32228c73b947bcc449919e4fdd0ef298.ppt
- Количество слайдов: 90

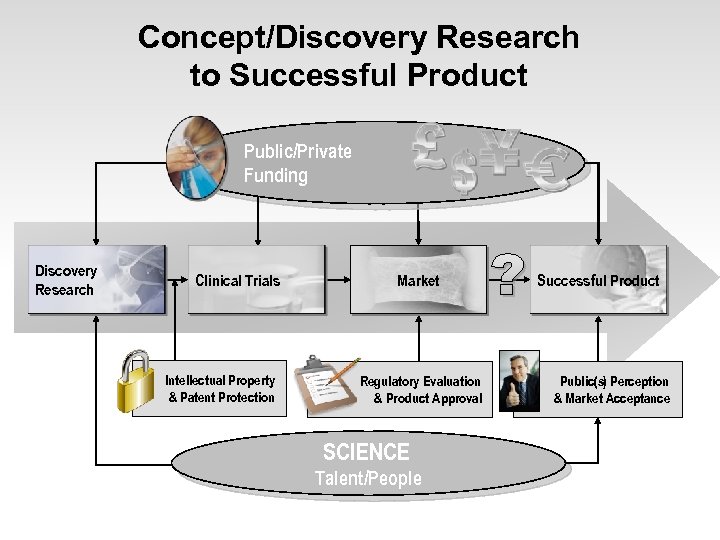
Concept/Discovery Research to Successful Product Public/Private Funding Discovery Research Clinical Trials Intellectual Property & Patent Protection Market Regulatory Evaluation & Product Approval SCIENCE Talent/People Successful Product Public(s) Perception & Market Acceptance

TERMIS-EU Industry Committee

TERMIS-AM Industry Committee

TERMIS-AM Industry Committee ‘Commercialization Hurdles’

TERMIS-AM Industry Committee ‘Funding’




European Regulatory environment of regenerative medicine TERMIS Industry Symposium 7 September 2012, Vienna (Austria) Presented by: Lucia D’Apote, Ph. D European Medicines Agency An agency of the European Union

Overview 11 Lucia D’Apote - EMA

12 Lucia D’Apote - EMA

Established in 1993, operational since 1995 13 Lucia D'Apote - EMA

The ATMP Regulation 14 Lucia D'Apote - EMA

ATMP Pipeline – what we see 15 Lucia D'Apote - EMA

Objective : Facilitate development of ATMPs and access to MA procedure 16 http: //www. ema. europa. eu/doc s/en_GB/document_library/Wor k_programme/2010/11/WC 500 099029. pdf

ATMP Pipeline – what we will see 17 Lucia D'Apote - EMA

Products 18
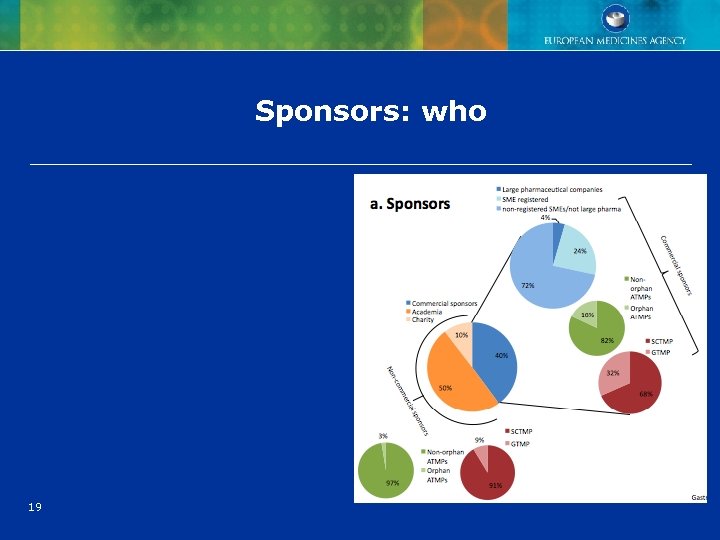
Sponsors: who 19

Sponsors: from where 20
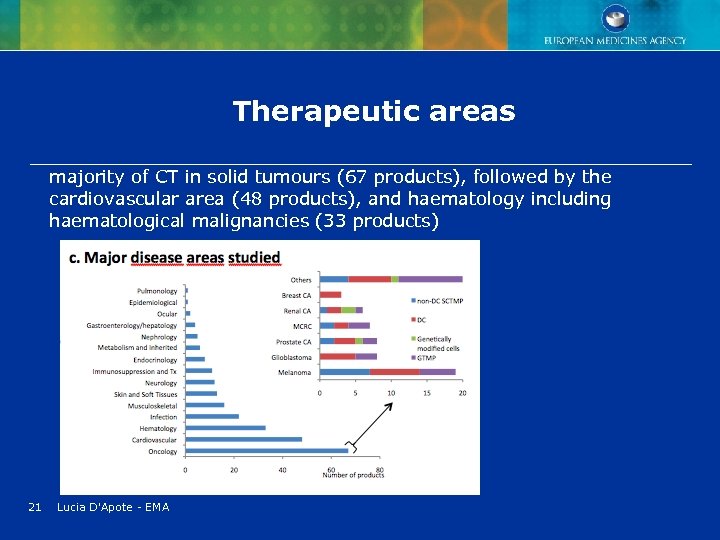
Therapeutic areas majority of CT in solid tumours (67 products), followed by the cardiovascular area (48 products), and haematology including haematological malignancies (33 products) 21 Lucia D'Apote - EMA

Orphan ATMPs 26 CT with Orphan ATMPs Mainly commercial sponsors Majority with cellbased products 75 ODD so far are ATMPs !!! 22 Lucia D'Apote - EMA

23 Lucia D’Apote - EMA

EU Marketing Authorisation (MA) for ATMPs A medicinal product may only be placed on the market in the EU, when a marketing authorisation has been issued by the European Commission (via the Centralised Procedure – EMA) or it is regulated by the competent authority of a EU Member State (hospital exemption) 24 EMA - Lucia D'APOTE 24
25 Lucia D'Apote - EMA

Risk based approach 26 Lucia D'Apote - EMA

Risk-management plan and follow-up safety/efficacy 27 Lucia D'Apote - EMA

Same evaluation, different MA? • Conditional approval vs Exceptional circumstances - to meet unmet medical needs of patients and in the interest of public health 28 Lucia D'Apote - EMA

Accellerated assessment 150 days - in order to meet, in particular the legitimate expectations of patients and to take account of the increasingly rapid progress of science and therapies, for medicinal products of major interest from the point of view of public health in and particular from the view point of therapeutic innovation. - There is no single definition of what constitutes major public health interest. This should be justified by the applicant on a case-by-case basis. 29 Lucia D'Apote - EMA

30 Lucia D’Apote - EMA

ATMP Translation: perceived challenges 31 Lucia D’Apote - EMA

Challenges with ATMPs: examples • Scientific challenges – Manufacturing constraints & quality issues – Non-clinical challenges – Clinical challenges Disclaimer: ATMPs are a very diverse group of products, so the challenges listed in the next slides are only examples! 32

Quality/manufacturing issues - Control of all starting and raw materials - Recombinant growth factors - Human cells/tissues + any human/animal reagents (e. g serum) History of cell-lines / vector constructs Appropriate characterisation and product testing (including potency assay*) - Poor definition and control of a product may directly effect safety & efficacy - Good control of the product is essential for manufacturing changes (e. g. product upscale) - * 33 Manufacture in GMP environment Potency assay: product specific, at least semi-quantitative, linking product testing with clinical effect /biological activity

Non-clinical challenges – What animal models to be used to test a human cell-based therapy or gene therapy product? – Use of a homologous model? / Disease models? / Other relevant animal models? – Proof of concept studies / toxicity studies – Dose finding studies? – Bio-distribution studies? – Germ line transmission for GTMP – Environmental risk / Shedding studies for GTMP 34

Clinical challenges – Dose finding studies – How to find the most effective dose, e. g. for a TEP? – Design of clinical trial – What is a suitable compatitor? – Blinding might be very difficult – Endpoints for TEP (how to measure structure repair? ) – Effect of concomitment treatment / surgery on Efficacy & Safety? – Long term efficacy and safety follow-up studies 35

Challenges with ATMPs • Scientific challenges – Yes! • But not all challenges are scientific! – Regulatory issues – Lack of regulatory expertise – Resources – Reimbursement issues – Competition with ‘hospital exempted ATMPs’ 36

Prospective product development 37 Lucia D'Apote - EMA Courtesy of dr. Paula Salmikangas, 2012

Retrospective product development Courtesy of dr. Paula Salmikangas, 2012 38 Lucia D'Apote - EMA

The way forward 39 Lucia D’Apote - EMA

Nature Reviews Drug Discovery, vol 9, March 2010, 185 -201 Regulatory Rapporteur, vol 8, July-August 2011, 4 -7 40

Advice during development 41 Lucia D'Apote - EMA

Regulatory strategy: save time Scientific advice Complying with SA/PA is significantly : associated with positive outcome Regnstrom J, Koenig F, Aronsson B, et al. (2010) Factors associated with success of market authorisation applications for pharmaceutical drugs submitted to the European Medicines Agency; EJCP 66: 39 -48 42 Lucia D'Apote - EMA
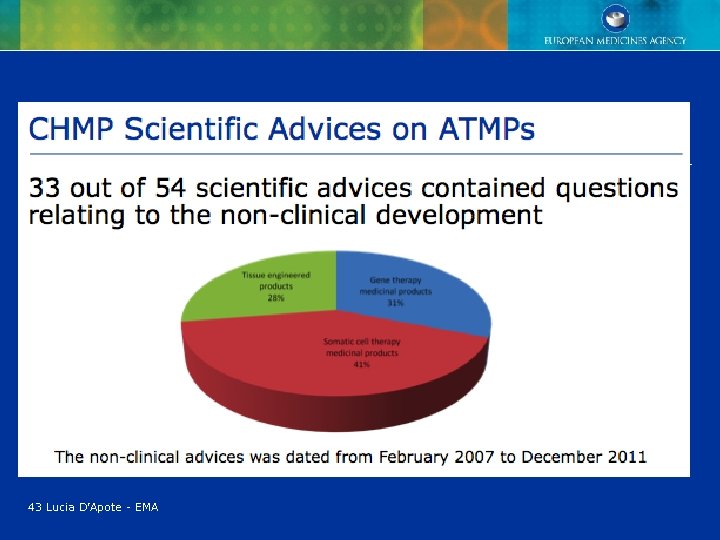
43 Lucia D’Apote - EMA
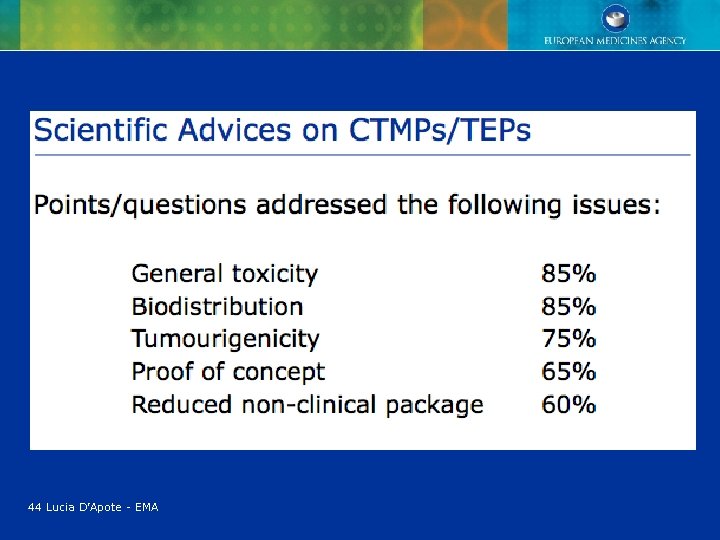
44 Lucia D’Apote - EMA

International Cooperation (EMA-FDA) http: //www. ema. europa. eu/docs/en_GB/document_libra ry/Other/2009/11/WC 500014868. pdf 45 Lucia D’Apote - EMA

Parallel Advice FDA Confidentiality Agreement FDA Applicant to address request to both Agencies Agreement principles since 2004 pilot Applicant initiative- exceptionally Agency initiative 46

Procedure Parallel Advice Initial discussion both Agencies Prime candidates-breakthrough products – no GL exist or GLs differ between Agencies Submit request in usual manner Timetable agreed between Agencies Tele-video-conference about D 60 47

Outcome No applicant involvement in draft reports Parallel separate advice given not ‘joint’ advice Confidentiality maintained Standard fee applies 48

Thank you for your attention! Lucia D’APOTE European Medicines Agency (EMA) lucia. dapote@ema. europa. eu 49 Lucia D'Apote - EMA


Outline

FDA Mission Statement

FDA Organization

OCTGT Activities
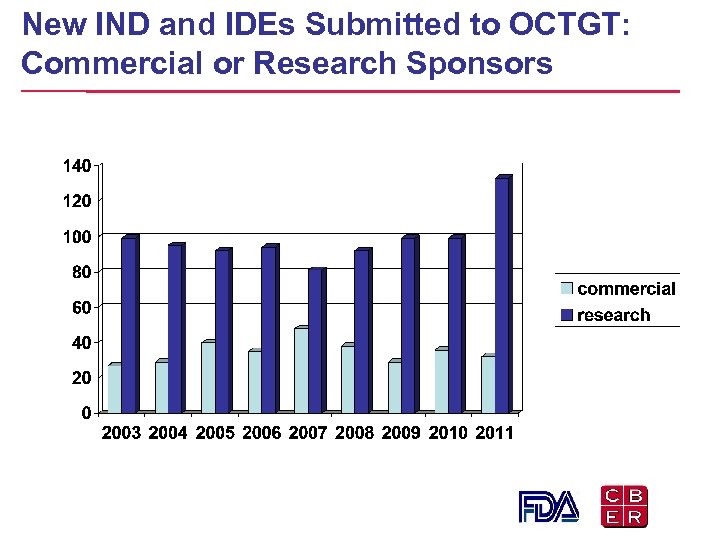
New IND and IDEs Submitted to OCTGT: Commercial or Research Sponsors

Examples of OCTGT Products





Recent CBER Guidances


Recent CTGTAC Advisory Committee Topics




Asia Pacific Economic Cooperation-Life Sciences Innovation Forum (APEC/LSIF)


Regulations vs. Standards



Use of Standards in CBER

International Clinical Trials

International Clinical Trials



Expanding Access to Investigational Drugs



OCTGT Regulatory Resources

Public Access to CBER

Contact Information




Regulatory Imperative Session Summary

Regulatory Imperative Session Summary

Regulatory Imperative Session Summary

A copy of the slides for Session 2 will be available online via the TERMIS website, www. termis. org.
32228c73b947bcc449919e4fdd0ef298.ppt