘concern’ – other lens contaminants also require removal § finger-borne contaminants ( lens handling) – inevitable surface deposition needs to be contained – a clean lens is usually more comfortable • Lens wettability is enhanced (see review by Butcko et al. , 2007) 5 L 1 -37
CONTACT LENS CLEANING RUB or NO RUB? NO RUB Why? • Rinsing alone does not detach adherent/penetrating fungi • Micro-organisms are not the only ‘concern’ – other lens contaminants also require removal § finger-borne contaminants ( lens handling) – inevitable surface deposition needs to be contained – a clean lens is usually more comfortable • Lens wettability is enhanced (see review by Butcko et al. , 2007) 5 L 1 -37
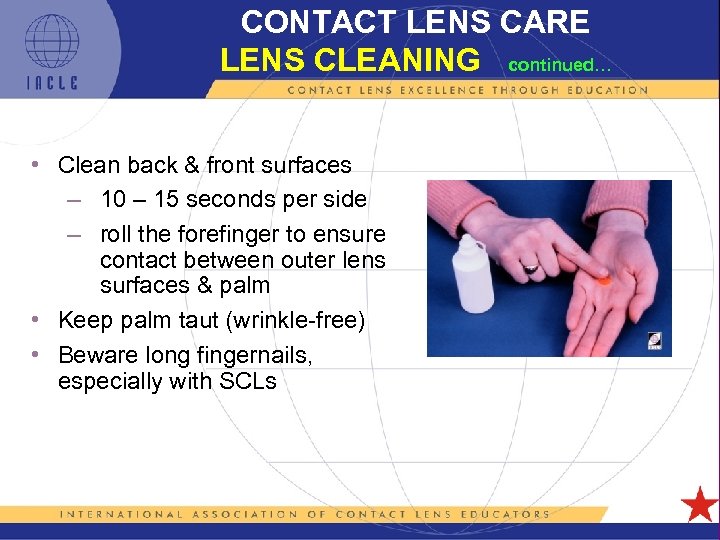 CONTACT LENS CARE LENS CLEANING continued… • Clean back & front surfaces – 10 – 15 seconds per side – roll the forefinger to ensure contact between outer lens surfaces & palm • Keep palm taut (wrinkle-free) • Beware long fingernails, especially with SCLs 5 L 1 -38
CONTACT LENS CARE LENS CLEANING continued… • Clean back & front surfaces – 10 – 15 seconds per side – roll the forefinger to ensure contact between outer lens surfaces & palm • Keep palm taut (wrinkle-free) • Beware long fingernails, especially with SCLs 5 L 1 -38
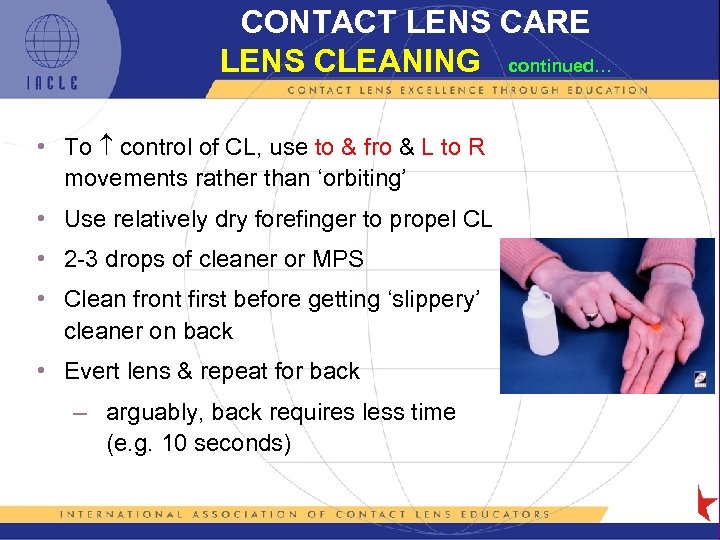 CONTACT LENS CARE LENS CLEANING continued… • To control of CL, use to & fro & L to R movements rather than ‘orbiting’ • Use relatively dry forefinger to propel CL • 2 -3 drops of cleaner or MPS • Clean front first before getting ‘slippery’ cleaner on back • Evert lens & repeat for back – arguably, back requires less time (e. g. 10 seconds) 5 L 1 -39
CONTACT LENS CARE LENS CLEANING continued… • To control of CL, use to & fro & L to R movements rather than ‘orbiting’ • Use relatively dry forefinger to propel CL • 2 -3 drops of cleaner or MPS • Clean front first before getting ‘slippery’ cleaner on back • Evert lens & repeat for back – arguably, back requires less time (e. g. 10 seconds) 5 L 1 -39
 CONTACT LENS CARE LENS RINSING • Rinse thoroughly using jet of sterile saline or MPS • Rub lens with forefinger to assist the removal of cleaner & loosened lens contaminants • Both lens surfaces should wet completely • No contaminants should be visible 5 L 1 -40
CONTACT LENS CARE LENS RINSING • Rinse thoroughly using jet of sterile saline or MPS • Rub lens with forefinger to assist the removal of cleaner & loosened lens contaminants • Both lens surfaces should wet completely • No contaminants should be visible 5 L 1 -40
 CONTACT LENS CARE LENS RINSING continued… • Lens shape should be normal & undistorted – suggests normal hydration levels • Shake-off excess rinsing solution before installing CLs in lens case • CLs should be rinsed once again after completion of the disinfection step, just prior to reinsertion 5 L 1 -41
CONTACT LENS CARE LENS RINSING continued… • Lens shape should be normal & undistorted – suggests normal hydration levels • Shake-off excess rinsing solution before installing CLs in lens case • CLs should be rinsed once again after completion of the disinfection step, just prior to reinsertion 5 L 1 -41
 CONTACT LENS CARE LENS RINSING Regardless of its quality, tap water has no rôle in the care of contact lenses (it is NOT a sterile product) Equally, bulk, unpreserved normal saline is unsuited to lens care once it has been opened for more than a few hours (wide-mouth bottles are best avoided altogether) 5 L 1 -42
CONTACT LENS CARE LENS RINSING Regardless of its quality, tap water has no rôle in the care of contact lenses (it is NOT a sterile product) Equally, bulk, unpreserved normal saline is unsuited to lens care once it has been opened for more than a few hours (wide-mouth bottles are best avoided altogether) 5 L 1 -42
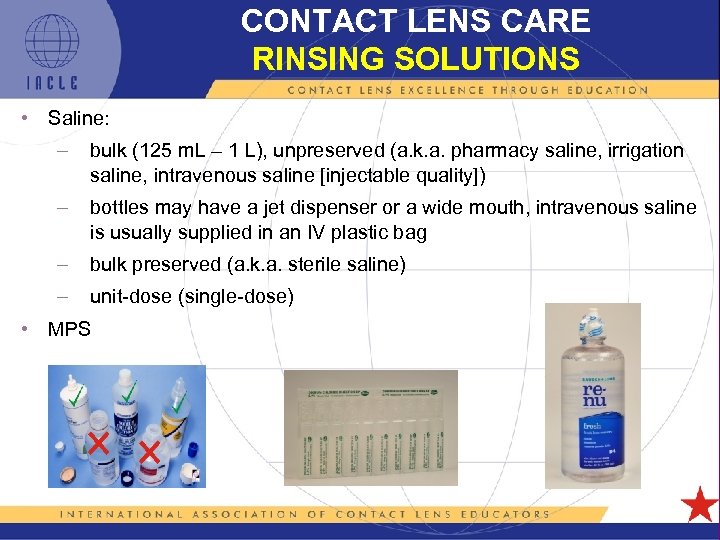 CONTACT LENS CARE RINSING SOLUTIONS • Saline: – bulk (125 m. L – 1 L), unpreserved (a. k. a. pharmacy saline, irrigation saline, intravenous saline [injectable quality]) – bottles may have a jet dispenser or a wide mouth, intravenous saline is usually supplied in an IV plastic bag – bulk preserved (a. k. a. sterile saline) – unit-dose (single-dose) • MPS 5 L 1 -43
CONTACT LENS CARE RINSING SOLUTIONS • Saline: – bulk (125 m. L – 1 L), unpreserved (a. k. a. pharmacy saline, irrigation saline, intravenous saline [injectable quality]) – bottles may have a jet dispenser or a wide mouth, intravenous saline is usually supplied in an IV plastic bag – bulk preserved (a. k. a. sterile saline) – unit-dose (single-dose) • MPS 5 L 1 -43
 CONTACT LENS CARE SALINE SOLUTIONS (Carney et al. , 1990) Property changes in saline solutions can occur with inappropriate storage conditions 5 L 1 -44
CONTACT LENS CARE SALINE SOLUTIONS (Carney et al. , 1990) Property changes in saline solutions can occur with inappropriate storage conditions 5 L 1 -44
 CONTACT LENS CARE ANTIMICROBIAL ACTIVITY After Wikipedia @ 2009 -Apr-23 Levels of efficacy: • Sterilization: Any process that kills or eliminates transmissible agents • Disinfection: The killing and/or the removal of some or all resident pathogenic organisms • Preservation: The killing, and/or the inhibition of growth, of selected micro-organisms 5 L 1 -45
CONTACT LENS CARE ANTIMICROBIAL ACTIVITY After Wikipedia @ 2009 -Apr-23 Levels of efficacy: • Sterilization: Any process that kills or eliminates transmissible agents • Disinfection: The killing and/or the removal of some or all resident pathogenic organisms • Preservation: The killing, and/or the inhibition of growth, of selected micro-organisms 5 L 1 -45
 ANTIMICROBIAL AGENTS MODES OF ACTION • Damage cell membrane • Damage cyst wall (encysted species) • Damage outer cytoplasmic membrane (bacteria) or plasma membrane (fungi) • Interference with synthesis of cell wall peptidoglycans • Alteration/binding/damage/interference with DNA • Inhibition of synthesis of folic acid, nucleotides, or protein • Dehydration of cell • Induction of autolysis • Potentiation of actions of co-located antimicrobials 5 L 1 -46
ANTIMICROBIAL AGENTS MODES OF ACTION • Damage cell membrane • Damage cyst wall (encysted species) • Damage outer cytoplasmic membrane (bacteria) or plasma membrane (fungi) • Interference with synthesis of cell wall peptidoglycans • Alteration/binding/damage/interference with DNA • Inhibition of synthesis of folic acid, nucleotides, or protein • Dehydration of cell • Induction of autolysis • Potentiation of actions of co-located antimicrobials 5 L 1 -46
 CONTACT LENS CARE PRESERVATIVE CHARACTERISTICS • Inhibit all microbial growth • Maintain the number of micro-organisms below a certain (safe) level • Act as a LCP ‘defence system’ • Must be: – compatible with ingredients, CLs, & container – non-toxic & non-irritating – stable over time More information on – effective against a broad spectrum PRESERVATION of organisms 5 L 1 -47
CONTACT LENS CARE PRESERVATIVE CHARACTERISTICS • Inhibit all microbial growth • Maintain the number of micro-organisms below a certain (safe) level • Act as a LCP ‘defence system’ • Must be: – compatible with ingredients, CLs, & container – non-toxic & non-irritating – stable over time More information on – effective against a broad spectrum PRESERVATION of organisms 5 L 1 -47
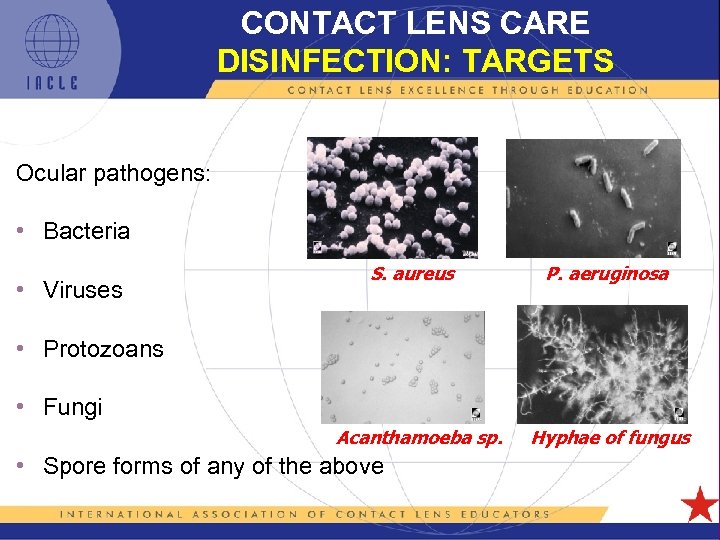 CONTACT LENS CARE DISINFECTION: TARGETS Ocular pathogens: • Bacteria • Viruses S. aureus P. aeruginosa • Protozoans • Fungi Acanthamoeba sp. • Spore forms of any of the above 5 L 1 -48 Hyphae of fungus
CONTACT LENS CARE DISINFECTION: TARGETS Ocular pathogens: • Bacteria • Viruses S. aureus P. aeruginosa • Protozoans • Fungi Acanthamoeba sp. • Spore forms of any of the above 5 L 1 -48 Hyphae of fungus
 CONTACT LENS CARE DISINFECTION: NEED Contact lenses may compromise the eye’s natural defence by: • Inhibiting tear film’s washing action • Introducing more micro-organisms via: – fingers – solutions – environment • Compromising the epithelial barrier function 5 L 1 -49
CONTACT LENS CARE DISINFECTION: NEED Contact lenses may compromise the eye’s natural defence by: • Inhibiting tear film’s washing action • Introducing more micro-organisms via: – fingers – solutions – environment • Compromising the epithelial barrier function 5 L 1 -49
 CONTACT LENS CARE CHARACTERISTICS OF A DISINFECTANT • Kills or deactivates potentially pathogenic organisms remaining on CLs • Rehydrates CLs (GP, SCL, & Si. Hy) & maintain CL hydration subsequently • Maintain microbial safety during storage • Must be: – compatible with LCP ingredients, CLs, & container – non-toxic & non-irritating to the eye – stable over time 5 L 1 -50
CONTACT LENS CARE CHARACTERISTICS OF A DISINFECTANT • Kills or deactivates potentially pathogenic organisms remaining on CLs • Rehydrates CLs (GP, SCL, & Si. Hy) & maintain CL hydration subsequently • Maintain microbial safety during storage • Must be: – compatible with LCP ingredients, CLs, & container – non-toxic & non-irritating to the eye – stable over time 5 L 1 -50
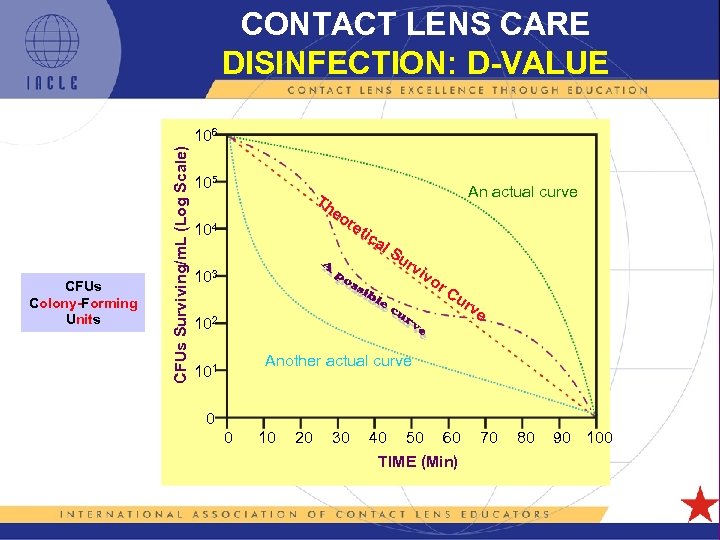 CONTACT LENS CARE DISINFECTION: D-VALUE CFUs Colony-Forming Units CFUs Surviving/m. L (Log Scale) 106 105 Th 104 An actual curve eo re tic al 103 Su rv iv or Cu rv 102 e Another actual curve 101 0 0 10 20 30 40 50 60 TIME (Min) 5 L 1 -51 70 80 90 100
CONTACT LENS CARE DISINFECTION: D-VALUE CFUs Colony-Forming Units CFUs Surviving/m. L (Log Scale) 106 105 Th 104 An actual curve eo re tic al 103 Su rv iv or Cu rv 102 e Another actual curve 101 0 0 10 20 30 40 50 60 TIME (Min) 5 L 1 -51 70 80 90 100
![CONTACT LENS CARE DISINFECTION: D-VALUE CFUs Surviving/m. L [Log Scale] 106 (about 16 min CONTACT LENS CARE DISINFECTION: D-VALUE CFUs Surviving/m. L [Log Scale] 106 (about 16 min](https://present5.com/presentation/c6d5bea17b858d082d0b9fb0af409ad3/image-52.jpg) CONTACT LENS CARE DISINFECTION: D-VALUE CFUs Surviving/m. L [Log Scale] 106 (about 16 min in this case) [a poor disinfectant or a resistant organism? ] 105 104 103 Su rv iv (a 90% reduction in the number of CFUs surviving) or 102 Cu rv e 101 0 0 10 20 30 40 50 60 70 TIME (Min) [Linear Scale] 5 L 1 -52 80 90 100 1 Log = 90% 2 Log = 99% 3 Log = 99. 9% 4 Log = 99. 99% 5 Log = 99. 999% If the inoculum is large, these figures become less impressive
CONTACT LENS CARE DISINFECTION: D-VALUE CFUs Surviving/m. L [Log Scale] 106 (about 16 min in this case) [a poor disinfectant or a resistant organism? ] 105 104 103 Su rv iv (a 90% reduction in the number of CFUs surviving) or 102 Cu rv e 101 0 0 10 20 30 40 50 60 70 TIME (Min) [Linear Scale] 5 L 1 -52 80 90 100 1 Log = 90% 2 Log = 99% 3 Log = 99. 9% 4 Log = 99. 99% 5 Log = 99. 999% If the inoculum is large, these figures become less impressive
 CONTACT LENS CARE FACTORS AFFECTING DISINFECTION • Microbial population size • Microbial growth conditions • Concentration of disinfectant • Environmental factors • Microbial resistance (natural & acquired) 5 L 1 -53
CONTACT LENS CARE FACTORS AFFECTING DISINFECTION • Microbial population size • Microbial growth conditions • Concentration of disinfectant • Environmental factors • Microbial resistance (natural & acquired) 5 L 1 -53
 CONTACT LENS CARE DISINFECTION: STANDARDS ISO 14729: Test Panel (TP) of Organisms • Fungi: – Candida albicans ATCC 10231 – Fusarium solani ATCC 36031 • Bacteria: – Pseudomonas aeruginosa ATCC 9027 – Serratia marcescens ATCC 13880 – Staphylococcus aureus ATCC 6538 Note: Currently (2011), Acanthamoeba spp. are not featured in the Test Panel. Therefore, there is no mandated requirement that a solution or product needs to meet in regard to Acanthamoeba spp. This may change following discussions between 2008 & 2010 5 L 1 -54
CONTACT LENS CARE DISINFECTION: STANDARDS ISO 14729: Test Panel (TP) of Organisms • Fungi: – Candida albicans ATCC 10231 – Fusarium solani ATCC 36031 • Bacteria: – Pseudomonas aeruginosa ATCC 9027 – Serratia marcescens ATCC 13880 – Staphylococcus aureus ATCC 6538 Note: Currently (2011), Acanthamoeba spp. are not featured in the Test Panel. Therefore, there is no mandated requirement that a solution or product needs to meet in regard to Acanthamoeba spp. This may change following discussions between 2008 & 2010 5 L 1 -54
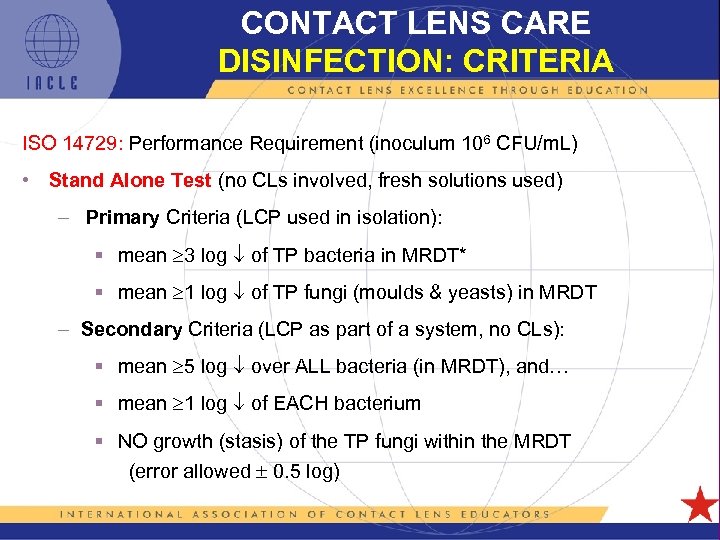 CONTACT LENS CARE DISINFECTION: CRITERIA ISO 14729: Performance Requirement (inoculum 106 CFU/m. L) • Stand Alone Test (no CLs involved, fresh solutions used) – Primary Criteria (LCP used in isolation): § mean 3 log of TP bacteria in MRDT* § mean 1 log of TP fungi (moulds & yeasts) in MRDT – Secondary Criteria (LCP as part of a system, no CLs): § mean 5 log over ALL bacteria (in MRDT), and… § mean 1 log of EACH bacterium § NO growth (stasis) of the TP fungi within the MRDT (error allowed 0. 5 log) 5 L 1 -55
CONTACT LENS CARE DISINFECTION: CRITERIA ISO 14729: Performance Requirement (inoculum 106 CFU/m. L) • Stand Alone Test (no CLs involved, fresh solutions used) – Primary Criteria (LCP used in isolation): § mean 3 log of TP bacteria in MRDT* § mean 1 log of TP fungi (moulds & yeasts) in MRDT – Secondary Criteria (LCP as part of a system, no CLs): § mean 5 log over ALL bacteria (in MRDT), and… § mean 1 log of EACH bacterium § NO growth (stasis) of the TP fungi within the MRDT (error allowed 0. 5 log) 5 L 1 -55
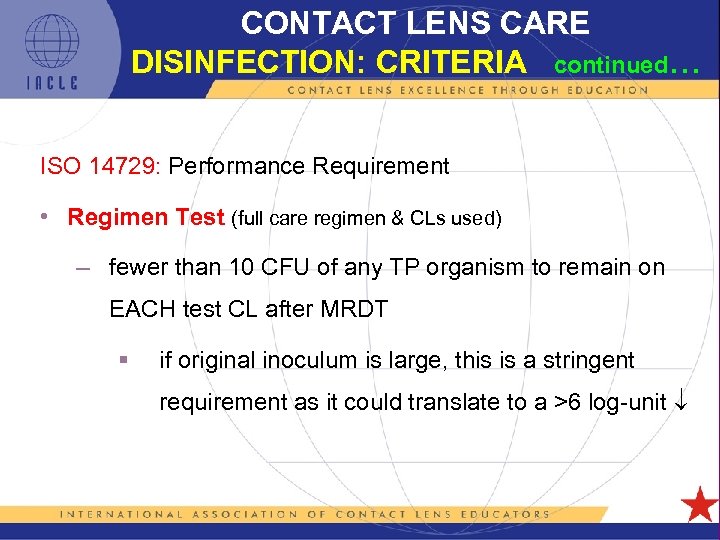 CONTACT LENS CARE DISINFECTION: CRITERIA continued… ISO 14729: Performance Requirement • Regimen Test (full care regimen & CLs used) – fewer than 10 CFU of any TP organism to remain on EACH test CL after MRDT § if original inoculum is large, this is a stringent requirement as it could translate to a >6 log-unit 5 L 1 -56
CONTACT LENS CARE DISINFECTION: CRITERIA continued… ISO 14729: Performance Requirement • Regimen Test (full care regimen & CLs used) – fewer than 10 CFU of any TP organism to remain on EACH test CL after MRDT § if original inoculum is large, this is a stringent requirement as it could translate to a >6 log-unit 5 L 1 -56
 CONTACT LENS CARE DISINFECTION METHODS • Heat (thermal unit, microwave oven, other) – now historic interest only • Chemical - oxidative (e. g. hydrogen peroxide) - cold chemical - various disinfectants 5 L 1 -57
CONTACT LENS CARE DISINFECTION METHODS • Heat (thermal unit, microwave oven, other) – now historic interest only • Chemical - oxidative (e. g. hydrogen peroxide) - cold chemical - various disinfectants 5 L 1 -57
 CONTACT LENS CARE A BALANCE: EFFICACY vs TOXICITY • Strong preservatives/disinfectants used only in products that NEVER come in contact with the eye, e. g. CL cleaners • MPSs must balance efficacy & toxicity – CLs carry MPSs into eye • disinfection regulations difficulty balancing these competing factors • So-called ‘disappearing’ disinfectants/preservatives used in some in-eye preparations & a LCP – they are not strong disinfectants 5 L 1 -58
CONTACT LENS CARE A BALANCE: EFFICACY vs TOXICITY • Strong preservatives/disinfectants used only in products that NEVER come in contact with the eye, e. g. CL cleaners • MPSs must balance efficacy & toxicity – CLs carry MPSs into eye • disinfection regulations difficulty balancing these competing factors • So-called ‘disappearing’ disinfectants/preservatives used in some in-eye preparations & a LCP – they are not strong disinfectants 5 L 1 -58
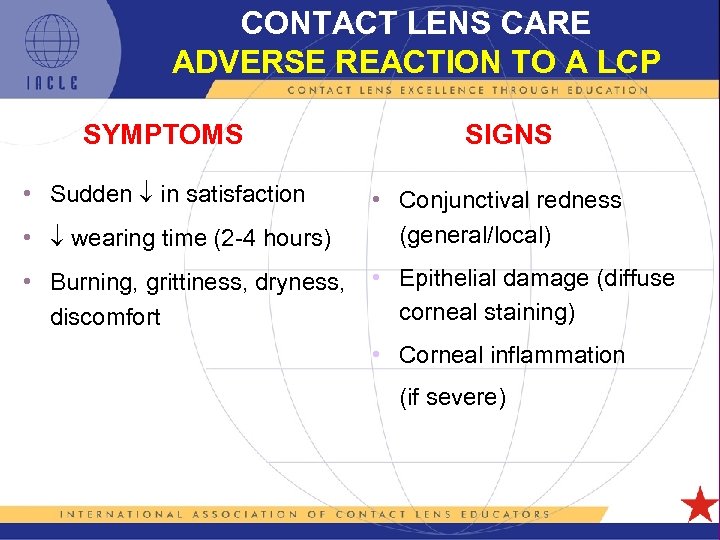 CONTACT LENS CARE ADVERSE REACTION TO A LCP SYMPTOMS SIGNS • Sudden in satisfaction • wearing time (2 -4 hours) • Conjunctival redness (general/local) • Burning, grittiness, dryness, discomfort • Epithelial damage (diffuse corneal staining) • Corneal inflammation (if severe) 5 L 1 -59
CONTACT LENS CARE ADVERSE REACTION TO A LCP SYMPTOMS SIGNS • Sudden in satisfaction • wearing time (2 -4 hours) • Conjunctival redness (general/local) • Burning, grittiness, dryness, discomfort • Epithelial damage (diffuse corneal staining) • Corneal inflammation (if severe) 5 L 1 -59
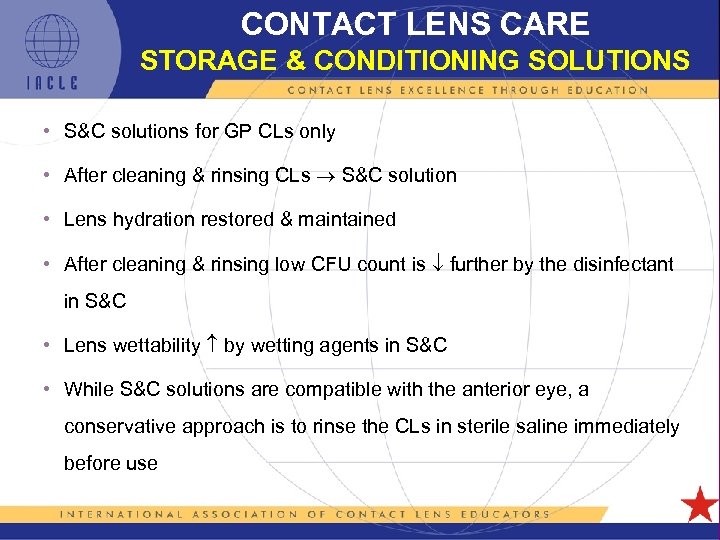 CONTACT LENS CARE STORAGE & CONDITIONING SOLUTIONS • S&C solutions for GP CLs only • After cleaning & rinsing CLs S&C solution • Lens hydration restored & maintained • After cleaning & rinsing low CFU count is further by the disinfectant in S&C • Lens wettability by wetting agents in S&C • While S&C solutions are compatible with the anterior eye, a conservative approach is to rinse the CLs in sterile saline immediately before use 5 L 1 -60
CONTACT LENS CARE STORAGE & CONDITIONING SOLUTIONS • S&C solutions for GP CLs only • After cleaning & rinsing CLs S&C solution • Lens hydration restored & maintained • After cleaning & rinsing low CFU count is further by the disinfectant in S&C • Lens wettability by wetting agents in S&C • While S&C solutions are compatible with the anterior eye, a conservative approach is to rinse the CLs in sterile saline immediately before use 5 L 1 -60
 CONTACT LENS CARE WETTING SOLUTIONS • GP CLs only • Solution is applied to GP CLs immediately before insertion • Solution contains: – surfactants § lens surface tension & lens wettability – viscosity-increasing agent(s) § cushions lens on insertion & lens lubricity § comfort on insertion • Preserved unless unit-dose (single-dose) • Rôle largely overtaken by S&C solutions or MPSs • Few products available currently 5 L 1 -61
CONTACT LENS CARE WETTING SOLUTIONS • GP CLs only • Solution is applied to GP CLs immediately before insertion • Solution contains: – surfactants § lens surface tension & lens wettability – viscosity-increasing agent(s) § cushions lens on insertion & lens lubricity § comfort on insertion • Preserved unless unit-dose (single-dose) • Rôle largely overtaken by S&C solutions or MPSs • Few products available currently 5 L 1 -61
 CONTACT LENS CARE PROTEIN REMOVAL • Targets tear film proteins, especially lysozyme • Most are proteolytic enzymes – some are inorganic • Some used separately, others suitable for use in MPSs or 1 -step peroxide systems • Some are peroxide-specific (compatible with H 2 O 2 & its low p. H) • Unnecessary for lenses discarded frequently 5 L 1 -62
CONTACT LENS CARE PROTEIN REMOVAL • Targets tear film proteins, especially lysozyme • Most are proteolytic enzymes – some are inorganic • Some used separately, others suitable for use in MPSs or 1 -step peroxide systems • Some are peroxide-specific (compatible with H 2 O 2 & its low p. H) • Unnecessary for lenses discarded frequently 5 L 1 -62
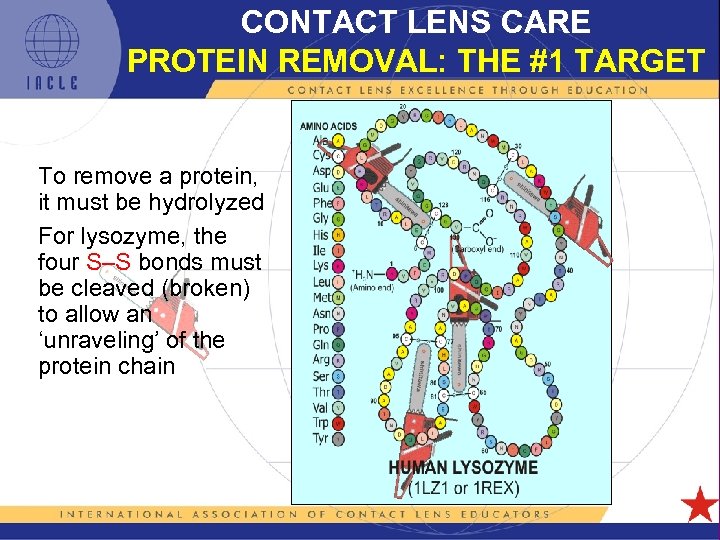 CONTACT LENS CARE PROTEIN REMOVAL: THE #1 TARGET To remove a protein, it must be hydrolyzed For lysozyme, the four S–S bonds must be cleaved (broken) to allow an ‘unraveling’ of the protein chain 5 L 1 -63
CONTACT LENS CARE PROTEIN REMOVAL: THE #1 TARGET To remove a protein, it must be hydrolyzed For lysozyme, the four S–S bonds must be cleaved (broken) to allow an ‘unraveling’ of the protein chain 5 L 1 -63
 CONTACT LENS CARE PROTEIN REMOVAL • Used periodically (typically weekly) – after clean & rinse, or… – concurrently in 1 -step care system • Heavy depositors require treatment frequency, especially group IV hydrogels – consider replacing CLs more often • CLs must be re-cleaned & re-rinsed after protein cleaning (ocular exposure to enzyme undesirable) 5 L 1 -64
CONTACT LENS CARE PROTEIN REMOVAL • Used periodically (typically weekly) – after clean & rinse, or… – concurrently in 1 -step care system • Heavy depositors require treatment frequency, especially group IV hydrogels – consider replacing CLs more often • CLs must be re-cleaned & re-rinsed after protein cleaning (ocular exposure to enzyme undesirable) 5 L 1 -64
 CONTACT LENS CARE PUT SIMPLY • Attain a ‘ready-to-wear’ state • Maintain that ‘ready-to-wear’ state 5 L 1 -65
CONTACT LENS CARE PUT SIMPLY • Attain a ‘ready-to-wear’ state • Maintain that ‘ready-to-wear’ state 5 L 1 -65
 CONTACT LENS CARE RE-WETTING DROPS/LUBRICANTS In-eye products: • Soothe dry, irritated, tired, or sensitive eyes • Preservative-free (unit-dose) or low-irritant preservative • Alleviate discomfort insufficiently lubricious tears – or insufficient tear volume • Alleviate dryness towards end of CL wear • Rehydrate CLs in situ • Flush/clean debris from lens surfaces & eye • tear protein denaturation & surface deposition • Viscous fluid envelope cushions CLs in situ 5 L 1 -66
CONTACT LENS CARE RE-WETTING DROPS/LUBRICANTS In-eye products: • Soothe dry, irritated, tired, or sensitive eyes • Preservative-free (unit-dose) or low-irritant preservative • Alleviate discomfort insufficiently lubricious tears – or insufficient tear volume • Alleviate dryness towards end of CL wear • Rehydrate CLs in situ • Flush/clean debris from lens surfaces & eye • tear protein denaturation & surface deposition • Viscous fluid envelope cushions CLs in situ 5 L 1 -66
 CONTACT LENS CARE RE-WETTING DROPS/LUBRICANTS Challenges: (after Tonge et al. , 2001) • Rapid fluid loss (patent tear drainage system) short anterior eye stay-time (short residency) – solution viscosity § drainage § absorption by conjunctival vasculature – alternatively, incorporating a mucoadhesive polymer may stay-time 5 L 1 -67
CONTACT LENS CARE RE-WETTING DROPS/LUBRICANTS Challenges: (after Tonge et al. , 2001) • Rapid fluid loss (patent tear drainage system) short anterior eye stay-time (short residency) – solution viscosity § drainage § absorption by conjunctival vasculature – alternatively, incorporating a mucoadhesive polymer may stay-time 5 L 1 -67
 CONTACT LENS CARE RE-WETTING DROPS/LUBRICANTS • Narrow & stable range of tear p. H • Cornea has low permeability to most solution constituents • If preserved, – tear stability, tear BUT & corneal and conjunctival cell integrity may be affected adversely – dry eye symptoms can be induced 5 L 1 -68
CONTACT LENS CARE RE-WETTING DROPS/LUBRICANTS • Narrow & stable range of tear p. H • Cornea has low permeability to most solution constituents • If preserved, – tear stability, tear BUT & corneal and conjunctival cell integrity may be affected adversely – dry eye symptoms can be induced 5 L 1 -68
 CONTACT LENS CARE LUBRICANTS: DO THEY WORK? “Neither lubricant tested was found to be significantly (Efron et al. , 1990) superior to saline” • Has the situation improved? – formulations are now more complex & sophisticated with claims of performance – Si. Hy CL wearers may benefit (see Notes) • Regular use can exacerbate rather than alleviate the original problem • Avoid using in-eye products just before CL removal (makes CLs slippery, & if hypertonic, tighter fitting) 5 L 1 -69
CONTACT LENS CARE LUBRICANTS: DO THEY WORK? “Neither lubricant tested was found to be significantly (Efron et al. , 1990) superior to saline” • Has the situation improved? – formulations are now more complex & sophisticated with claims of performance – Si. Hy CL wearers may benefit (see Notes) • Regular use can exacerbate rather than alleviate the original problem • Avoid using in-eye products just before CL removal (makes CLs slippery, & if hypertonic, tighter fitting) 5 L 1 -69
 CONTACT LENS CARE RE-WETTING DROPS/LUBRICANTS Does the need for these products actually overlap: • An underlying ocular condition? or • Ocular surface disease, e. g. dry eye? 5 L 1 -70
CONTACT LENS CARE RE-WETTING DROPS/LUBRICANTS Does the need for these products actually overlap: • An underlying ocular condition? or • Ocular surface disease, e. g. dry eye? 5 L 1 -70
 CONTACT LENS CARE EPHEMERA Many gadgets, ‘innovations’, novel ideas, resurrection of old ideas, etc. appear regularly, few survive. The reasons for failure include: • Does not work or not sufficiently efficacious • Product is actually flawed or even dangerous • Too expensive • Failed to get regulatory approval • Research studies reach adverse conclusions • Competitor product wins marketing war • Supplying company fails • Key component(s) no longer available • Product’s function becomes redundant 5 L 1 -71 Planetary-gear devices (swishers)
CONTACT LENS CARE EPHEMERA Many gadgets, ‘innovations’, novel ideas, resurrection of old ideas, etc. appear regularly, few survive. The reasons for failure include: • Does not work or not sufficiently efficacious • Product is actually flawed or even dangerous • Too expensive • Failed to get regulatory approval • Research studies reach adverse conclusions • Competitor product wins marketing war • Supplying company fails • Key component(s) no longer available • Product’s function becomes redundant 5 L 1 -71 Planetary-gear devices (swishers)
 CONTACT LENS CARE COMPLIANCE Mc. Monnies (1988): With new wearers, compliance problems may start at the delivery visit Radford et al. (1993): After instruction & suitable reinstruction, an 85% compliance rate is achievable Claydon et al. (1996): In a reasonably compliant group of wearers, additional education had no significant effect on compliance levels Overall compliance with lens care instructions is 40% to 74% 5 L 1 -72
CONTACT LENS CARE COMPLIANCE Mc. Monnies (1988): With new wearers, compliance problems may start at the delivery visit Radford et al. (1993): After instruction & suitable reinstruction, an 85% compliance rate is achievable Claydon et al. (1996): In a reasonably compliant group of wearers, additional education had no significant effect on compliance levels Overall compliance with lens care instructions is 40% to 74% 5 L 1 -72
 CONTACT LENS CARE NON-COMPLIANCE Non-Compliance: • Failure to return for after-care • Not adhering to prescribed CL wearing schedule • life of CLs >recommended • Failure to understand the requirements for, or significance of, the various lens care steps • Skipping the cleaning and/or disinfection steps • Using quantities of LCPs than recommended • Poor general hygiene • Failure to clean or replace lens case (Woods et al. , 2010) • Not following manufacturers’ instructions (lenses, LCPs) 5 L 1 -73
CONTACT LENS CARE NON-COMPLIANCE Non-Compliance: • Failure to return for after-care • Not adhering to prescribed CL wearing schedule • life of CLs >recommended • Failure to understand the requirements for, or significance of, the various lens care steps • Skipping the cleaning and/or disinfection steps • Using quantities of LCPs than recommended • Poor general hygiene • Failure to clean or replace lens case (Woods et al. , 2010) • Not following manufacturers’ instructions (lenses, LCPs) 5 L 1 -73
 CONTACT LENS CARE NON-COMPLIANCE • Non-compliance is probably the greatest source of CLrelated complications and is a barrier to long-term successful CL wear • Lowest reported rate is 26% • Highest rates reported: – 60% - 85% • Importantly, non-compliance complication rates 5 L 1 -74
CONTACT LENS CARE NON-COMPLIANCE • Non-compliance is probably the greatest source of CLrelated complications and is a barrier to long-term successful CL wear • Lowest reported rate is 26% • Highest rates reported: – 60% - 85% • Importantly, non-compliance complication rates 5 L 1 -74
 CONTACT LENS CARE NON-COMPLIANCE • 70% non-compliance rate was reported among 215 microbial keratitis (MK) cases (Abry et al. , 2010) – 96% were wearing disposable CLs – 30% were Si. Hy CLs – only 45% of MK patients rubbed & rinsed CLs • Wearer perceptions indicator of behaviour: – 86% rated themselves compliant, only 76% were good or average ( 24% non-compliant) – 80% were risk aware but this had no effect on negative behaviour (Bui et al. , 2010) 5 L 1 -75
CONTACT LENS CARE NON-COMPLIANCE • 70% non-compliance rate was reported among 215 microbial keratitis (MK) cases (Abry et al. , 2010) – 96% were wearing disposable CLs – 30% were Si. Hy CLs – only 45% of MK patients rubbed & rinsed CLs • Wearer perceptions indicator of behaviour: – 86% rated themselves compliant, only 76% were good or average ( 24% non-compliant) – 80% were risk aware but this had no effect on negative behaviour (Bui et al. , 2010) 5 L 1 -75
 CONTACT LENS CARE FACTORS AFFECTING COMPLIANCE Positively: • Patient education • Practitioner attitudes & communication skills • Use & supply of clear, illustrated instructions • Simplicity of the lens care system • Review procedures at every opportunity 5 L 1 -76
CONTACT LENS CARE FACTORS AFFECTING COMPLIANCE Positively: • Patient education • Practitioner attitudes & communication skills • Use & supply of clear, illustrated instructions • Simplicity of the lens care system • Review procedures at every opportunity 5 L 1 -76
 CONTACT LENS CARE FACTORS AFFECTING COMPLIANCE Negatively: • Poor or no education from the practitioner – failure of patient/practitioner ‘partnership’ • Being <30 & using CLs for cosmesis or convenience • Being 10 -30 or >50 or with more than 2 years of lenswearing experience • Differing advice from different practices • Being a risk taker 5 L 1 -77
CONTACT LENS CARE FACTORS AFFECTING COMPLIANCE Negatively: • Poor or no education from the practitioner – failure of patient/practitioner ‘partnership’ • Being <30 & using CLs for cosmesis or convenience • Being 10 -30 or >50 or with more than 2 years of lenswearing experience • Differing advice from different practices • Being a risk taker 5 L 1 -77
 CONTACT LENS CARE FACTORS AFFECTING COMPLIANCE (after Shannon, 1987) Negatively: • Complexity of the procedures recommended • Length of time required to perform prescribed tasks • Cost of the regimen • Poor understanding of instructions • Poor, or no, patient-practitioner relationship 5 L 1 -78
CONTACT LENS CARE FACTORS AFFECTING COMPLIANCE (after Shannon, 1987) Negatively: • Complexity of the procedures recommended • Length of time required to perform prescribed tasks • Cost of the regimen • Poor understanding of instructions • Poor, or no, patient-practitioner relationship 5 L 1 -78
 CONTACT LENS CARE FACTORS AFFECTING COMPLIANCE Negatively: • Wearer ‘discovering’ a more ‘convenient’ way or an ‘easier’ way • Laziness • Erroneous information provided by well-intentioned, ‘helpful’ friends • Following instructions correctly for different, irrelevant, LCP and/or CLs • The awe-struck wearer who is afraid to question the practitioner or confirm what they think they heard • Inappropriate practitioner attitudes that act as barriers to communication 5 L 1 -79
CONTACT LENS CARE FACTORS AFFECTING COMPLIANCE Negatively: • Wearer ‘discovering’ a more ‘convenient’ way or an ‘easier’ way • Laziness • Erroneous information provided by well-intentioned, ‘helpful’ friends • Following instructions correctly for different, irrelevant, LCP and/or CLs • The awe-struck wearer who is afraid to question the practitioner or confirm what they think they heard • Inappropriate practitioner attitudes that act as barriers to communication 5 L 1 -79
 CONTACT LENS CARE FACTORS NOT AFFECTING COMPLIANCE • Additional education (esp. if level of compliance is relatively high already) (Claydon et al. , 1996, 1997) • Shannon (1987): – age (other studies suggest age is a factor) – wearer’s sex – occupation – perception of threat or consequence of disease – race – highest education level achieved – socio-economic group 5 L 1 -80
CONTACT LENS CARE FACTORS NOT AFFECTING COMPLIANCE • Additional education (esp. if level of compliance is relatively high already) (Claydon et al. , 1996, 1997) • Shannon (1987): – age (other studies suggest age is a factor) – wearer’s sex – occupation – perception of threat or consequence of disease – race – highest education level achieved – socio-economic group 5 L 1 -80
 CONTACT LENS CARE OTHER COMPLIANCE ISSUES • What to do if a red eye is noted? • Swimming in CLs against advice • CL storage if not used for extended periods (should they be stored at all? ) • Mixing & matching LCPs - different systems, different manufacturers • Being swayed from prescribed products/procedures by third parties (other professionals, e. g. pharmacists) 5 L 1 -81
CONTACT LENS CARE OTHER COMPLIANCE ISSUES • What to do if a red eye is noted? • Swimming in CLs against advice • CL storage if not used for extended periods (should they be stored at all? ) • Mixing & matching LCPs - different systems, different manufacturers • Being swayed from prescribed products/procedures by third parties (other professionals, e. g. pharmacists) 5 L 1 -81
 CONTACT LENS CARE OTHER COMPLIANCE ISSUES • Defaulting to ‘No Rub’ when ‘Rub’ was prescribed – ‘corner cutting’, laziness/haste, ignorance (after Woods et al. , 2010) • Re-use and/or topping-up (topping-off) of remaining spent solution is a flawed pursuit & a false economy – the Fusarium spp. issue of 2006 – the Acanthamoeba spp. issue of 2007 — use of tap water was also implicated 5 L 1 -82
CONTACT LENS CARE OTHER COMPLIANCE ISSUES • Defaulting to ‘No Rub’ when ‘Rub’ was prescribed – ‘corner cutting’, laziness/haste, ignorance (after Woods et al. , 2010) • Re-use and/or topping-up (topping-off) of remaining spent solution is a flawed pursuit & a false economy – the Fusarium spp. issue of 2006 – the Acanthamoeba spp. issue of 2007 — use of tap water was also implicated 5 L 1 -82
 CONTACT LENS CARE OTHER COMPLIANCE ISSUES continued… Convenience of a system may determine: • Patient compliance • Frequency of lens wear (intermittent? ) • Patient satisfaction • Continued use of recommended care system components (a more convenient alternative? ) 5 L 1 -83
CONTACT LENS CARE OTHER COMPLIANCE ISSUES continued… Convenience of a system may determine: • Patient compliance • Frequency of lens wear (intermittent? ) • Patient satisfaction • Continued use of recommended care system components (a more convenient alternative? ) 5 L 1 -83
 CONTACT LENS CARE FDA RECOMMENDATIONS 2010 • Follow recommended wearing schedule • Do not substitute sterile saline for MPSs • Rub & rinse CLs as directed by your eye care professional (ECP) • Do not ‘top-up’ (top-off) solutions in your case. Always discard all of the leftover solution after each use. Never reuse any lens solution • Clean, rinse, & air-dry your lens case (upside-down) each time CLs removed • Do not expose your CLs to water: tap, bottled, distilled, lake, ocean, etc. • Contact your ECP if symptoms of eye irritation or infection occur 5 L 1 -84
CONTACT LENS CARE FDA RECOMMENDATIONS 2010 • Follow recommended wearing schedule • Do not substitute sterile saline for MPSs • Rub & rinse CLs as directed by your eye care professional (ECP) • Do not ‘top-up’ (top-off) solutions in your case. Always discard all of the leftover solution after each use. Never reuse any lens solution • Clean, rinse, & air-dry your lens case (upside-down) each time CLs removed • Do not expose your CLs to water: tap, bottled, distilled, lake, ocean, etc. • Contact your ECP if symptoms of eye irritation or infection occur 5 L 1 -84
 CONTACT LENS CARE LENS STORAGE & LENS CASE CARE Contact lenses should be stored in: • A clean contact lens storage case • Fresh disinfecting solution Lens case care is an important but often ignored aspect of contact lens care Lens cases are a potential source of lens recontamination Microbial contaminants create biofilms in lens cases for self-protection 5 L 1 -85
CONTACT LENS CARE LENS STORAGE & LENS CASE CARE Contact lenses should be stored in: • A clean contact lens storage case • Fresh disinfecting solution Lens case care is an important but often ignored aspect of contact lens care Lens cases are a potential source of lens recontamination Microbial contaminants create biofilms in lens cases for self-protection 5 L 1 -85
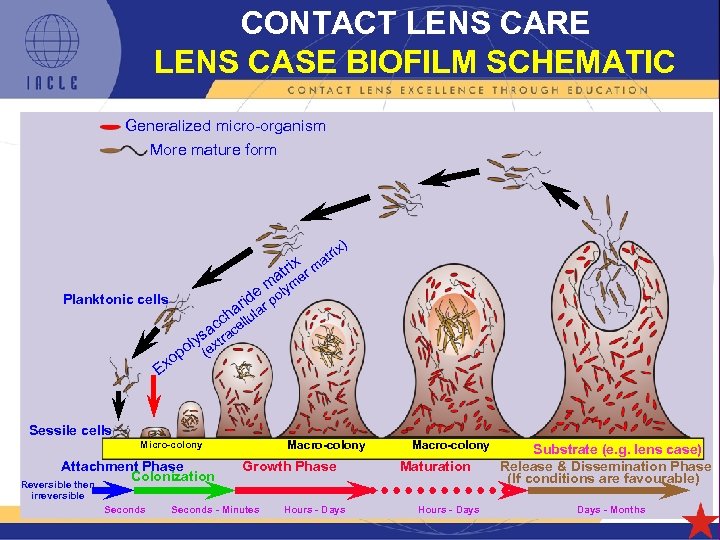 CONTACT LENS CARE LENS CASE BIOFILM SCHEMATIC Generalized micro-organism More mature form x) ri at rix m at er m lym e o rid ar p a Planktonic cells h l cc cellu a o Ex s ra oly (ext p Sessile cells Macro-colony Micro-colony Attachment Phase Colonization Reversible then Growth Phase Macro-colony Maturation Substrate (e. g. lens case) Release & Dissemination Phase (If conditions are favourable) irreversible Seconds - Minutes Hours - Days 5 L 1 -86 Hours - Days - Months
CONTACT LENS CARE LENS CASE BIOFILM SCHEMATIC Generalized micro-organism More mature form x) ri at rix m at er m lym e o rid ar p a Planktonic cells h l cc cellu a o Ex s ra oly (ext p Sessile cells Macro-colony Micro-colony Attachment Phase Colonization Reversible then Growth Phase Macro-colony Maturation Substrate (e. g. lens case) Release & Dissemination Phase (If conditions are favourable) irreversible Seconds - Minutes Hours - Days 5 L 1 -86 Hours - Days - Months
 CONTACT LENS CARE COMPLIANCE & LENS CASE CARE • Lens case hygiene, care, & replacement have been identified as a common compliance issue • More frequent case contamination reported with 1 -step peroxide systems than with 2 -step peroxide or MPSs • Use of tap water at any stage of lens care NOT RECOMMENDED • CL cases became contaminated after just 1 week & moderately to heavily contaminated after 2 weeks – 47% of cases were contaminated with Gram+ bacteria – 21% with Gram– bacteria 5 L 1 -87
CONTACT LENS CARE COMPLIANCE & LENS CASE CARE • Lens case hygiene, care, & replacement have been identified as a common compliance issue • More frequent case contamination reported with 1 -step peroxide systems than with 2 -step peroxide or MPSs • Use of tap water at any stage of lens care NOT RECOMMENDED • CL cases became contaminated after just 1 week & moderately to heavily contaminated after 2 weeks – 47% of cases were contaminated with Gram+ bacteria – 21% with Gram– bacteria 5 L 1 -87
 CONTACT LENS CARE LENS CASE CARE • Rinsing alone is incapable of removing adherent organisms • Hot water & air-drying bacterial contamination • Recommendations: – water temperatures >70°C, or… – unneutralized peroxide for >20 min, or… – bleach (sodium hypochlorite [Na. OCl]) for >20 min and… – periodic scrubbing with dishwashing detergent, bleach, or peroxide & water using a stiff brush to disrupt any biofilm established inside the case & lid, & case/lid screw threads 5 L 1 -88
CONTACT LENS CARE LENS CASE CARE • Rinsing alone is incapable of removing adherent organisms • Hot water & air-drying bacterial contamination • Recommendations: – water temperatures >70°C, or… – unneutralized peroxide for >20 min, or… – bleach (sodium hypochlorite [Na. OCl]) for >20 min and… – periodic scrubbing with dishwashing detergent, bleach, or peroxide & water using a stiff brush to disrupt any biofilm established inside the case & lid, & case/lid screw threads 5 L 1 -88
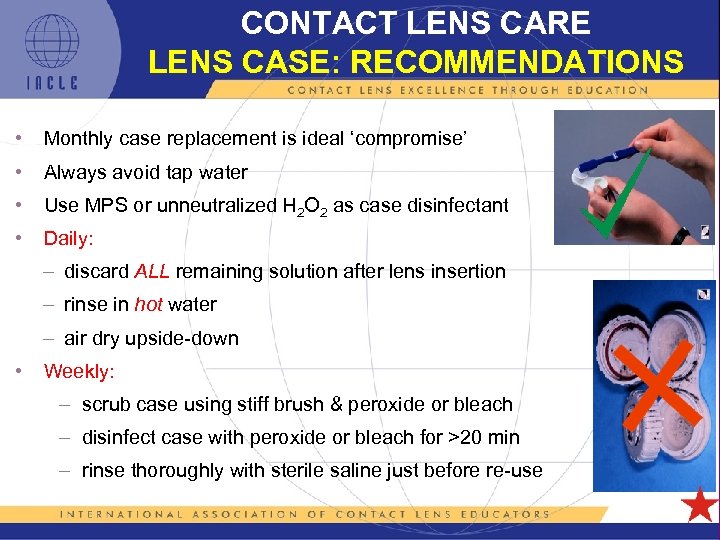 CONTACT LENS CARE LENS CASE: RECOMMENDATIONS • Monthly case replacement is ideal ‘compromise’ • Always avoid tap water • Use MPS or unneutralized H 2 O 2 as case disinfectant • Daily: – discard ALL remaining solution after lens insertion – rinse in hot water – air dry upside-down • Weekly: – scrub case using stiff brush & peroxide or bleach – disinfect case with peroxide or bleach for >20 min – rinse thoroughly with sterile saline just before re-use 5 L 1 -89
CONTACT LENS CARE LENS CASE: RECOMMENDATIONS • Monthly case replacement is ideal ‘compromise’ • Always avoid tap water • Use MPS or unneutralized H 2 O 2 as case disinfectant • Daily: – discard ALL remaining solution after lens insertion – rinse in hot water – air dry upside-down • Weekly: – scrub case using stiff brush & peroxide or bleach – disinfect case with peroxide or bleach for >20 min – rinse thoroughly with sterile saline just before re-use 5 L 1 -89
 CONTACT LENS CARE LENS CASE CARE: OTHER ISSUES • The supply of new lens case with each bottle of solution has met with limited success – some CL wearers are ‘collectors’. Each new case becomes part of a ‘collection’ of unused cases thwarting the original intention – ‘collecting’ is encouraged by cases that are well-designed, attractive, & well finished – conversely, cases made as low-cost, disposable items may be perceived poorly. Functionally, they may also be inadequate, e. g. they might leak • The cost of complex cases, e. g. vented peroxide cases, may make the cost of frequent disposability prohibitive 5 L 1 -90
CONTACT LENS CARE LENS CASE CARE: OTHER ISSUES • The supply of new lens case with each bottle of solution has met with limited success – some CL wearers are ‘collectors’. Each new case becomes part of a ‘collection’ of unused cases thwarting the original intention – ‘collecting’ is encouraged by cases that are well-designed, attractive, & well finished – conversely, cases made as low-cost, disposable items may be perceived poorly. Functionally, they may also be inadequate, e. g. they might leak • The cost of complex cases, e. g. vented peroxide cases, may make the cost of frequent disposability prohibitive 5 L 1 -90
 CONTACT LENS CARE LENS CASE CARE: OTHER ISSUES • In a ‘connected’ world, confusion can result from: – different marketing policies applying in different countries to identical products – identical products marketed under different names and/or packaging – different products marketed under identical names in different countries • Nanotechnology is now being deployed in the CL world – used in some lens cases, e. g. silver (Ag) is in the case polymer & plays anti-microbial & anti-biofilm rôles 5 L 1 -91
CONTACT LENS CARE LENS CASE CARE: OTHER ISSUES • In a ‘connected’ world, confusion can result from: – different marketing policies applying in different countries to identical products – identical products marketed under different names and/or packaging – different products marketed under identical names in different countries • Nanotechnology is now being deployed in the CL world – used in some lens cases, e. g. silver (Ag) is in the case polymer & plays anti-microbial & anti-biofilm rôles 5 L 1 -91
 CONTACT LENS CARE WEAR MODALITY & LENS TYPE Lens Replacement Scheduling: • >12 months – has no place in CL practice • Conventional wear ( 12 months) - discourage • Frequent/programmed replacement (>1 month replacement) • Disposable (1 week to 1 month) • Daily Disposable • Other (as prescribed or by non-compliance) 5 L 1 -92
CONTACT LENS CARE WEAR MODALITY & LENS TYPE Lens Replacement Scheduling: • >12 months – has no place in CL practice • Conventional wear ( 12 months) - discourage • Frequent/programmed replacement (>1 month replacement) • Disposable (1 week to 1 month) • Daily Disposable • Other (as prescribed or by non-compliance) 5 L 1 -92
 CONTACT LENS CARE WEAR MODALITY & LENS TYPE Lens Wear Modalities: • Daily wear (DW) – DD (single-use) – Disposable – Programmed replacement – Conventional • Flexible wear (FW) - disposable • Extended wear (EW) - disposable • Continuous wear (CW) – single-use 5 L 1 -93
CONTACT LENS CARE WEAR MODALITY & LENS TYPE Lens Wear Modalities: • Daily wear (DW) – DD (single-use) – Disposable – Programmed replacement – Conventional • Flexible wear (FW) - disposable • Extended wear (EW) - disposable • Continuous wear (CW) – single-use 5 L 1 -93
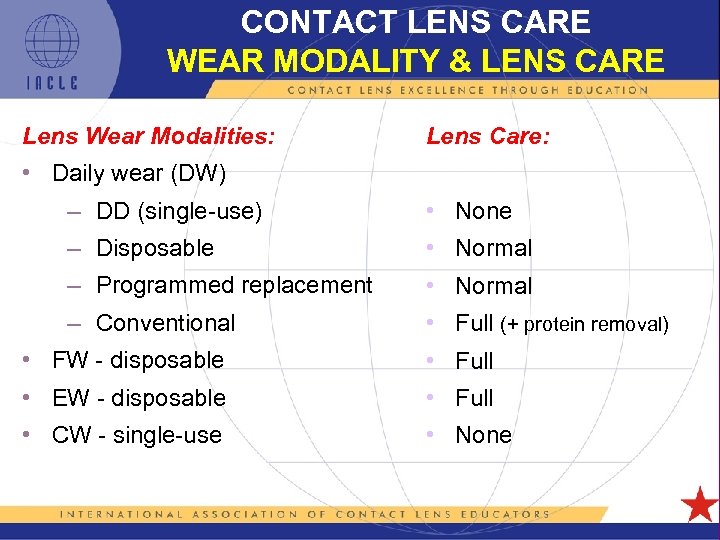 CONTACT LENS CARE WEAR MODALITY & LENS CARE Lens Wear Modalities: Lens Care: • Daily wear (DW) – DD (single-use) • None – Disposable • Normal – Programmed replacement • Normal – Conventional • Full (+ protein removal) • FW - disposable • Full • EW - disposable • Full • CW - single-use • None 5 L 1 -94
CONTACT LENS CARE WEAR MODALITY & LENS CARE Lens Wear Modalities: Lens Care: • Daily wear (DW) – DD (single-use) • None – Disposable • Normal – Programmed replacement • Normal – Conventional • Full (+ protein removal) • FW - disposable • Full • EW - disposable • Full • CW - single-use • None 5 L 1 -94
 CONTACT LENS CARE SELECTING A CARE REGIMEN • Wearing schedule (5/7, 7/7, DW, FW, EW, CW) • Lens material (FDA group, Si. Hy, GP, Hybrid) • Lens replacement schedule (DD, 2 wk, 1 mth, 3 mth, annual, other) • Convenience (safety still a consideration) • Ocular sensitivity (personal/family history, known issues) 5 L 1 -95
CONTACT LENS CARE SELECTING A CARE REGIMEN • Wearing schedule (5/7, 7/7, DW, FW, EW, CW) • Lens material (FDA group, Si. Hy, GP, Hybrid) • Lens replacement schedule (DD, 2 wk, 1 mth, 3 mth, annual, other) • Convenience (safety still a consideration) • Ocular sensitivity (personal/family history, known issues) 5 L 1 -95
 CONTACT LENS CARE LEGAL ISSUES Potentially, the use and/or recommendation of unapproved lens care procedures and/or products, or off-label recommendations for approved products, has legal implications for the contact lens practice & practitioner. The implications may be serial 5 L 1 -96
CONTACT LENS CARE LEGAL ISSUES Potentially, the use and/or recommendation of unapproved lens care procedures and/or products, or off-label recommendations for approved products, has legal implications for the contact lens practice & practitioner. The implications may be serial 5 L 1 -96
 CONTACT LENS CARE SILICONE HYDROGELS 0 After a decade of development, silicone hydrogel CLs (a. k. a. siloxane hydrogels, Si. Hy CLs) were launched in 1999 • Initially, the Si. Hy manufacturers simply recommended their own LCPs • Anecdotal information suggested that more superficial corneal staining was being observed with some combinations of Si. Hy CLs & MPSs • The matter was brought to a head by a report by Epstein (2002) & the series of claims & counter claims that ensued (2002 -2004). Some of the ensuing responses had marketing overtones • Most patients & practitioners were unaware of the issue because the ‘staining’ peaked after about 2 hours of lens wear & comfort remained unaffected, • Investigations revealed that some LCP formulations did result in more superficial staining with some Si. Hy lenses. Initial assumptions that the disinfectant was the cause were shown to be false because other products with the same disinfectant remained problemfree. This suggested specific formulations (or combinations of excipients) were the issue 5 L 1 -97
CONTACT LENS CARE SILICONE HYDROGELS 0 After a decade of development, silicone hydrogel CLs (a. k. a. siloxane hydrogels, Si. Hy CLs) were launched in 1999 • Initially, the Si. Hy manufacturers simply recommended their own LCPs • Anecdotal information suggested that more superficial corneal staining was being observed with some combinations of Si. Hy CLs & MPSs • The matter was brought to a head by a report by Epstein (2002) & the series of claims & counter claims that ensued (2002 -2004). Some of the ensuing responses had marketing overtones • Most patients & practitioners were unaware of the issue because the ‘staining’ peaked after about 2 hours of lens wear & comfort remained unaffected, • Investigations revealed that some LCP formulations did result in more superficial staining with some Si. Hy lenses. Initial assumptions that the disinfectant was the cause were shown to be false because other products with the same disinfectant remained problemfree. This suggested specific formulations (or combinations of excipients) were the issue 5 L 1 -97
 CONTACT LENS CARE SILICONE HYDROGELS 1 • The reports exposed an unmet need for Si. Hy LCPs if the ‘problems’ reported were to be addressed adequately • Subsequently, LCPs targeting Si. Hy CLs were released • Throughout this saga, hydrogen peroxide’s status as the yardstick against which other LCPs should be measured, was confirmed, even when used with Si. Hy CLs • For practitioners, it was also a salutatory lesson on how easy it is to jump to logical but erroneous conclusions when inadequate ‘research’ (or no research) in undertaken • Industry’s seizure of the obvious but ultimately hollow marketing opportunities, also provided a valuable lesson 5 L 1 -98
CONTACT LENS CARE SILICONE HYDROGELS 1 • The reports exposed an unmet need for Si. Hy LCPs if the ‘problems’ reported were to be addressed adequately • Subsequently, LCPs targeting Si. Hy CLs were released • Throughout this saga, hydrogen peroxide’s status as the yardstick against which other LCPs should be measured, was confirmed, even when used with Si. Hy CLs • For practitioners, it was also a salutatory lesson on how easy it is to jump to logical but erroneous conclusions when inadequate ‘research’ (or no research) in undertaken • Industry’s seizure of the obvious but ultimately hollow marketing opportunities, also provided a valuable lesson 5 L 1 -98
 CONTACT LENS CARE TO RUB OR NOT TO RUB • The benefits of CL cleaning by rubbing have been detailed already & have been long understood • The Fusarium spp. & Acanthamoeba spp. events of 2005 -2007 revealed several potential problems compounded by the usual compliance issues • The down-side of the wide acceptance & promotion of a ‘No Rub’ approach became apparent • All stakeholders, many of whom never supported ‘No Rub’, now agree that rubbing is required • Erasing the entrenched ‘No Rub’ culture will take time & effort by all, especially practitioners – • omitting rubbing is the easiest form of ‘non-compliance’ Regulation & approval of Patient Instruction inserts is an obvious next step – differing regulations around the world are unhelpful 5 L 1 -99
CONTACT LENS CARE TO RUB OR NOT TO RUB • The benefits of CL cleaning by rubbing have been detailed already & have been long understood • The Fusarium spp. & Acanthamoeba spp. events of 2005 -2007 revealed several potential problems compounded by the usual compliance issues • The down-side of the wide acceptance & promotion of a ‘No Rub’ approach became apparent • All stakeholders, many of whom never supported ‘No Rub’, now agree that rubbing is required • Erasing the entrenched ‘No Rub’ culture will take time & effort by all, especially practitioners – • omitting rubbing is the easiest form of ‘non-compliance’ Regulation & approval of Patient Instruction inserts is an obvious next step – differing regulations around the world are unhelpful 5 L 1 -99
 CONTACT LENS CARE TINTED LENSES • As an added CL ‘feature’, modern CL tinting technologies were developed with a full knowledge of LCPs • Hydrogen peroxide systems pose no special risk to tints • Usually, so-called ‘crazy’ or ‘fun’ CLs are made of conventional materials using accepted tinting & artwork technologies. Such CLs require conventional lens care (a fact lost on far too many ‘buyers’) – commonly, these CLs are sourced from unregulated suppliers with no CL professional involvement – this means little or no lens care & unsafe wearing habits. Wearers swapping CLs is reportedly common. Overall, this situation is a danger to the wearer’s health & the reputation of the CL industry – many jurisdictions have resorted to specific legislation targeting the illegitimate & unsafe supply of non-Rx CLs 5 L 1 -100
CONTACT LENS CARE TINTED LENSES • As an added CL ‘feature’, modern CL tinting technologies were developed with a full knowledge of LCPs • Hydrogen peroxide systems pose no special risk to tints • Usually, so-called ‘crazy’ or ‘fun’ CLs are made of conventional materials using accepted tinting & artwork technologies. Such CLs require conventional lens care (a fact lost on far too many ‘buyers’) – commonly, these CLs are sourced from unregulated suppliers with no CL professional involvement – this means little or no lens care & unsafe wearing habits. Wearers swapping CLs is reportedly common. Overall, this situation is a danger to the wearer’s health & the reputation of the CL industry – many jurisdictions have resorted to specific legislation targeting the illegitimate & unsafe supply of non-Rx CLs 5 L 1 -100
 CONTACT LENS CARE LONG-TERM STORAGE OF CLs • Home or CL practice, long-term CL storage is potentially risky • Unneutralized H 2 O 2 ideal medium for CL storage. However, its long -term stability, while high, requires a vented container. Decomposition gaseous oxygen pressure in sealed container - case rupture possible • Because safe, long-term storage is difficult technically, it should be actively discouraged. In era of disposable/frequent replacement, this should be easier to achieve – generally, those with unusual Rxs not available as disposable CLs, need to wear their CLs regularly & are therefore likely to need to store CLs 5 L 1 -101
CONTACT LENS CARE LONG-TERM STORAGE OF CLs • Home or CL practice, long-term CL storage is potentially risky • Unneutralized H 2 O 2 ideal medium for CL storage. However, its long -term stability, while high, requires a vented container. Decomposition gaseous oxygen pressure in sealed container - case rupture possible • Because safe, long-term storage is difficult technically, it should be actively discouraged. In era of disposable/frequent replacement, this should be easier to achieve – generally, those with unusual Rxs not available as disposable CLs, need to wear their CLs regularly & are therefore likely to need to store CLs 5 L 1 -101
 CONTACT LENS CARE LONG-TERM STORAGE OF CLs • For CLs that cannot be discarded but can be disinfected thermally (@ 70° - 80°C) thermal disinfection is the safest – – • before disinfection, CLs should be sealed in a glass vial with a silicone bung (plug) & a crimped, tear-off, metal seal once disinfected, the vial should remain unopened until needed If unsuited to heating, a regimen of a MPS in a new case (biofilm-free) with scheduled, regular solution changes is required. This is difficult to orchestrate: – the solution must be ‘in date’ and within its ‘discard after’ period – some form of assurance/confirmation (written) that the necessary steps have been performed, is required — solution changes need to be scheduled for every 1 -2 weeks (for a case that remains unopened in the interim) – this is a demanding regimen (non-compliance likely!) – once resealed, the case should be agitated vigorously to ensure fresh solution bathes all inside surfaces, lid, case/lid threads, etc. 5 L 1 -102
CONTACT LENS CARE LONG-TERM STORAGE OF CLs • For CLs that cannot be discarded but can be disinfected thermally (@ 70° - 80°C) thermal disinfection is the safest – – • before disinfection, CLs should be sealed in a glass vial with a silicone bung (plug) & a crimped, tear-off, metal seal once disinfected, the vial should remain unopened until needed If unsuited to heating, a regimen of a MPS in a new case (biofilm-free) with scheduled, regular solution changes is required. This is difficult to orchestrate: – the solution must be ‘in date’ and within its ‘discard after’ period – some form of assurance/confirmation (written) that the necessary steps have been performed, is required — solution changes need to be scheduled for every 1 -2 weeks (for a case that remains unopened in the interim) – this is a demanding regimen (non-compliance likely!) – once resealed, the case should be agitated vigorously to ensure fresh solution bathes all inside surfaces, lid, case/lid threads, etc. 5 L 1 -102
 CONTACT LENS CARE LONG-TERM STORAGE OF CLs • An alternative is to use unneutralized 3% peroxide in a new, vented case with 3 -monthly (a conservative figure) scheduled solution changes – the same provisos detailed previously apply, especially ensuring the new solution bathes the insides of the case completely – current peroxide solutions are well stabilized and can easily perform this task (a vented case still required) § longer storage is possible & safe but scheduling longer intervals is more difficult § obviously, CLs require thorough neutralization & rinsing before further use • In summary: Discard the CLs – it’s the easiest solution 5 L 1 -103
CONTACT LENS CARE LONG-TERM STORAGE OF CLs • An alternative is to use unneutralized 3% peroxide in a new, vented case with 3 -monthly (a conservative figure) scheduled solution changes – the same provisos detailed previously apply, especially ensuring the new solution bathes the insides of the case completely – current peroxide solutions are well stabilized and can easily perform this task (a vented case still required) § longer storage is possible & safe but scheduling longer intervals is more difficult § obviously, CLs require thorough neutralization & rinsing before further use • In summary: Discard the CLs – it’s the easiest solution 5 L 1 -103
 CONTACT LENS CARE IN-OFFICE (TRIAL SET) DISINFECTION • Similar to all other long-term storage – problems for GP & hydrogel lenses are similar – a CL practice must set the standard for infection control • Disposable CLs have problem significantly. However, not all trial CLs are disposable & GP CLs are unlikely to be disposable in the foreseeable future • If trial CLs can be disinfected thermally, this is preferred – prions are not inactivated by heat used in this context – any CLs used on a known CJD/v. CJD case should be destroyed – undiagnosed CJD cases are a risk § there are no known cases of CL-mediated CJD – yet! 5 L 1 -104
CONTACT LENS CARE IN-OFFICE (TRIAL SET) DISINFECTION • Similar to all other long-term storage – problems for GP & hydrogel lenses are similar – a CL practice must set the standard for infection control • Disposable CLs have problem significantly. However, not all trial CLs are disposable & GP CLs are unlikely to be disposable in the foreseeable future • If trial CLs can be disinfected thermally, this is preferred – prions are not inactivated by heat used in this context – any CLs used on a known CJD/v. CJD case should be destroyed – undiagnosed CJD cases are a risk § there are no known cases of CL-mediated CJD – yet! 5 L 1 -104
 CONTACT LENS CARE IN-OFFICE (TRIAL SET) DISINFECTION • Alternatives include: – peroxide storage in a vented lens case with scheduled 3 -monthly solution changes § as neutralization takes time, and is required before reuse, it must be scheduled immediately before any relevant appointment. The latter can not always be anticipated – storage in a MPS with 2 -weekly solution changes as described previously. As none of these solutions are especially strong disinfectants, thorough cleaning & rinsing before storage, and documentation of the steps taken are mandatory § • as the vial/case/container is likely to be reused many times, consideration of case hygiene/replacement and/or biofilm formation are also required Although obvious, the need for the case/vial lids to remain on & sealed needs to be emphasized to all staff in the practice 5 L 1 -105
CONTACT LENS CARE IN-OFFICE (TRIAL SET) DISINFECTION • Alternatives include: – peroxide storage in a vented lens case with scheduled 3 -monthly solution changes § as neutralization takes time, and is required before reuse, it must be scheduled immediately before any relevant appointment. The latter can not always be anticipated – storage in a MPS with 2 -weekly solution changes as described previously. As none of these solutions are especially strong disinfectants, thorough cleaning & rinsing before storage, and documentation of the steps taken are mandatory § • as the vial/case/container is likely to be reused many times, consideration of case hygiene/replacement and/or biofilm formation are also required Although obvious, the need for the case/vial lids to remain on & sealed needs to be emphasized to all staff in the practice 5 L 1 -105
 CONTACT LENS CARE SUMMARY • The need for routine lens care remains essentially the same as previously • Contact lenses need to be rubbed during the CL cleaning step • It is probable that regulatory requirements for lens care products will be made more stringent in the near future • Long-term, the cost of CLs & the costs of LCPs will have to be reconciled 5 L 1 -106
CONTACT LENS CARE SUMMARY • The need for routine lens care remains essentially the same as previously • Contact lenses need to be rubbed during the CL cleaning step • It is probable that regulatory requirements for lens care products will be made more stringent in the near future • Long-term, the cost of CLs & the costs of LCPs will have to be reconciled 5 L 1 -106
 GP LENS CARE PRODUCT UPDATES Due to research advances and changing market conditions, new LCP releases and older product retirements are regular events IACLE is keen to keep its resources up-to-date. Therefore, we invite members and other interested parties to inform IACLE of changes in the LCP marketplace Please forward information to: iacle@iacle. org As IACLE’s presentations are electronic resources, they can be updated easily & promptly IACLE will inform members of resource updates 5 L 1 -107
GP LENS CARE PRODUCT UPDATES Due to research advances and changing market conditions, new LCP releases and older product retirements are regular events IACLE is keen to keep its resources up-to-date. Therefore, we invite members and other interested parties to inform IACLE of changes in the LCP marketplace Please forward information to: iacle@iacle. org As IACLE’s presentations are electronic resources, they can be updated easily & promptly IACLE will inform members of resource updates 5 L 1 -107
 IACLE RESOURCES ERRORS, OMISSIONS, IMPROVEMENTS IACLE also invites users of its resources to help it evolve and improve them If you become aware of errors or omissions, or you have suggestions for improvements, you are invited to submit them to : iacle@iacle. org for consideration Contributions incorporated will be acknowledged in the relevant presentation’s Notes pane(s) 5 L 1 -108
IACLE RESOURCES ERRORS, OMISSIONS, IMPROVEMENTS IACLE also invites users of its resources to help it evolve and improve them If you become aware of errors or omissions, or you have suggestions for improvements, you are invited to submit them to : iacle@iacle. org for consideration Contributions incorporated will be acknowledged in the relevant presentation’s Notes pane(s) 5 L 1 -108
 IACLE INDUSTRY SPONSORS 5 L 1 -109
IACLE INDUSTRY SPONSORS 5 L 1 -109
 5 L 1 -110
5 L 1 -110
 ALL REFERENCES USED (In alphabetical order) 5 L 1 -111
ALL REFERENCES USED (In alphabetical order) 5 L 1 -111
 Abry F et al. (2010) reported in Sivak A (2010), ARVO 2010 Part 2 available at: http: //www. siliconehydrogels. org/meeting_synopsis/index. asp (accessed on 2010 -Dec-22). Bayer S et al. (2004). Effect of rewetting drops use on comfort and protein deposition of silicone hydrogel (Focus Night and Day) contact lenses. ARVO Abstracts Book Vol. 1: 1575. Boost M et al. (2010). Do multipurpose contact lens disinfecting solutions work effectively against non-FDA/ISO recommended strains of bacteria and fungi? Ophthal Physiol Opt. 30: 12 – 19. Bowden FW et al. (1989). Patterns of lens care practices and lens product contamination in contact lens associated microbial keratitis. CLAO J. 15: 49 – 54. Bright FV et al. (2011). PHMB and PQ-1 impact on a liposome corneal surface membrane model. Invest Ophthalmol Vis Sci. 52: E-abstract 6491. Bui TH et al. (2010). Patient compliance during contact lens wear: perceptions, awareness, and behaviour. Eye & CL. 36(6): 334 – 339. Butcko V et al. (2007). Microbial keratitis and the role of rub and rinsing. Eye & CL. 33(6): 421 – 423. Carney LG et al. (1990). Do contact lens solutions stand the test of time? II The aging of salines. CL Spectrum. 5(8): 53 56. Carnt N et al. (2010). Contact lens user profile, attitudes and level of compliance to lens care. Cont Lens Ant Eye. 33: 183188. Chun MW & Weissman BA (1987). Compliance in contact lens care. Am J Optom Physiol Opt. 64: 274 – 278. Clark G et al. (1994). Microbial contamination of cases used for storing contact lenses. J Infect. 28: 293 - 304. Claydon BE et al. (1996). A prospective study of non-compliance in contact lens wear. J BCLA. 19(4): 133 -140. Claydon BE et al. (1997). A prospective study of the effect of education on non-compliant behaviour in contact lens wear. Ophthalmic Physiol Optics. 17(2): 137 - 146. Collins M (1990). Failure to care for CLs becoming alarming. In: Contact Lenses a supplement to Australian Optometry Nov. : 11 – 12. Collins MJ & Carney LG (1986 a). Compliance with care and maintenance procedures amongst contact lens wearers. Clin Exp Optom. 69: 174 – 177. Collins MJ & Carney LG (1986 b). Patient compliance and its influence on contact lens wearing problems. Am J Optom Physiol Optics. 63: 952 – 956. 5 L 1 -112
Abry F et al. (2010) reported in Sivak A (2010), ARVO 2010 Part 2 available at: http: //www. siliconehydrogels. org/meeting_synopsis/index. asp (accessed on 2010 -Dec-22). Bayer S et al. (2004). Effect of rewetting drops use on comfort and protein deposition of silicone hydrogel (Focus Night and Day) contact lenses. ARVO Abstracts Book Vol. 1: 1575. Boost M et al. (2010). Do multipurpose contact lens disinfecting solutions work effectively against non-FDA/ISO recommended strains of bacteria and fungi? Ophthal Physiol Opt. 30: 12 – 19. Bowden FW et al. (1989). Patterns of lens care practices and lens product contamination in contact lens associated microbial keratitis. CLAO J. 15: 49 – 54. Bright FV et al. (2011). PHMB and PQ-1 impact on a liposome corneal surface membrane model. Invest Ophthalmol Vis Sci. 52: E-abstract 6491. Bui TH et al. (2010). Patient compliance during contact lens wear: perceptions, awareness, and behaviour. Eye & CL. 36(6): 334 – 339. Butcko V et al. (2007). Microbial keratitis and the role of rub and rinsing. Eye & CL. 33(6): 421 – 423. Carney LG et al. (1990). Do contact lens solutions stand the test of time? II The aging of salines. CL Spectrum. 5(8): 53 56. Carnt N et al. (2010). Contact lens user profile, attitudes and level of compliance to lens care. Cont Lens Ant Eye. 33: 183188. Chun MW & Weissman BA (1987). Compliance in contact lens care. Am J Optom Physiol Opt. 64: 274 – 278. Clark G et al. (1994). Microbial contamination of cases used for storing contact lenses. J Infect. 28: 293 - 304. Claydon BE et al. (1996). A prospective study of non-compliance in contact lens wear. J BCLA. 19(4): 133 -140. Claydon BE et al. (1997). A prospective study of the effect of education on non-compliant behaviour in contact lens wear. Ophthalmic Physiol Optics. 17(2): 137 - 146. Collins M (1990). Failure to care for CLs becoming alarming. In: Contact Lenses a supplement to Australian Optometry Nov. : 11 – 12. Collins MJ & Carney LG (1986 a). Compliance with care and maintenance procedures amongst contact lens wearers. Clin Exp Optom. 69: 174 – 177. Collins MJ & Carney LG (1986 b). Patient compliance and its influence on contact lens wearing problems. Am J Optom Physiol Optics. 63: 952 – 956. 5 L 1 -112
 Davis LJ (1995). Lens hygiene and care system contamination of asymptomatic rigid gas permeable lens wearers. ICLC. 22: 217 – 221. Dumbleton KA et al. (2010). Relationship between compliance with lens replacement and contact lens-related problems in silicone hydrogel wearers. Poster Amer Acad Optom Annual Meeting, San Francisco Nov 2010. Efron N et al. , (1990). Do in-eye lubricants for contact lens wearers really work? CL Ant Eye. 5: 14 - 19. Epstein AB (2002). SPK with daily wear of silicone hydrogel lenses and MPS. CL Spectrum. 17(11): 30. Gleason WJ (1999). Contact Lens Regulations and Compliance. CL Spectrum. May or visit: http: //www. clspectrum. com/article. aspx? article=&loc=archive%5 C 1999%5 Cmay%5 C 0599034. htm Hickson-Curran SB et al. (2010). Making the case for daily disposable contact lenses: patient non-compliance with storage case hygiene and replacement. Poster Amer Acad Optom Annual Meeting, San Francisco Nov 2010. Hind HW (1979). Contact lens solutions: Yesterday, Today, and Tomorrow. CL Forum. 4(100): 17 – 27. Houang E et al. (2001). Microbial keratitis in Hong Kong: relationship to climate, environment and contact-lens disinfection. Trans R Soc Trop Med Hyg. 95: 361 - 367. Houlsby RD et al. (1984). Microbiological evaluation of soft contact lens disinfecting solutions. J Am Optom Assoc. 55(3): 205 – 211. Jones L, Senchyna M (2007). Soft contact lens solutions review: Part 1 - components of modern care regimens. Optometry in Practice 8: 45 - 56. Kilvington S (2000). Through a glass darkly – Contact lenses and personal hygiene. Microbiol Today. 27(5): 66 – 69. Ky W et al. (1998). Clinical survey of lens care in contact lens patients. 24(4): 216 – 219 & comment 194. Lakkis C et al. (2010) reported in Sivak A (2010), ARVO 2010 Part 2 available at: http: //www. siliconehydrogels. org/meeting_synopsis/index. asp (accessed on 2010 -Dec-22). Larragoiti ND et al. (1994). A comparative study of techniques for decreasing contact lens storage case contamination. J Am Optom Assoc. 65(3): 161 – 163. Leluan P et al. (1991). Amoebic and bacterial contamination of contact lens storage cases. Contactologia 13: 137 – 141. Mayers M et al. (2010). Compliance and contamination in contact lens wear. Poster Amer Acad Optom Annual Meeting, San Francisco Nov 2010. Mc. Geehon M (1988). Guidelines to handling problem patients. CL Forum. 13(4): 23 – 28. Mc. Monnies CW (1988). Is there a way through the maintenance minefield? J BCLA. 12 (Science Meeting): 26 – 31. 5 L 1 -113
Davis LJ (1995). Lens hygiene and care system contamination of asymptomatic rigid gas permeable lens wearers. ICLC. 22: 217 – 221. Dumbleton KA et al. (2010). Relationship between compliance with lens replacement and contact lens-related problems in silicone hydrogel wearers. Poster Amer Acad Optom Annual Meeting, San Francisco Nov 2010. Efron N et al. , (1990). Do in-eye lubricants for contact lens wearers really work? CL Ant Eye. 5: 14 - 19. Epstein AB (2002). SPK with daily wear of silicone hydrogel lenses and MPS. CL Spectrum. 17(11): 30. Gleason WJ (1999). Contact Lens Regulations and Compliance. CL Spectrum. May or visit: http: //www. clspectrum. com/article. aspx? article=&loc=archive%5 C 1999%5 Cmay%5 C 0599034. htm Hickson-Curran SB et al. (2010). Making the case for daily disposable contact lenses: patient non-compliance with storage case hygiene and replacement. Poster Amer Acad Optom Annual Meeting, San Francisco Nov 2010. Hind HW (1979). Contact lens solutions: Yesterday, Today, and Tomorrow. CL Forum. 4(100): 17 – 27. Houang E et al. (2001). Microbial keratitis in Hong Kong: relationship to climate, environment and contact-lens disinfection. Trans R Soc Trop Med Hyg. 95: 361 - 367. Houlsby RD et al. (1984). Microbiological evaluation of soft contact lens disinfecting solutions. J Am Optom Assoc. 55(3): 205 – 211. Jones L, Senchyna M (2007). Soft contact lens solutions review: Part 1 - components of modern care regimens. Optometry in Practice 8: 45 - 56. Kilvington S (2000). Through a glass darkly – Contact lenses and personal hygiene. Microbiol Today. 27(5): 66 – 69. Ky W et al. (1998). Clinical survey of lens care in contact lens patients. 24(4): 216 – 219 & comment 194. Lakkis C et al. (2010) reported in Sivak A (2010), ARVO 2010 Part 2 available at: http: //www. siliconehydrogels. org/meeting_synopsis/index. asp (accessed on 2010 -Dec-22). Larragoiti ND et al. (1994). A comparative study of techniques for decreasing contact lens storage case contamination. J Am Optom Assoc. 65(3): 161 – 163. Leluan P et al. (1991). Amoebic and bacterial contamination of contact lens storage cases. Contactologia 13: 137 – 141. Mayers M et al. (2010). Compliance and contamination in contact lens wear. Poster Amer Acad Optom Annual Meeting, San Francisco Nov 2010. Mc. Geehon M (1988). Guidelines to handling problem patients. CL Forum. 13(4): 23 – 28. Mc. Monnies CW (1988). Is there a way through the maintenance minefield? J BCLA. 12 (Science Meeting): 26 – 31. 5 L 1 -113
 Morgan PB et al. (2011). International Contact Lens Prescribing in 2010. CL Spectrum. January 2011. Available at: http: //www. clspectrum. com/article. aspx? article=105084 Peterson R et al. (2010). Impact of a rub and rinse on solution-induced corneal staining. Optom Vis Sci. 87: 1030 – 1036. Powell CH et al. (2010). ARVO Fort Lauderdale, May 2010. Synopsis by Sivak A Oct 2010 Meeting Synopsis on the silicone hydrogels website (www. siliconehydrogels. org) Radford CF et al. (1993). Contact lens hygiene compliance in a university population. J BCLA. 16(3): 105 – 111. Seal D et al. (1999). Acanthamoeba keratitis in Scotland: Risk factors of contact lens wearers. Cont Lens Ant Eye. 22(2): 58 - 68. Schwartz CA (1987). What’s on their minds when they don’t comply? Review Optom. 130: 49. Shannon BJ (1987). Don’t quit with the fit. CL Forum. 12: 46 – 48. Shih KL et al. (1985). The microbial benefit of cleaning and rinsing contact lenses. ICLC. 12(4): 235 – 242. Shih KL et al. (1991). Disinfecting activities of non-peroxide soft contact lens cold disinfection solutions. CLAO J. 17(3): 165 – 168. Sokol JL et al. 1990. A study of patient compliance in a contact lens wearing population. CLAO J. 16(3): 209 – 213. Stapleton F et al. (1995 a). Epidemiology of Pseudomonas aeruginosa keratitis in contact lens wearers. Epidemiol Infect. 114(3): 395 – 402. Stone R (1988). Why contact lens groups? CL Spectrum. 3: 38 - 41. Sweeney DF et al. (1992). Contamination of 500 ml bottles of unpreserved saline. Clin Exp Optom. 75(2): 67 – 75. Tonge et al. (2001). Contact lens care: Part 6: - Comfort drops, artificial tears and dry-eye therapies. Optician 222(5817): 27 – 33. Turner FD et al. (1993). Compliance and contact lens care: A new assessment method. Optom Vis Sci. 70(12): 998 – 1004. Wilson LA et al. (1990). Microbial contamination of contact lens storage cases and solutions. Am J Ophthalmol. 110: 193 198. Wilson LA et al. (1991). Comparative Efficacies of Soft Contact Lens Disinfectant Solutions Against Microbial Films in Lens Cases. Arch Ophthalmol 109: 1155 – 1157. Woods CA et al. (2010). Compliance with lens care and contact lens case care and replacement. Poster Amer Acad Optom Annual Meeting, San Francisco Nov 2010. 5 L 1 -114
Morgan PB et al. (2011). International Contact Lens Prescribing in 2010. CL Spectrum. January 2011. Available at: http: //www. clspectrum. com/article. aspx? article=105084 Peterson R et al. (2010). Impact of a rub and rinse on solution-induced corneal staining. Optom Vis Sci. 87: 1030 – 1036. Powell CH et al. (2010). ARVO Fort Lauderdale, May 2010. Synopsis by Sivak A Oct 2010 Meeting Synopsis on the silicone hydrogels website (www. siliconehydrogels. org) Radford CF et al. (1993). Contact lens hygiene compliance in a university population. J BCLA. 16(3): 105 – 111. Seal D et al. (1999). Acanthamoeba keratitis in Scotland: Risk factors of contact lens wearers. Cont Lens Ant Eye. 22(2): 58 - 68. Schwartz CA (1987). What’s on their minds when they don’t comply? Review Optom. 130: 49. Shannon BJ (1987). Don’t quit with the fit. CL Forum. 12: 46 – 48. Shih KL et al. (1985). The microbial benefit of cleaning and rinsing contact lenses. ICLC. 12(4): 235 – 242. Shih KL et al. (1991). Disinfecting activities of non-peroxide soft contact lens cold disinfection solutions. CLAO J. 17(3): 165 – 168. Sokol JL et al. 1990. A study of patient compliance in a contact lens wearing population. CLAO J. 16(3): 209 – 213. Stapleton F et al. (1995 a). Epidemiology of Pseudomonas aeruginosa keratitis in contact lens wearers. Epidemiol Infect. 114(3): 395 – 402. Stone R (1988). Why contact lens groups? CL Spectrum. 3: 38 - 41. Sweeney DF et al. (1992). Contamination of 500 ml bottles of unpreserved saline. Clin Exp Optom. 75(2): 67 – 75. Tonge et al. (2001). Contact lens care: Part 6: - Comfort drops, artificial tears and dry-eye therapies. Optician 222(5817): 27 – 33. Turner FD et al. (1993). Compliance and contact lens care: A new assessment method. Optom Vis Sci. 70(12): 998 – 1004. Wilson LA et al. (1990). Microbial contamination of contact lens storage cases and solutions. Am J Ophthalmol. 110: 193 198. Wilson LA et al. (1991). Comparative Efficacies of Soft Contact Lens Disinfectant Solutions Against Microbial Films in Lens Cases. Arch Ophthalmol 109: 1155 – 1157. Woods CA et al. (2010). Compliance with lens care and contact lens case care and replacement. Poster Amer Acad Optom Annual Meeting, San Francisco Nov 2010. 5 L 1 -114
 CONTACT LENS CARE PRESERVATION STANDARDS ISO 14730: Preservative Efficacy Test (tests effectiveness up to 30 days, no CLs involved, microbial re-challenge @ 14 days, tests for initial efficacy & shelf-life. Test is basis of 30 -day lens storage) Test Panel (TP) of Organisms (inoculum 1 X 107 to X 108 CFU/m. L): • Fungi: – Candida albicans ATCC 10231 – Aspergillus niger ATCC 16404 • Bacteria: – Pseudomonas aeruginosa ATCC 9027 – Staphylococcus aureus ATCC 6538 – Escherichia coli ATCC 8739 5 L 1 -115
CONTACT LENS CARE PRESERVATION STANDARDS ISO 14730: Preservative Efficacy Test (tests effectiveness up to 30 days, no CLs involved, microbial re-challenge @ 14 days, tests for initial efficacy & shelf-life. Test is basis of 30 -day lens storage) Test Panel (TP) of Organisms (inoculum 1 X 107 to X 108 CFU/m. L): • Fungi: – Candida albicans ATCC 10231 – Aspergillus niger ATCC 16404 • Bacteria: – Pseudomonas aeruginosa ATCC 9027 – Staphylococcus aureus ATCC 6538 – Escherichia coli ATCC 8739 5 L 1 -115
 CONTACT LENS CARE PRESERVATION CRITERIA ISO 14730: Performance Requirement (inoculum 107 to 108) Bacteria • A preservative acts as a LCP ‘defence system’ At 14 days & before re-challenge – by a mean value of 3 log of TP bacteria • After the 14 day re-challenge, assessed at day 28 § by a mean value of 3 log of TP bacteria Molds & Yeasts • • Return to At 14 days & before re-challenge DISINFECTION – the number of organisms recovered per m. L is equal to or fewer than initial numbers (error allowed 0. 5 log) After the 14 day re-challenge, assessed at day 28 – the numbers to remain at or below level after the re-challenge (error allowed 0. 5 log) These criteria to be met throughout labeled shelf-life 5 L 1 -116
CONTACT LENS CARE PRESERVATION CRITERIA ISO 14730: Performance Requirement (inoculum 107 to 108) Bacteria • A preservative acts as a LCP ‘defence system’ At 14 days & before re-challenge – by a mean value of 3 log of TP bacteria • After the 14 day re-challenge, assessed at day 28 § by a mean value of 3 log of TP bacteria Molds & Yeasts • • Return to At 14 days & before re-challenge DISINFECTION – the number of organisms recovered per m. L is equal to or fewer than initial numbers (error allowed 0. 5 log) After the 14 day re-challenge, assessed at day 28 – the numbers to remain at or below level after the re-challenge (error allowed 0. 5 log) These criteria to be met throughout labeled shelf-life 5 L 1 -116
 CONTACT LENS CARE A BRIEF HISTORY 0 • Early glass scleral CLs maintained with soap & water • Glass-PMMA hybrid sclerals & early PMMA corneal lenses (early 1940 s) used with sodium bicarbonate (Na. HCO 3) solution to try to address corneal oedema not lens condition (Hind, 1979) • First true LCPs addressed lens wettability, not cleanliness. Many contained polyvinyl alcohol (PVA). It was then realized that other factors such as soiled/deposited lens surfaces also affected lens wettability. Lens cleaners followed & modern lens care was born • Eventually, suites of LCPs that cleaned, disinfected, and wet PMMA lenses became available from several manufacturers 5 L 1 -117
CONTACT LENS CARE A BRIEF HISTORY 0 • Early glass scleral CLs maintained with soap & water • Glass-PMMA hybrid sclerals & early PMMA corneal lenses (early 1940 s) used with sodium bicarbonate (Na. HCO 3) solution to try to address corneal oedema not lens condition (Hind, 1979) • First true LCPs addressed lens wettability, not cleanliness. Many contained polyvinyl alcohol (PVA). It was then realized that other factors such as soiled/deposited lens surfaces also affected lens wettability. Lens cleaners followed & modern lens care was born • Eventually, suites of LCPs that cleaned, disinfected, and wet PMMA lenses became available from several manufacturers 5 L 1 -117
 CONTACT LENS CARE A BRIEF HISTORY 1 • Thermal disinfection used on the first HEMA SCLs (1950 s): – lens case immersed in boiling water – denaturation of tear protein induced adverse reactions/hypersensitivity – lens deposits comfort & lens life – eventually, enzymatic protein removal treatments addressed these issues partially – still the cheapest & most effective antimicrobial treatment but prions remain effective – later, automatic electric wet, and later still, electric dry heat units were developed deploying lower temperatures (70 -85°C) to reduce denaturation – still applicable to HEMA trial (diagnostic) lenses 5 L 1 -118
CONTACT LENS CARE A BRIEF HISTORY 1 • Thermal disinfection used on the first HEMA SCLs (1950 s): – lens case immersed in boiling water – denaturation of tear protein induced adverse reactions/hypersensitivity – lens deposits comfort & lens life – eventually, enzymatic protein removal treatments addressed these issues partially – still the cheapest & most effective antimicrobial treatment but prions remain effective – later, automatic electric wet, and later still, electric dry heat units were developed deploying lower temperatures (70 -85°C) to reduce denaturation – still applicable to HEMA trial (diagnostic) lenses 5 L 1 -118
 CONTACT LENS CARE A BRIEF HISTORY 2 • Hydrogen peroxide disinfection was introduced when non. HEMA SCLs (45 -55% water initially, later 79 - 85%) appeared on the market: – initially, pharmacy grade 3% peroxide used but variable quality & stabilizers used in some, led to unwanted discolouration & physical property changes – effective neutralization an issue (See notes) 5 L 1 -119
CONTACT LENS CARE A BRIEF HISTORY 2 • Hydrogen peroxide disinfection was introduced when non. HEMA SCLs (45 -55% water initially, later 79 - 85%) appeared on the market: – initially, pharmacy grade 3% peroxide used but variable quality & stabilizers used in some, led to unwanted discolouration & physical property changes – effective neutralization an issue (See notes) 5 L 1 -119
 CONTACT LENS CARE A BRIEF HISTORY 3 • Next were the so-called cold chemical systems in which CLs (rigid & soft) were immersed after cleaning & rinsing: – disinfectants used included: mercurials (e. g. thimerosal), chlorhexidine (a biguanide, e. g. chlorhexidine gluconate), quaternary ammonium compounds or QACs (e. g. ATAC or ATEAC) – a common excipient then & now was EDTA. Later, benzalkonium chloride (BAK – rigid lens only), sorbic acid, chlorbutanol (an alcohol), benzyl, ethyl, & isopropyl alcohols were also used § initially, EDTA included as a disinfectant/preservative enhancer, later its calcium-chelating properties were also harnessed – adverse reaction rates to individual ingredients or combinations of ingredients were significant especially in hydrogel wearers. Research resulted in the evolution of the modern polymeric biguanides & QACs that now underpin the majority of non -peroxide LCPs 5 L 1 -120
CONTACT LENS CARE A BRIEF HISTORY 3 • Next were the so-called cold chemical systems in which CLs (rigid & soft) were immersed after cleaning & rinsing: – disinfectants used included: mercurials (e. g. thimerosal), chlorhexidine (a biguanide, e. g. chlorhexidine gluconate), quaternary ammonium compounds or QACs (e. g. ATAC or ATEAC) – a common excipient then & now was EDTA. Later, benzalkonium chloride (BAK – rigid lens only), sorbic acid, chlorbutanol (an alcohol), benzyl, ethyl, & isopropyl alcohols were also used § initially, EDTA included as a disinfectant/preservative enhancer, later its calcium-chelating properties were also harnessed – adverse reaction rates to individual ingredients or combinations of ingredients were significant especially in hydrogel wearers. Research resulted in the evolution of the modern polymeric biguanides & QACs that now underpin the majority of non -peroxide LCPs 5 L 1 -120
 CONTACT LENS CARE A BRIEF HISTORY 4 • Chlorine–based systems have appeared & disappeared – usually in tablet form, these products proved to be less efficacious than desired, e. g. they were less effective against fungi & Acanthamoeba spp. [especially encysted]) – efficacy was by chlorine’s predilection for binding to biological soil, hence removing it from solution – they were simple & economic • A chlorhexidine-based tablet system was introduced but the ocular reaction rate to the product limited its marketability • UV-generating (usually UV-C), ozone generating, & microwave ovenbased disinfection systems have also appeared. Few remain 5 L 1 -121
CONTACT LENS CARE A BRIEF HISTORY 4 • Chlorine–based systems have appeared & disappeared – usually in tablet form, these products proved to be less efficacious than desired, e. g. they were less effective against fungi & Acanthamoeba spp. [especially encysted]) – efficacy was by chlorine’s predilection for binding to biological soil, hence removing it from solution – they were simple & economic • A chlorhexidine-based tablet system was introduced but the ocular reaction rate to the product limited its marketability • UV-generating (usually UV-C), ozone generating, & microwave ovenbased disinfection systems have also appeared. Few remain 5 L 1 -121
 CONTACT LENS CARE A BRIEF HISTORY 5 • Modern MPSs are based on polymeric biguanides, polymeric QACs, or more recently, both. Their large molecular structure deters accumulation within intact hydrogel or Si. Hy CLs eye exposure to man-made chemical entities – most non-peroxide lens care systems are based on polihexanide (PHX, PAPB, PHMB [polymeric biguanides]) or polyquaternium-1 (PQ-1 or polidronium chloride – a polymeric QAC), or a combination of these – antimicrobial efficacy of MPSs is also something of a compromise – a balance of efficacy & toxicity 5 L 1 -122
CONTACT LENS CARE A BRIEF HISTORY 5 • Modern MPSs are based on polymeric biguanides, polymeric QACs, or more recently, both. Their large molecular structure deters accumulation within intact hydrogel or Si. Hy CLs eye exposure to man-made chemical entities – most non-peroxide lens care systems are based on polihexanide (PHX, PAPB, PHMB [polymeric biguanides]) or polyquaternium-1 (PQ-1 or polidronium chloride – a polymeric QAC), or a combination of these – antimicrobial efficacy of MPSs is also something of a compromise – a balance of efficacy & toxicity 5 L 1 -122
 CONTACT LENS CARE A BRIEF HISTORY 6 • Chemically, peroxide systems have changed little over time – most are now 1 -step (tablet or disc) • A sodium chlorite-hydrogen peroxide disinfectant & a sodium chlorite preserved (a so-called ‘disappearing preservative’) series of in-eye products round out the current LCP market • Other additions include anti-acanthamoebal compounds (e. g. MAPD). Such inclusions can be expected to become more common in future LCPs • EDTA is still a common inclusion in LCPs • Many solutions, especially cleaners, still include viscosity-increasing agents 5 L 1 -123
CONTACT LENS CARE A BRIEF HISTORY 6 • Chemically, peroxide systems have changed little over time – most are now 1 -step (tablet or disc) • A sodium chlorite-hydrogen peroxide disinfectant & a sodium chlorite preserved (a so-called ‘disappearing preservative’) series of in-eye products round out the current LCP market • Other additions include anti-acanthamoebal compounds (e. g. MAPD). Such inclusions can be expected to become more common in future LCPs • EDTA is still a common inclusion in LCPs • Many solutions, especially cleaners, still include viscosity-increasing agents 5 L 1 -123
 CONTACT LENS CARE A BRIEF HISTORY 7 Why have some products survived? Possibilities include: • Success at balancing efficacy & toxicity, i. e. product is effective against microorganisms & relatively ineffective against the anterior eye • Strong marketing of an acceptable product • Availability of starter kits at little or no cost. However, unpublished studies have shown that even free kits do not necessarily ensure market penetration/success • Competitive pricing of an acceptable product – intuitively, nothing will save an unacceptable product. Sometimes however, ‘failure’ takes time • Addressing a niche market that is unattractive to competitors (often due to small size or highly specialized nature) • Adequate & relevant market research used to steer product development • Luck – having the right (appropriate) product at the right time 5 L 1 -124
CONTACT LENS CARE A BRIEF HISTORY 7 Why have some products survived? Possibilities include: • Success at balancing efficacy & toxicity, i. e. product is effective against microorganisms & relatively ineffective against the anterior eye • Strong marketing of an acceptable product • Availability of starter kits at little or no cost. However, unpublished studies have shown that even free kits do not necessarily ensure market penetration/success • Competitive pricing of an acceptable product – intuitively, nothing will save an unacceptable product. Sometimes however, ‘failure’ takes time • Addressing a niche market that is unattractive to competitors (often due to small size or highly specialized nature) • Adequate & relevant market research used to steer product development • Luck – having the right (appropriate) product at the right time 5 L 1 -124
 CONTACT LENS CARE A BRIEF HISTORY 8 Why have some products failed? Possibilities include: • Failure to balance efficacy & toxicity • Poor marketing of an acceptable product • Uncompetitive pricing of an acceptable product • Addressing a niche market that is too small to be sustained • Inadequate and/or poorly targeted market research used to steer product development • Failure to ‘move with the times’ – product(s) perceived as ‘old’ • Inadequately researched product • Failure to take ‘worst case scenarios’ into account, e. g. user non-compliance • Unforeseen changes in external factors, e. g. reticulated mains water disinfection levels, that alter the anticipated user environment • Bad luck 5 L 1 -125
CONTACT LENS CARE A BRIEF HISTORY 8 Why have some products failed? Possibilities include: • Failure to balance efficacy & toxicity • Poor marketing of an acceptable product • Uncompetitive pricing of an acceptable product • Addressing a niche market that is too small to be sustained • Inadequate and/or poorly targeted market research used to steer product development • Failure to ‘move with the times’ – product(s) perceived as ‘old’ • Inadequately researched product • Failure to take ‘worst case scenarios’ into account, e. g. user non-compliance • Unforeseen changes in external factors, e. g. reticulated mains water disinfection levels, that alter the anticipated user environment • Bad luck 5 L 1 -125
 5 L 1 -126
5 L 1 -126


