ff3b4a79c5bd6421943b8f11e96ed14a.ppt
- Количество слайдов: 16

Concept Foundation: Making Medabon® Available Helena von Hertzen MD Senior Advisor FIGO Initiative on Unsafe Abortion Regional meetings, 2011

What is Concept Foundation? Concept Foundation was established as an international, charitable, not-for-profit organization in Thailand in 1989, through a grant to HRP/WHO (PATH, UNFPA, the World Bank and several foundations) to “make various reproductive health and family planning products available to public sector of low and middle income countries at affordable, or at least preferential prices, for their safe and effective use. ” FIGO Initiative on Unsafe Abortion Regional meetings, 2011

Concept Foundation - WHO Concept Foundation has a general collaboration agreement with WHO in reproductive health, which allows it to translate the research work of WHO into products for commercialization for the benefit of people who access their medicines through the public sector in developing countries worldwide. FIGO Initiative on Unsafe Abortion Regional meetings 2011

Goals of Concept Foundation is pursueing the following goals Goal 1 2 3 4 5 Access to Safe Medical Abortion Access to High Quality Drugs for Maternal Health – misoprostol (Gynuity); - prequalification programme (WHO) Access to Hormonal Contraception Access to other Reproductive Health products Supporting PDPs on IP & access to markets FIGO Initiative on Unsafe Abortion Regional meetings, 2011

Concept’s PPP on Medabon® Concept is making available a co-packaged mifepristone (1 x 200 mg tablet) & misoprostol (4 x 200μg tablets) = Medabon® through its pharmaceutical partner, Sun Pharma, Mumbai, India. Activities are ongoing, or planned, to register it in 26 developing countries or countries in transition; and introduce it in 11 countries. FIGO initiative on unsafe abortion
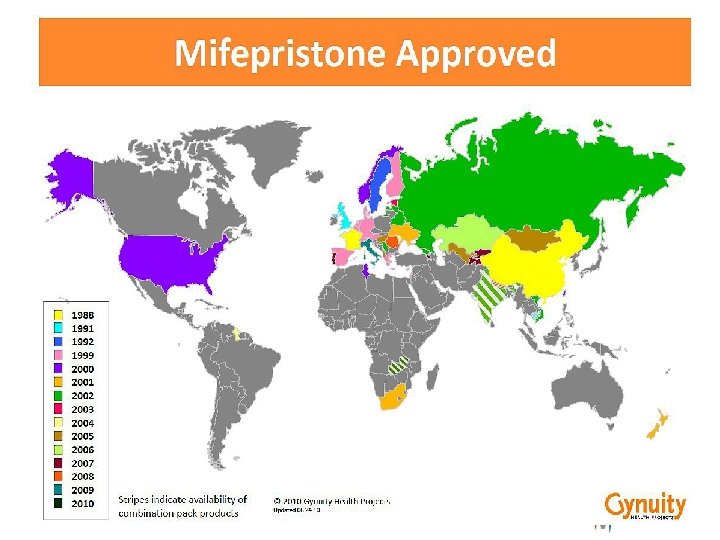

Product availability BUT, - What is the quality of the products that are available? - Do mifepristone and misoprostol manufacturers meet international quality and safety requirements? - What do we know about the stability of misoprostol formulations in tropical climates or even in 25°C after production ? Concept Foundation is undertaking a study with the support of Gynuity Health Projects to investigate the quality of medical abortion drugs on the market.
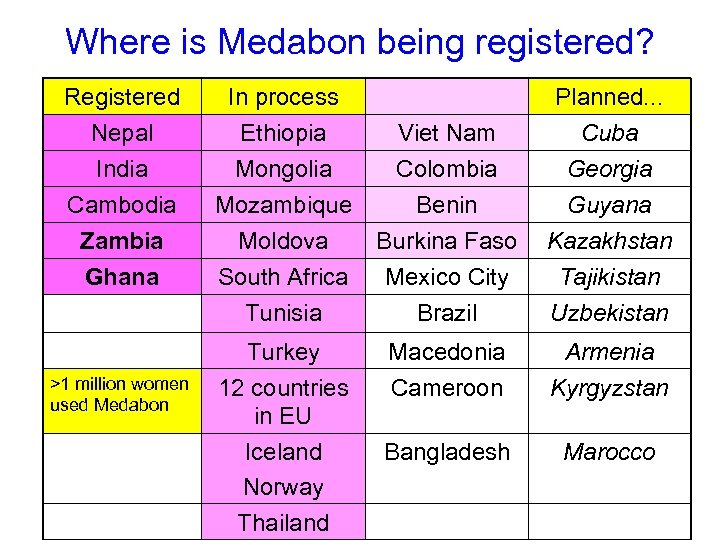
Where is Medabon being registered? Registered In process Nepal Ethiopia Viet Nam Cuba India Mongolia Colombia Georgia Cambodia Mozambique Benin Guyana Zambia Moldova Burkina Faso Kazakhstan Ghana South Africa Mexico City Tajikistan Tunisia Brazil Uzbekistan Turkey Macedonia Armenia 12 countries in EU Cameroon Kyrgyzstan Iceland Norway Bangladesh Marocco >1 million women used Medabon Thailand Planned. . .
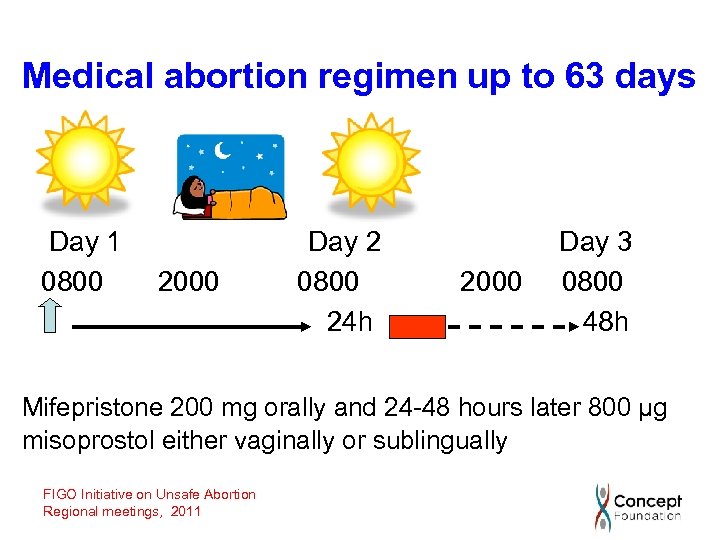
Medical abortion regimen up to 63 days Day 1 0800 2000 Day 2 0800 24 h 2000 Day 3 0800 48 h Mifepristone 200 mg orally and 24 -48 hours later 800 µg misoprostol either vaginally or sublingually FIGO Initiative on Unsafe Abortion Regional meetings, 2011

Need for information PATH, Ipas, Concept and WHO/HRP developed materials for health care providers and programme managers who are working to introduce Medabon®. This material is also intended to reach policy makers and the media and include: • • Medabon®: Medical & Service Delivery Guidelines Medical Abortion & Medabon®: Key Talking Points Medabon®: Frequently Asked Questions (FAQ) Medabon®: What You Need to Know (sample patient brochure) Medabon®: Background for Providers of Emergency Care Medabon®: A Framework for Introduction Medical Abortion: Selected References On site: www. medabon. info FIGO Initiative on Unsafe Abortion Regional meetings, 2011
FIGO Initiative on Unsafe Abortion Regional meetings, July-August 2009

The need for information - providers/policy makers WHO organized a meeting of experienced researchers and clinicians working on medical abortion. They responded to a list of the most frequently asked questions about medical abortion from health-care personnel providing abortion services. They reviewed those questions and compiled answers based on scientific literature and their own experience. www. who. int/reproductivehealth/publications/ medical_abortion/index. html FIGO Initiative on Unsafe Abortion Regional meetings, 2011
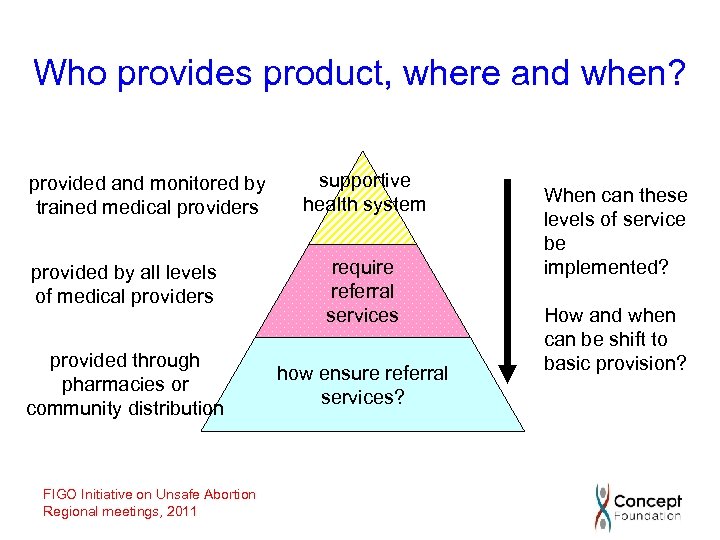
Who provides product, where and when? provided and monitored by trained medical providers supportive health system provided by all levels of medical providers require referral services provided through pharmacies or community distribution how ensure referral services? FIGO Initiative on Unsafe Abortion Regional meetings, 2011 When can these levels of service be implemented? How and when can be shift to basic provision?

Objectives of an introductory strategy • Provision of medical abortion in the context of CAC in all facilities meeting the requirements of the national guidelines. In the initial phase this will be undertaken in selected healthcare facilities. • Development of a training curriculum for the provision of comprehensive abortion care, in particular of MVA and medical abortion and including values clarification. • Development of IEC materials for professionals and users. • Development of an advocacy strategy for CAC. • Implementation of operations research to allow planning for scaling up to other health care facilities. FIGO Initiative on Unsafe Abortion Regional meetings, 2011

An optimal approach to product introduction To achieve the stated objectives, it is necessary to develop and implement an appropriately designed introductory process. This requires: • a systematic and incremental approach; and • coordination and collaboration between the public health system and all key stakeholders synchronization of activities is the key to building an appropriate and supportive health system for the provision of medical abortion. This is ongoing in Nepal, Cambodia and Zambia, and starting in Ghana. FIGO Initiative on Unsafe Abortion Regional meetings, 2011
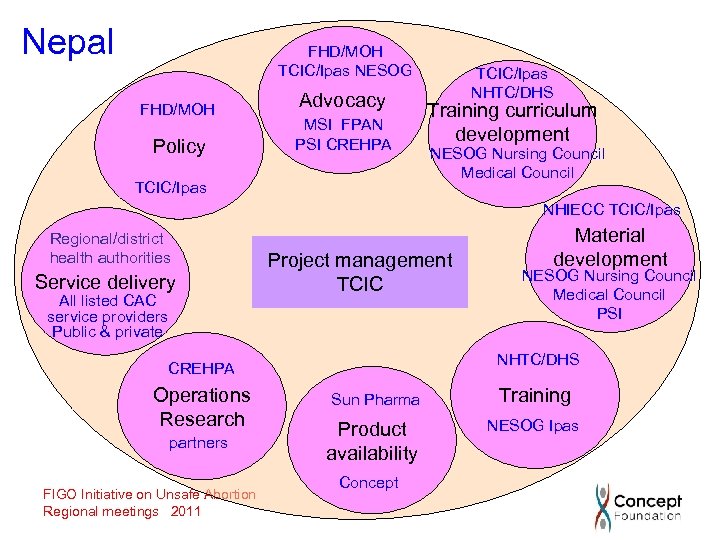
Nepal FHD/MOH TCIC/Ipas NESOG FHD/MOH Policy Advocacy MSI FPAN PSI CREHPA TCIC/Ipas NHTC/DHS Training curriculum development NESOG Nursing Council Medical Council NHIECC TCIC/Ipas Regional/district health authorities Service delivery All listed CAC service providers Public & private Project management TCIC partners FIGO Initiative on Unsafe Abortion Regional meetings 2011 NESOG Nursing Council Medical Council PSI NHTC/DHS CREHPA Operations Research Material development Sun Pharma Training Product availability NESOG Ipas Concept
ff3b4a79c5bd6421943b8f11e96ed14a.ppt