9ef6d82b0d9b417b13e4cbd965b67997.ppt
- Количество слайдов: 27
 computational studies of cathinone and cathinone derivatives KIARA LIPSEY MENTOR: TIFFANI HOLMES, PH. D. LOUIS STOKES ALLIANCE FOR MINORITY PARTICIPATION SUMMER RESEARCH PROGRAM FORT VALLEY STATE UNIVERSITY JULY 31, 2014
computational studies of cathinone and cathinone derivatives KIARA LIPSEY MENTOR: TIFFANI HOLMES, PH. D. LOUIS STOKES ALLIANCE FOR MINORITY PARTICIPATION SUMMER RESEARCH PROGRAM FORT VALLEY STATE UNIVERSITY JULY 31, 2014
 Table of Contents Ø Khat……………………………………. 1 Ø Cathinone…………………………………. . 2 Ø Synthetic Cathinone…………………………. 3 Ø Government Regulations………………………. . 4 Ø Possible Poisonings…………………………. . 5 Ø Pharmacological Effects………………………. 6 Ø Cathinone Derivatives…………………………. 7 Ø Previous Studies……………………………. . 8
Table of Contents Ø Khat……………………………………. 1 Ø Cathinone…………………………………. . 2 Ø Synthetic Cathinone…………………………. 3 Ø Government Regulations………………………. . 4 Ø Possible Poisonings…………………………. . 5 Ø Pharmacological Effects………………………. 6 Ø Cathinone Derivatives…………………………. 7 Ø Previous Studies……………………………. . 8
 Is a shrub or small tree Khat indigenous to East Africa and the Arabian Peninsula. Chewing the khat leaves results in release of the psychoactive alkaloids cathinone and cathine. In 1975 Cathinone was isolated from the Khat leaves and determined to be its principal psychoactive component.
Is a shrub or small tree Khat indigenous to East Africa and the Arabian Peninsula. Chewing the khat leaves results in release of the psychoactive alkaloids cathinone and cathine. In 1975 Cathinone was isolated from the Khat leaves and determined to be its principal psychoactive component.
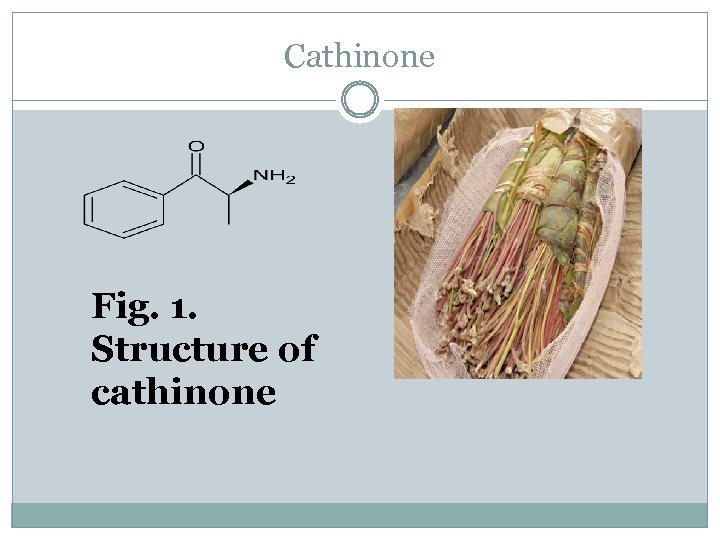 Cathinone Fig. 1. Structure of cathinone
Cathinone Fig. 1. Structure of cathinone
 Synthetic cathinones are found in Synthetic Cathinones bath salts. Bath salts refers to an emerging family of drugs containing one or more synthetic chemicals related to cathinone an amphetamine like stimulant found naturally in the Khat plant. Mephedrone and methylene dioxyprovalerone are currently the predominantly abused synthetic cathinone.
Synthetic cathinones are found in Synthetic Cathinones bath salts. Bath salts refers to an emerging family of drugs containing one or more synthetic chemicals related to cathinone an amphetamine like stimulant found naturally in the Khat plant. Mephedrone and methylene dioxyprovalerone are currently the predominantly abused synthetic cathinone.
 Bath Salt Incidents ABC News Article May 2012 Randy Eugene, a 31 -year-old homeless man, was shot to death after he had consumed 75 percent of the flesh on Poppo's face. A suspect who had torn off his clothes was hit by a taxi and then beat the occupants of the vehicle. It took 15 officers to stop him. Behavior was consistent with other incidents involving bath salts in Miami.
Bath Salt Incidents ABC News Article May 2012 Randy Eugene, a 31 -year-old homeless man, was shot to death after he had consumed 75 percent of the flesh on Poppo's face. A suspect who had torn off his clothes was hit by a taxi and then beat the occupants of the vehicle. It took 15 officers to stop him. Behavior was consistent with other incidents involving bath salts in Miami.
 Law makers cannot keep pace Government Regulations with bath salt producers To control the spread problem , the Drug Enforcement Agency issued a temporary ban in October on three of the most common drugs: mephedrone methylone MDPV (methylene dioxyprovalerone)
Law makers cannot keep pace Government Regulations with bath salt producers To control the spread problem , the Drug Enforcement Agency issued a temporary ban in October on three of the most common drugs: mephedrone methylone MDPV (methylene dioxyprovalerone)
 Possible Poisioning After the consumption of bath salts, users have reported symptoms of poisoning. Some symptoms are: Paranoia and violent behavior Hallucinations Delusions Suicidal thoughts Panic attacks Increased heart rate Increased blood pressure
Possible Poisioning After the consumption of bath salts, users have reported symptoms of poisoning. Some symptoms are: Paranoia and violent behavior Hallucinations Delusions Suicidal thoughts Panic attacks Increased heart rate Increased blood pressure
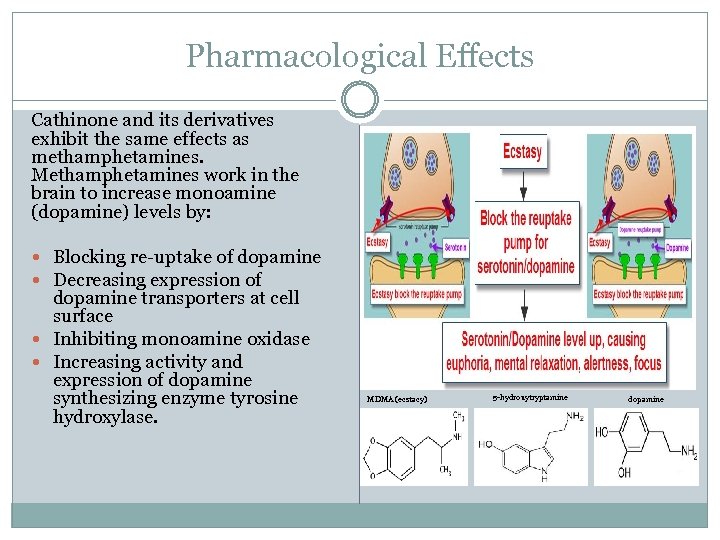 Pharmacological Effects Cathinone and its derivatives exhibit the same effects as methamphetamines. Methamphetamines work in the brain to increase monoamine (dopamine) levels by: Blocking re-uptake of dopamine Decreasing expression of dopamine transporters at cell surface Inhibiting monoamine oxidase Increasing activity and expression of dopamine synthesizing enzyme tyrosine hydroxylase. MDMA (ecstacy) 5 -hydroxytryptamine dopamine
Pharmacological Effects Cathinone and its derivatives exhibit the same effects as methamphetamines. Methamphetamines work in the brain to increase monoamine (dopamine) levels by: Blocking re-uptake of dopamine Decreasing expression of dopamine transporters at cell surface Inhibiting monoamine oxidase Increasing activity and expression of dopamine synthesizing enzyme tyrosine hydroxylase. MDMA (ecstacy) 5 -hydroxytryptamine dopamine
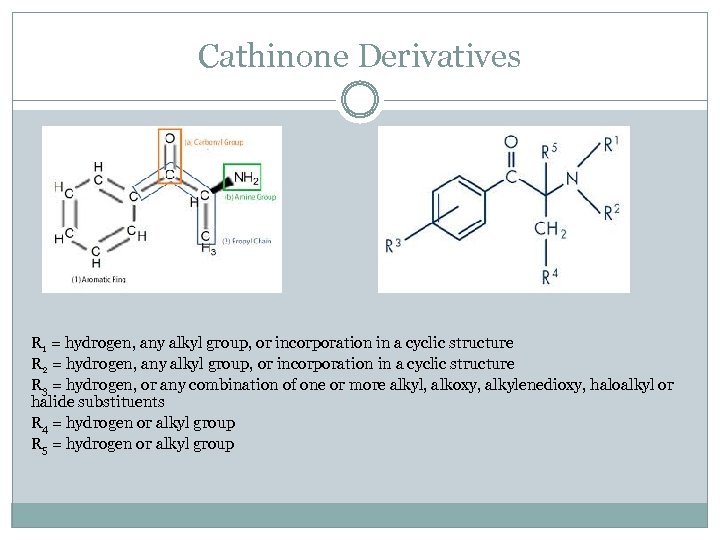 Cathinone Derivatives R 1 = hydrogen, any alkyl group, or incorporation in a cyclic structure R 2 = hydrogen, any alkyl group, or incorporation in a cyclic structure R 3 = hydrogen, or any combination of one or more alkyl, alkoxy, alkylenedioxy, haloalkyl or halide substituents R 4 = hydrogen or alkyl group R 5 = hydrogen or alkyl group
Cathinone Derivatives R 1 = hydrogen, any alkyl group, or incorporation in a cyclic structure R 2 = hydrogen, any alkyl group, or incorporation in a cyclic structure R 3 = hydrogen, or any combination of one or more alkyl, alkoxy, alkylenedioxy, haloalkyl or halide substituents R 4 = hydrogen or alkyl group R 5 = hydrogen or alkyl group
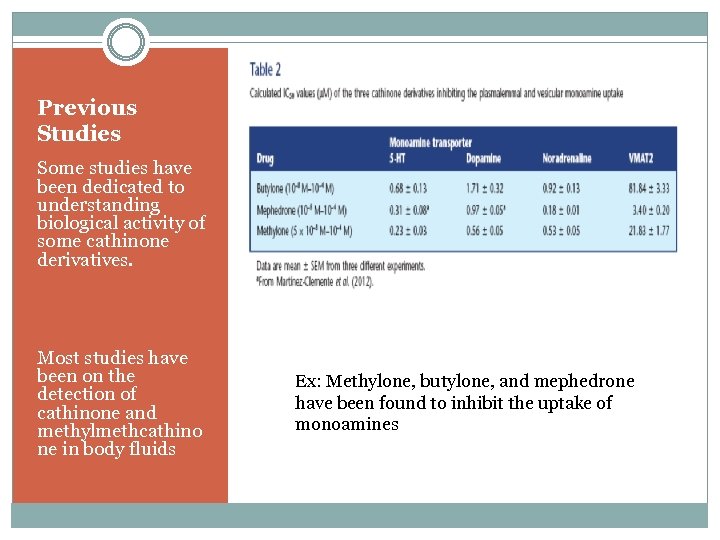 Previous Studies Some studies have been dedicated to understanding biological activity of some cathinone derivatives. Most studies have been on the detection of cathinone and methylmethcathino ne in body fluids Ex: Methylone, butylone, and mephedrone have been found to inhibit the uptake of monoamines
Previous Studies Some studies have been dedicated to understanding biological activity of some cathinone derivatives. Most studies have been on the detection of cathinone and methylmethcathino ne in body fluids Ex: Methylone, butylone, and mephedrone have been found to inhibit the uptake of monoamines
 Aim To determine the electronic structure of compounds not yet synthesized. The electronic structures will allow for the prediction of the biological activity of these compounds.
Aim To determine the electronic structure of compounds not yet synthesized. The electronic structures will allow for the prediction of the biological activity of these compounds.
 Methods Modeled structures using Gauss. View 5 Carried out structure optimization and vibrational frequency calculations using the Gaussian 09 program NBO analysis Calculated molecular descriptors from output Dipole Moment Energy of HOMO Energy of LUMO Polarizability
Methods Modeled structures using Gauss. View 5 Carried out structure optimization and vibrational frequency calculations using the Gaussian 09 program NBO analysis Calculated molecular descriptors from output Dipole Moment Energy of HOMO Energy of LUMO Polarizability
 Programs Gauss View SSH Secure Shell Client
Programs Gauss View SSH Secure Shell Client
 Density Functional Theory (DFT) widely used method for theoretical calculations of the structure of atoms, molecules, surfaces and their interactions. Methods Basis Set set of functions used to describe the molecular orbitals or density of a compound. Example: B 3 LYP/6 -31 G (d) B 3 LYP/6 -31 G (d, p) B 3 LYP/6 -311++G (d, p)
Density Functional Theory (DFT) widely used method for theoretical calculations of the structure of atoms, molecules, surfaces and their interactions. Methods Basis Set set of functions used to describe the molecular orbitals or density of a compound. Example: B 3 LYP/6 -31 G (d) B 3 LYP/6 -31 G (d, p) B 3 LYP/6 -311++G (d, p)
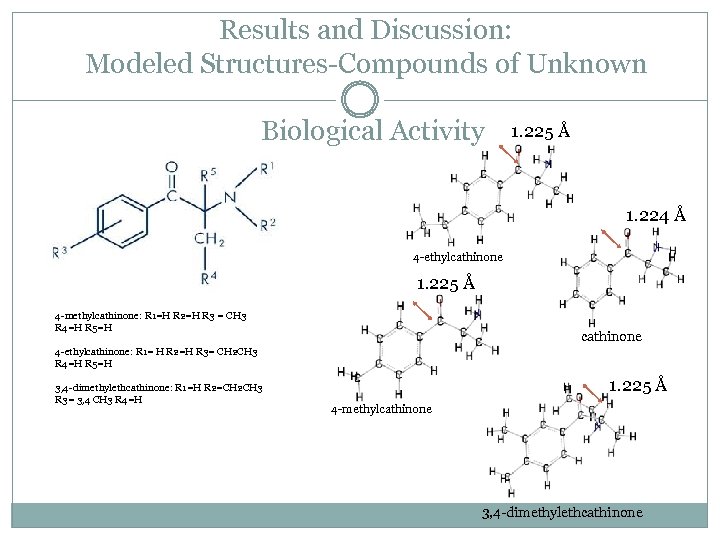 Results and Discussion: Modeled Structures-Compounds of Unknown Biological Activity 1. 225 Å 1. 224 Å 4 -ethylcathinone 1. 225 Å 4 -methylcathinone: R 1=H R 2=H R 3 = CH 3 R 4=H R 5=H cathinone 4 -ethylcathinone: R 1= H R 2=H R 3= CH 2 CH 3 R 4=H R 5=H 3, 4 -dimethylethcathinone: R 1=H R 2=CH 2 CH 3 R 3= 3, 4 CH 3 R 4=H 1. 225 Å 4 -methylcathinone 3, 4 -dimethylethcathinone
Results and Discussion: Modeled Structures-Compounds of Unknown Biological Activity 1. 225 Å 1. 224 Å 4 -ethylcathinone 1. 225 Å 4 -methylcathinone: R 1=H R 2=H R 3 = CH 3 R 4=H R 5=H cathinone 4 -ethylcathinone: R 1= H R 2=H R 3= CH 2 CH 3 R 4=H R 5=H 3, 4 -dimethylethcathinone: R 1=H R 2=CH 2 CH 3 R 3= 3, 4 CH 3 R 4=H 1. 225 Å 4 -methylcathinone 3, 4 -dimethylethcathinone
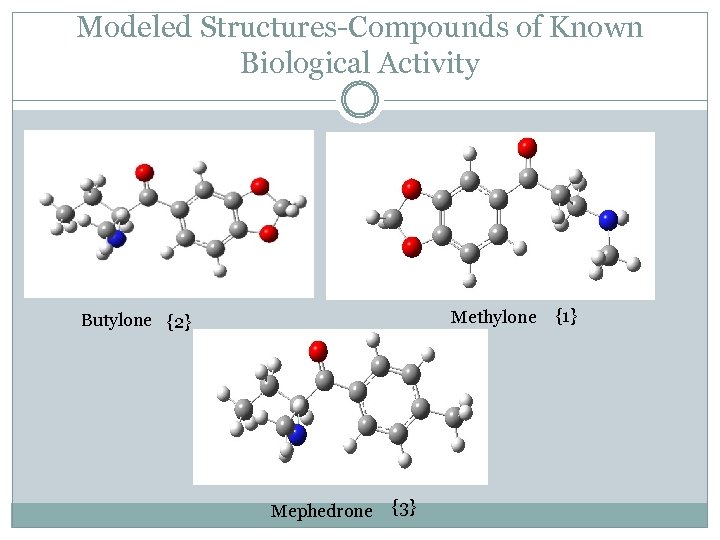 Modeled Structures-Compounds of Known Biological Activity Methylone Butylone {2} Mephedrone {3} {1}
Modeled Structures-Compounds of Known Biological Activity Methylone Butylone {2} Mephedrone {3} {1}
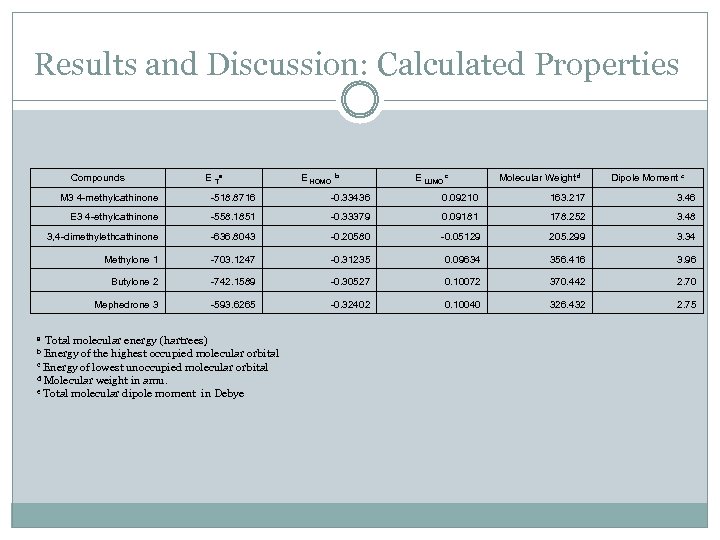 Results and Discussion: Calculated Properties Compounds E Ta E HOMO b E LUMO c Molecular Weight d Dipole Moment e M 3 4 -methylcathinone -518. 8716 -0. 33436 0. 09210 163. 217 3. 46 E 3 4 -ethylcathinone -558. 1851 -0. 33379 0. 09181 178. 252 3. 48 3, 4 -dimethylethcathinone -636. 8043 -0. 20580 -0. 05129 Methylone 1 -703. 1247 -0. 31235 0. 09634 356. 416 3. 96 Butylone 2 -742. 1589 -0. 30527 0. 10072 370. 442 2. 70 Mephedrone 3 -593. 6265 -0. 32402 0. 10040 326. 432 2. 75 Total molecular energy (hartrees) Energy of the highest occupied molecular orbital c Energy of lowest unoccupied molecular orbital d Molecular weight in amu. e Total molecular dipole moment in Debye a b 205. 299 3. 34
Results and Discussion: Calculated Properties Compounds E Ta E HOMO b E LUMO c Molecular Weight d Dipole Moment e M 3 4 -methylcathinone -518. 8716 -0. 33436 0. 09210 163. 217 3. 46 E 3 4 -ethylcathinone -558. 1851 -0. 33379 0. 09181 178. 252 3. 48 3, 4 -dimethylethcathinone -636. 8043 -0. 20580 -0. 05129 Methylone 1 -703. 1247 -0. 31235 0. 09634 356. 416 3. 96 Butylone 2 -742. 1589 -0. 30527 0. 10072 370. 442 2. 70 Mephedrone 3 -593. 6265 -0. 32402 0. 10040 326. 432 2. 75 Total molecular energy (hartrees) Energy of the highest occupied molecular orbital c Energy of lowest unoccupied molecular orbital d Molecular weight in amu. e Total molecular dipole moment in Debye a b 205. 299 3. 34
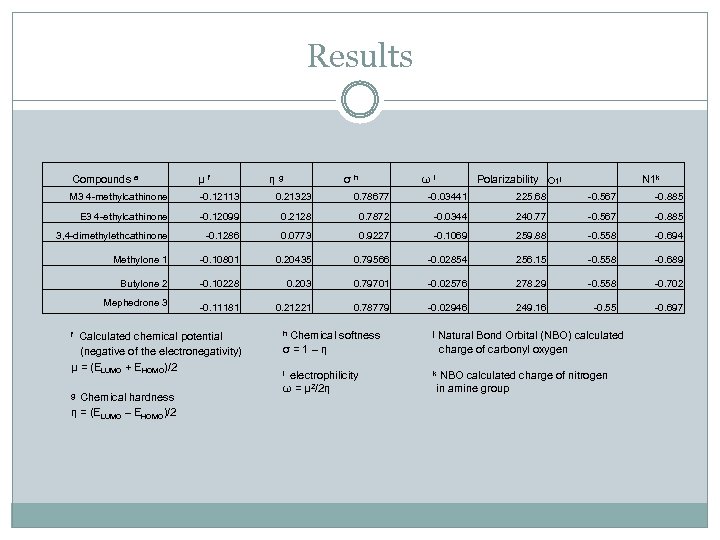 Results Compounds a μ f M 3 4 -methylcathinone -0. 12113 0. 21323 0. 78677 -0. 03441 225. 68 -0. 567 -0. 885 E 3 4 -ethylcathinone -0. 12099 0. 2128 0. 7872 -0. 0344 240. 77 -0. 567 -0. 885 3, 4 -dimethylethcathinone -0. 1286 0. 0773 0. 9227 -0. 1069 259. 88 -0. 558 -0. 694 Methylone 1 -0. 10801 0. 20435 0. 79566 -0. 02854 256. 15 -0. 558 -0. 689 Butylone 2 -0. 10228 0. 203 0. 79701 -0. 02576 278. 29 -0. 558 -0. 702 -0. 11181 0. 21221 0. 78779 -0. 02946 249. 16 -0. 55 -0. 697 Mephedrone 3 η g σ h ω i Polarizability O 1 j N 1 k f Calculated chemical potential h Chemical softness j Natural Bond Orbital (NBO) calculated (negative of the electronegativity) μ = (ELUMO + EHOMO)/2 g Chemical hardness η = (ELUMO – EHOMO)/2 σ = 1 – η i electrophilicity ω = μ 2/2η charge of carbonyl oxygen k NBO calculated charge of nitrogen in amine group
Results Compounds a μ f M 3 4 -methylcathinone -0. 12113 0. 21323 0. 78677 -0. 03441 225. 68 -0. 567 -0. 885 E 3 4 -ethylcathinone -0. 12099 0. 2128 0. 7872 -0. 0344 240. 77 -0. 567 -0. 885 3, 4 -dimethylethcathinone -0. 1286 0. 0773 0. 9227 -0. 1069 259. 88 -0. 558 -0. 694 Methylone 1 -0. 10801 0. 20435 0. 79566 -0. 02854 256. 15 -0. 558 -0. 689 Butylone 2 -0. 10228 0. 203 0. 79701 -0. 02576 278. 29 -0. 558 -0. 702 -0. 11181 0. 21221 0. 78779 -0. 02946 249. 16 -0. 55 -0. 697 Mephedrone 3 η g σ h ω i Polarizability O 1 j N 1 k f Calculated chemical potential h Chemical softness j Natural Bond Orbital (NBO) calculated (negative of the electronegativity) μ = (ELUMO + EHOMO)/2 g Chemical hardness η = (ELUMO – EHOMO)/2 σ = 1 – η i electrophilicity ω = μ 2/2η charge of carbonyl oxygen k NBO calculated charge of nitrogen in amine group
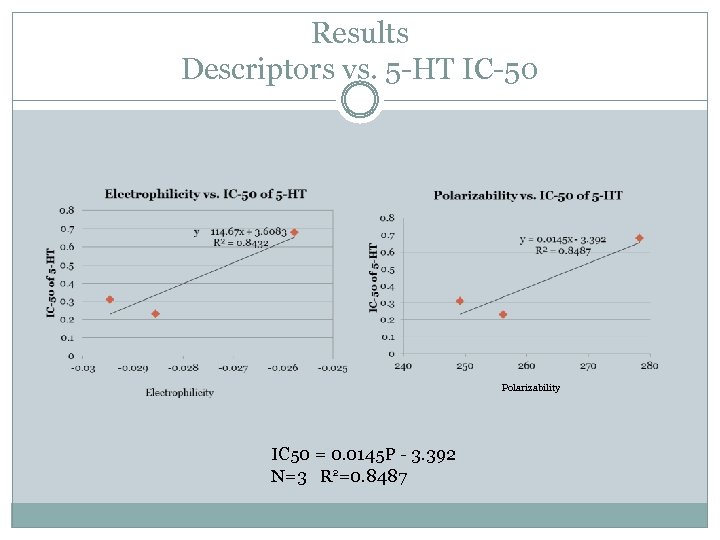 Results Descriptors vs. 5 -HT IC-50 Polarizability IC 50 = 0. 0145 P - 3. 392 N=3 R 2=0. 8487
Results Descriptors vs. 5 -HT IC-50 Polarizability IC 50 = 0. 0145 P - 3. 392 N=3 R 2=0. 8487
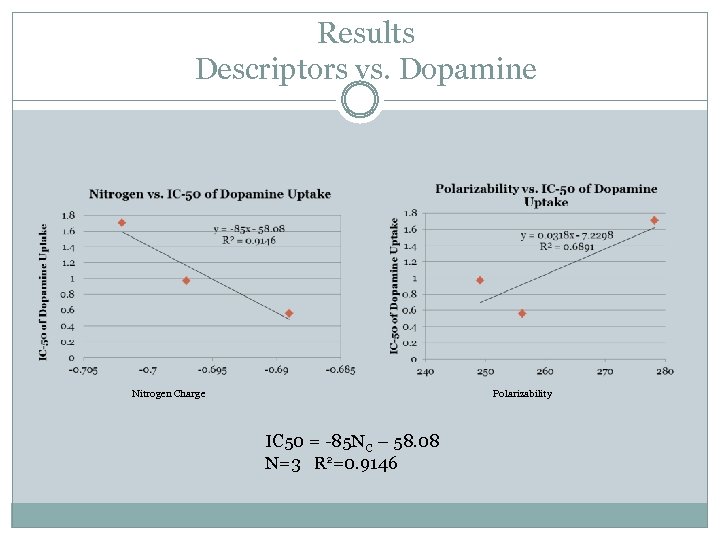 Results Descriptors vs. Dopamine Nitrogen Charge Polarizability IC 50 = -85 NC – 58. 08 N=3 R 2=0. 9146
Results Descriptors vs. Dopamine Nitrogen Charge Polarizability IC 50 = -85 NC – 58. 08 N=3 R 2=0. 9146
 Conclusions No significant changes in derivative bond lengths Good correlation between polarizability and IC-50 of 5 -HT. Good correlation between inhibition of dopamine uptake and nitrogen charge.
Conclusions No significant changes in derivative bond lengths Good correlation between polarizability and IC-50 of 5 -HT. Good correlation between inhibition of dopamine uptake and nitrogen charge.
 Future Work Model validation Increase the size of the compound training set. More experimental data from a single source is needed. Identify a test set of compounds.
Future Work Model validation Increase the size of the compound training set. More experimental data from a single source is needed. Identify a test set of compounds.
 References
References
 References
References
![References [19] References [19]](https://present5.com/presentation/9ef6d82b0d9b417b13e4cbd965b67997/image-26.jpg) References [19]"Face-Eating Cannibal Attack May Be Latest in String of 'Bath Salts' Incidents. " ABC News Network, n. d. Web. 16 July 2014.
References [19]"Face-Eating Cannibal Attack May Be Latest in String of 'Bath Salts' Incidents. " ABC News Network, n. d. Web. 16 July 2014.
 Acknowledgements Fort Valley State University’s Department of Chemistry FVSU’s Peach State Louis Stokes Alliance for Minority Participation Program Dr. T. M. Holmes Dr. Dwayne Daniels Dr. Robin Bright
Acknowledgements Fort Valley State University’s Department of Chemistry FVSU’s Peach State Louis Stokes Alliance for Minority Participation Program Dr. T. M. Holmes Dr. Dwayne Daniels Dr. Robin Bright


