17bef05a13da9890a650bf02edb3b2d9.ppt
- Количество слайдов: 30
 Consent, Data Sharing, and Returning Results: Decision Making in Managing Biobanks Kelly A. Edwards, Ph. D Associate Professor, Bioethics Co-Director, Regulatory Support & Bioethics Core, ITHS 1
Consent, Data Sharing, and Returning Results: Decision Making in Managing Biobanks Kelly A. Edwards, Ph. D Associate Professor, Bioethics Co-Director, Regulatory Support & Bioethics Core, ITHS 1
 Take Home Points • Trustworthy practices in research are going to be critical to long-term success • Old practice paradigms may no longer preserve public trust – Upfront regulatory review – Heavy burden on consent procedures – Focus on individual privacy and identifiability • Transformed data management and research practices are needed 2
Take Home Points • Trustworthy practices in research are going to be critical to long-term success • Old practice paradigms may no longer preserve public trust – Upfront regulatory review – Heavy burden on consent procedures – Focus on individual privacy and identifiability • Transformed data management and research practices are needed 2
 Ethics Issues and Questions Consent: – What information management systems will best enhance or extend traditional consent mechanisms? Communications: – In what ways might technical systems enhance on-going engagement with participants and/or the public? Auditability: – What are the benefits and risks of rich audit trails and data use tracking systems? How should we respond to errors or lapses? 3
Ethics Issues and Questions Consent: – What information management systems will best enhance or extend traditional consent mechanisms? Communications: – In what ways might technical systems enhance on-going engagement with participants and/or the public? Auditability: – What are the benefits and risks of rich audit trails and data use tracking systems? How should we respond to errors or lapses? 3
 Ethics Issues and Questions Governance: – How do we incorporate, and track, participant preferences into research governance decisionmaking? Sustainability: – What are the best ways to engage the public about the open-ended nature of translational research? 4
Ethics Issues and Questions Governance: – How do we incorporate, and track, participant preferences into research governance decisionmaking? Sustainability: – What are the best ways to engage the public about the open-ended nature of translational research? 4
 CTSA Clinical Research Ethics Committee Biobank Work Group Goals: Serve as a resource to CTSAs and build new knowledge and tools • Progress: – Building a google website to share governance and community engagement materials – Serving as a consult service for individuals and groups with questions – Applying for outside funding for demonstration projects to assess outcomes 5
CTSA Clinical Research Ethics Committee Biobank Work Group Goals: Serve as a resource to CTSAs and build new knowledge and tools • Progress: – Building a google website to share governance and community engagement materials – Serving as a consult service for individuals and groups with questions – Applying for outside funding for demonstration projects to assess outcomes 5
 Bioethics Consult Service • Questions about repository governance? • Contact our consult service: – www. iths. org to bioethics consult request – Seattle Children’s Operator: 206 -9872000 6
Bioethics Consult Service • Questions about repository governance? • Contact our consult service: – www. iths. org to bioethics consult request – Seattle Children’s Operator: 206 -9872000 6
 What is Governance? “The process of policy orientation that guides research under ethical and scientific norms so that the results can be used for the benefit of population health. ” –P 3 G Consortium Lexicon: p 3 gobservatory. org “The agreements, procedures, conventions, or policies that define who gets power, how decisions are taken and how accountability is rendered. ” –Principles for Good Governance: www. iog. ca 7
What is Governance? “The process of policy orientation that guides research under ethical and scientific norms so that the results can be used for the benefit of population health. ” –P 3 G Consortium Lexicon: p 3 gobservatory. org “The agreements, procedures, conventions, or policies that define who gets power, how decisions are taken and how accountability is rendered. ” –Principles for Good Governance: www. iog. ca 7
 Governance Decisions • Purpose • Consent – Specific or broad • Population • Data Access • Protections • Return Results • Participation – By whom and how – To participants? – To repository? • Oversight – By whom and how 8
Governance Decisions • Purpose • Consent – Specific or broad • Population • Data Access • Protections • Return Results • Participation – By whom and how – To participants? – To repository? • Oversight – By whom and how 8
 Step by step start-up guide § What will you collect? – Area of focus – Study design – Ownership and access § How will you collect it? – – Regulatory compliance Operations and resources Registry considerations Repository considerations § What else do you need? – Public relations and materials – Making samples available to researchers http: //resourcerepository. org/documents/1862/registry/repositorystart-upguide/
Step by step start-up guide § What will you collect? – Area of focus – Study design – Ownership and access § How will you collect it? – – Regulatory compliance Operations and resources Registry considerations Repository considerations § What else do you need? – Public relations and materials – Making samples available to researchers http: //resourcerepository. org/documents/1862/registry/repositorystart-upguide/
 10
10
 What are the risks? • • • Bad guys Data invaders Security breach Carelessness Forensic uses • People doing things we do not agree with • “Usual” harms: – Violation of privacy – Discrimination • Less common harms: – – – Tying up resources Self-concept damage Group stigmatization Perceived deception Lack of respect Lack of recognition 11
What are the risks? • • • Bad guys Data invaders Security breach Carelessness Forensic uses • People doing things we do not agree with • “Usual” harms: – Violation of privacy – Discrimination • Less common harms: – – – Tying up resources Self-concept damage Group stigmatization Perceived deception Lack of respect Lack of recognition 11
 Current Public Climate for Research “Where did you go with my DNA? ” - NYT
Current Public Climate for Research “Where did you go with my DNA? ” - NYT
 Lessons from these Stories? • Regulations are the floor – We may need other standards to guide us • “Business as usual” practices can cause harm – We cannot anticipate what “harm” looks like • Engage the public – Be transparent about research practices and intentions – Communicate openly and clearly – Ask permission before using samples if outside original scope or intentions 13
Lessons from these Stories? • Regulations are the floor – We may need other standards to guide us • “Business as usual” practices can cause harm – We cannot anticipate what “harm” looks like • Engage the public – Be transparent about research practices and intentions – Communicate openly and clearly – Ask permission before using samples if outside original scope or intentions 13
 Returning to Old Fashioned Research Ethics • Respect for Persons – How can our research processes enact respect? • Beneficence – How can we assure our research is achieving benefits? And clear benefits for whom? • Justice – How can we proceed equitably and fairly while addressing current injustices in the system? 14
Returning to Old Fashioned Research Ethics • Respect for Persons – How can our research processes enact respect? • Beneficence – How can we assure our research is achieving benefits? And clear benefits for whom? • Justice – How can we proceed equitably and fairly while addressing current injustices in the system? 14
 Chain of trust: Doing science without eye contact 15
Chain of trust: Doing science without eye contact 15
 Demonstrating Respect What other ways can we use to demonstrate respect? – Increased communication – Increased choice – Benefit sharing – Saying “thank you” 16
Demonstrating Respect What other ways can we use to demonstrate respect? – Increased communication – Increased choice – Benefit sharing – Saying “thank you” 16
 Informed Consent: Options • • • Opt-out Opt-in Specific designations of use Consent at admission (if hospital-based) Consent post-op Re-consent for specific use How should we decide what to use? What will accomplish the goal of informed choice? 17
Informed Consent: Options • • • Opt-out Opt-in Specific designations of use Consent at admission (if hospital-based) Consent post-op Re-consent for specific use How should we decide what to use? What will accomplish the goal of informed choice? 17
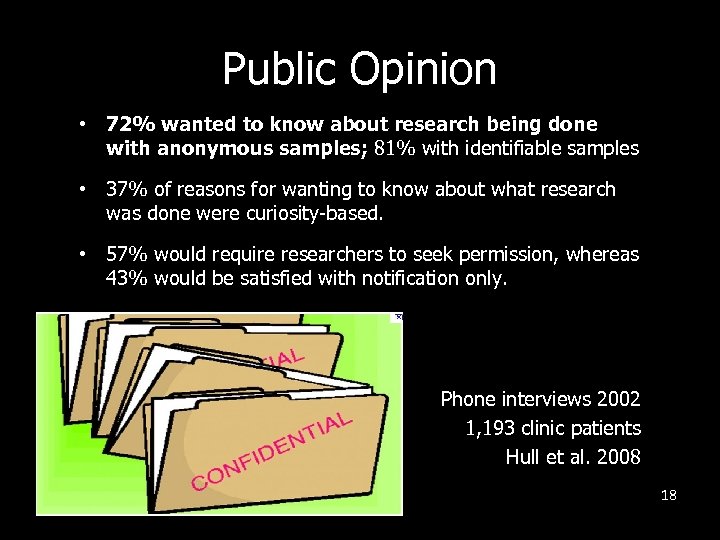 Public Opinion • 72% wanted to know about research being done with anonymous samples; 81% with identifiable samples • 37% of reasons for wanting to know about what research was done were curiosity-based. • 57% would require researchers to seek permission, whereas 43% would be satisfied with notification only. Phone interviews 2002 1, 193 clinic patients Hull et al. 2008 18
Public Opinion • 72% wanted to know about research being done with anonymous samples; 81% with identifiable samples • 37% of reasons for wanting to know about what research was done were curiosity-based. • 57% would require researchers to seek permission, whereas 43% would be satisfied with notification only. Phone interviews 2002 1, 193 clinic patients Hull et al. 2008 18
 Public Opinion • 90% were concerned about privacy protections • 60% would participate in a biobank if asked • 48% would provide consent for all research if approved by an oversight board, 42% wanted to be asked for each 2008 public survey N= 4659 (58. 4% response) Kaufman et al. 2009 19
Public Opinion • 90% were concerned about privacy protections • 60% would participate in a biobank if asked • 48% would provide consent for all research if approved by an oversight board, 42% wanted to be asked for each 2008 public survey N= 4659 (58. 4% response) Kaufman et al. 2009 19
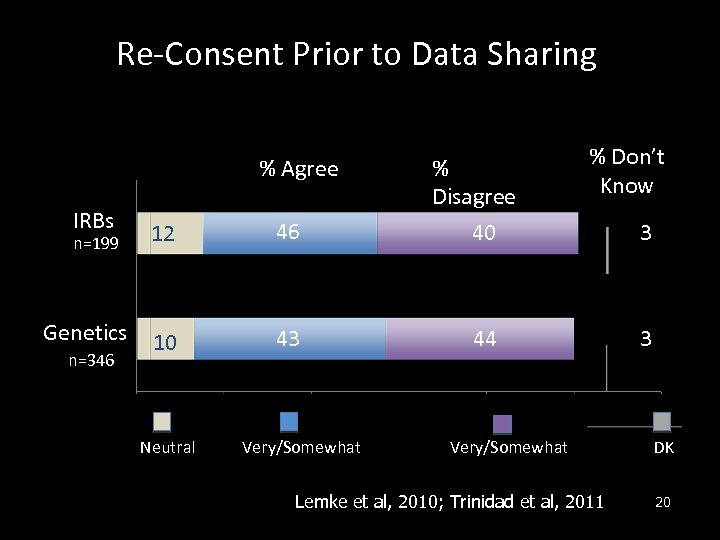 Re-Consent Prior to Data Sharing % Agree IRBs n=199 Genetics n=346 12 46 10 43 Neutral Very/Somewhat % Disagree 40 % Don’t Know 44 Very/Somewhat Lemke et al, 2010; Trinidad et al, 2011 3 3 DK 20
Re-Consent Prior to Data Sharing % Agree IRBs n=199 Genetics n=346 12 46 10 43 Neutral Very/Somewhat % Disagree 40 % Don’t Know 44 Very/Somewhat Lemke et al, 2010; Trinidad et al, 2011 3 3 DK 20
 Re-contact, Re-consent We should explore new methods of recontact (automated, electronic communication), which: – Keep participants engaged and informed about research activities – May contribute to science literacy – Builds and sustains relationships, which are important to trust – Creates good will in public programs and research enterprise 21
Re-contact, Re-consent We should explore new methods of recontact (automated, electronic communication), which: – Keep participants engaged and informed about research activities – May contribute to science literacy – Builds and sustains relationships, which are important to trust – Creates good will in public programs and research enterprise 21
 Managing Choice Dynamically 22
Managing Choice Dynamically 22
 Returning Results: Current Consensus (NHLBI) • Results should be offered if: – there is established analytic validity. – the associated risk for disease is replicable and significant. – the disease has important health implications, such as premature death or substantial morbidity. – proven therapeutic or preventive interventions are available. • Assuming that participants have agreed to receive results. • Results should never be forced on research participants. Bookman et al. (2006) Am J Med Genet 23
Returning Results: Current Consensus (NHLBI) • Results should be offered if: – there is established analytic validity. – the associated risk for disease is replicable and significant. – the disease has important health implications, such as premature death or substantial morbidity. – proven therapeutic or preventive interventions are available. • Assuming that participants have agreed to receive results. • Results should never be forced on research participants. Bookman et al. (2006) Am J Med Genet 23
 Disclose or Not: Barriers Remain Conceptual: – What is the fundamental purpose of research? Should disclosure be part of research practice? – What counts as a benefit? A harm? How much certainty do we need to act? Practical: – CLIA-certified laboratories – Paid staff to follow up – Finding participants over time 24
Disclose or Not: Barriers Remain Conceptual: – What is the fundamental purpose of research? Should disclosure be part of research practice? – What counts as a benefit? A harm? How much certainty do we need to act? Practical: – CLIA-certified laboratories – Paid staff to follow up – Finding participants over time 24
 Different Kinds of Results • Already in clinical use – Example: BRCA 1 mutation (Breast/ovarian cancer risk) • Potential clinical use – Example: Association of gene variant with prostate cancer risk • Clinical interest – Example: Association of gene variant with cardiovascular disease risk • Research/general interest – Example: Association with height 25
Different Kinds of Results • Already in clinical use – Example: BRCA 1 mutation (Breast/ovarian cancer risk) • Potential clinical use – Example: Association of gene variant with prostate cancer risk • Clinical interest – Example: Association of gene variant with cardiovascular disease risk • Research/general interest – Example: Association with height 25
 Cloud Sourcing Data • Return all data – raw data – to patients for further, independent use 26
Cloud Sourcing Data • Return all data – raw data – to patients for further, independent use 26
 Benefits of Public Participation Public participation in research can: – Improve recruitment – Enhance data collection – Focus analysis and interpretation – Facilitate dissemination – Creates trust Staley K. (2009) Exploring Impact: Public involvement in NHS, 27 public health and social care research. INVOLVE, Eastleigh.
Benefits of Public Participation Public participation in research can: – Improve recruitment – Enhance data collection – Focus analysis and interpretation – Facilitate dissemination – Creates trust Staley K. (2009) Exploring Impact: Public involvement in NHS, 27 public health and social care research. INVOLVE, Eastleigh.
 Resources § ISBER – http: //www. isber. org/ibc. html § NCI Best practices – http: //biospecimens. cancer. gov/practices/default. a sp § NCRR Clinical Translational Science Award – Biobank Working Group. Resource share site TBA. § ITHS Bioethics Consult Service – www. iths. org
Resources § ISBER – http: //www. isber. org/ibc. html § NCI Best practices – http: //biospecimens. cancer. gov/practices/default. a sp § NCRR Clinical Translational Science Award – Biobank Working Group. Resource share site TBA. § ITHS Bioethics Consult Service – www. iths. org
 Acknowledgments Center for Genomics & Healthcare Equality (NHGRI) – Wylie Burke, Malia Fullerton, Rose James, Helene Starks (UW) – Bert Boyer & Scarlett Hopkins (University of Alaska, Fairbanks) Genetic Alliance – Sharon Terry, CEO and Liz Horn, Director of Biobank Institute for Translational Health Sciences (NCRR) – Nick Anderson, Sarah Greene, Holly Tabor, Ben Wilfond, Jen Wroblewski Testing Justice Project (Greenwall Foundation) – Sara Goering and Suzanne Holland TIES Project (UCD and Office of Research Integrity) – Gail Geller (Hopkins), Rich Sharp (Cleveland), Mark Yarborough (Colorado), and several community health leaders (Alok Sarwal, Grant Jones, et al) 29
Acknowledgments Center for Genomics & Healthcare Equality (NHGRI) – Wylie Burke, Malia Fullerton, Rose James, Helene Starks (UW) – Bert Boyer & Scarlett Hopkins (University of Alaska, Fairbanks) Genetic Alliance – Sharon Terry, CEO and Liz Horn, Director of Biobank Institute for Translational Health Sciences (NCRR) – Nick Anderson, Sarah Greene, Holly Tabor, Ben Wilfond, Jen Wroblewski Testing Justice Project (Greenwall Foundation) – Sara Goering and Suzanne Holland TIES Project (UCD and Office of Research Integrity) – Gail Geller (Hopkins), Rich Sharp (Cleveland), Mark Yarborough (Colorado), and several community health leaders (Alok Sarwal, Grant Jones, et al) 29
 Challenges of Biobanking Research • Who owns the data? • How do we continue to have stewardship over data collected in good faith? • How can we get meaningful consent? • How should we weigh the trade-offs of privacy risks against research utility? • When, if ever, should results be returned? 30
Challenges of Biobanking Research • Who owns the data? • How do we continue to have stewardship over data collected in good faith? • How can we get meaningful consent? • How should we weigh the trade-offs of privacy risks against research utility? • When, if ever, should results be returned? 30


