b0c7895c3c83bc93d065dbfd91697462.ppt
- Количество слайдов: 14

Compulsory Licensing in Thailand Inthira Yamabhai Researcher, Health Intervention and Technology Assessment Program Bureau of Policy and Strategy-Ministry of Public Health, Thailand Five years from the Decision to the action – is the 2003 August 30 Decision “the expeditious solution” for access to medicines we need? 25 September, 2008
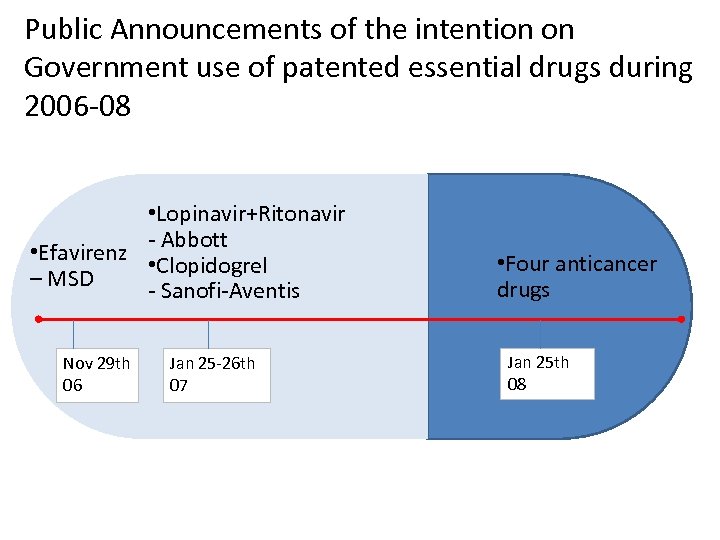
Public Announcements of the intention on Government use of patented essential drugs during 2006 -08 • Lopinavir+Ritonavir - Abbott • Efavirenz • Clopidogrel – MSD - Sanofi-Aventis Nov 29 th 06 Jan 25 -26 th 07 • Four anticancer drugs Jan 25 th 08
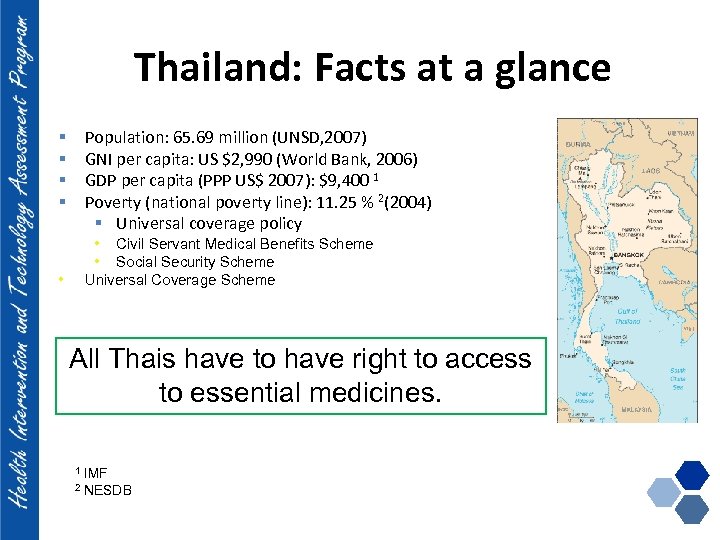
Thailand: Facts at a glance Population: 65. 69 million (UNSD, 2007) GNI per capita: US $2, 990 (World Bank, 2006) GDP per capita (PPP US$ 2007): $9, 400 1 Poverty (national poverty line): 11. 25 % 2(2004) § Universal coverage policy § § • Civil Servant Medical Benefits Scheme • Social Security Scheme Universal Coverage Scheme • All Thais have to have right to access to essential medicines. 1 2 IMF NESDB
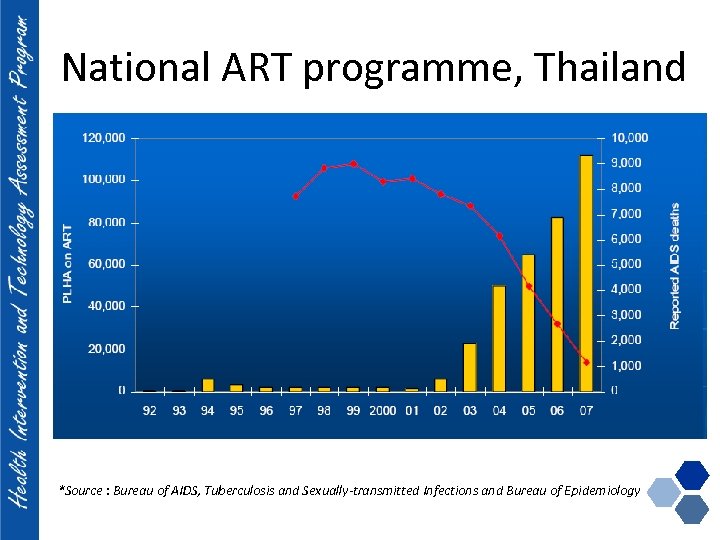
National ART programme, Thailand *Source : Bureau of AIDS, Tuberculosis and Sexually-transmitted Infections and Bureau of Epidemiology
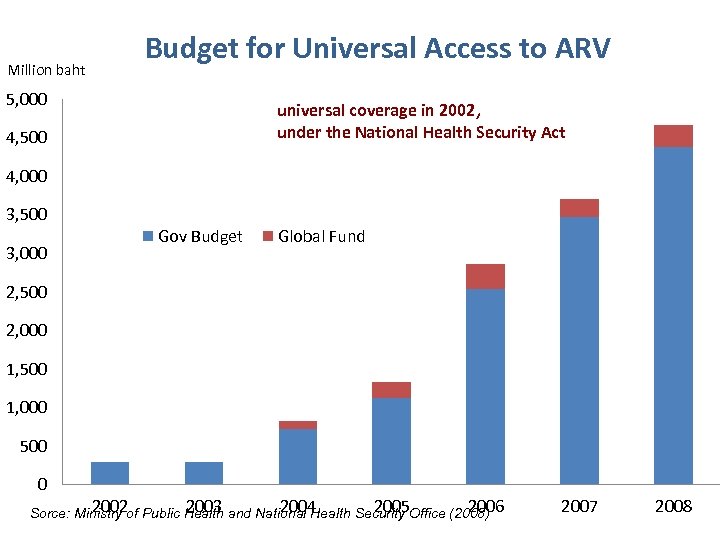
Million baht Budget for Universal Access to ARV 5, 000 universal coverage in 2002, under the National Health Security Act 4, 500 4, 000 3, 500 3, 000 Gov Budget Global Fund 2, 500 2, 000 1, 500 1, 000 500 0 2002 2003 2004 2005 2006 Sorce: Ministry of Public Health and National Health Security Office (2008) 2007 2008
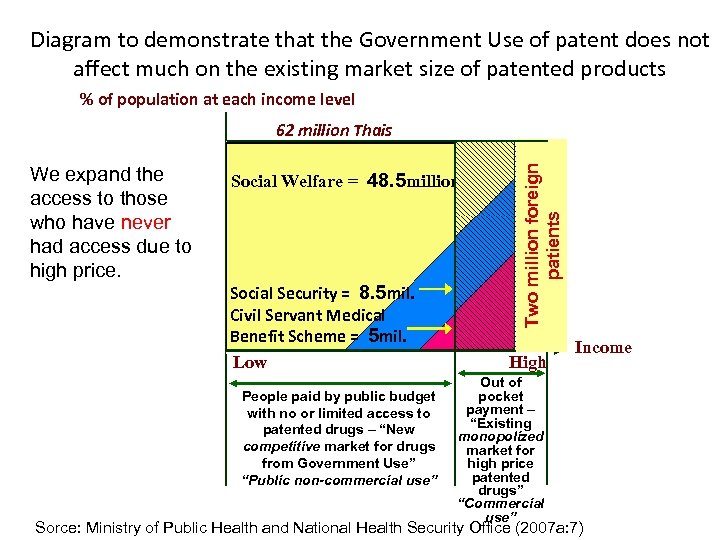
Diagram to demonstrate that the Government Use of patent does not affect much on the existing market size of patented products % of population at each income level We expand the access to those who have never had access due to high price. Social Welfare = 48. 5 million Social Security = 8. 5 mil. Civil Servant Medical Benefit Scheme = 5 mil. Low People paid by public budget with no or limited access to patented drugs – “New competitive market for drugs from Government Use” “Public non-commercial use” Two million foreign patients 62 million Thais High Out of pocket payment – “Existing monopolized market for high price patented drugs” “Commercial use” Income Sorce: Ministry of Public Health and National Health Security Office (2007 a: 7)
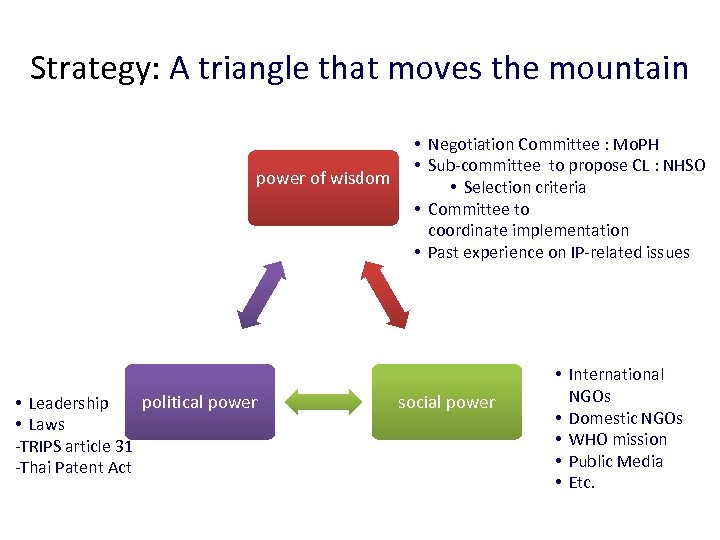
Strategy: A triangle that moves the mountain power of wisdom political power • Leadership • Laws -TRIPS article 31 -Thai Patent Act • Negotiation Committee : Mo. PH • Sub-committee to propose CL : NHSO • Selection criteria • Committee to coordinate implementation • Past experience on IP-related issues social power • International NGOs • Domestic NGOs • WHO mission • Public Media • Etc.
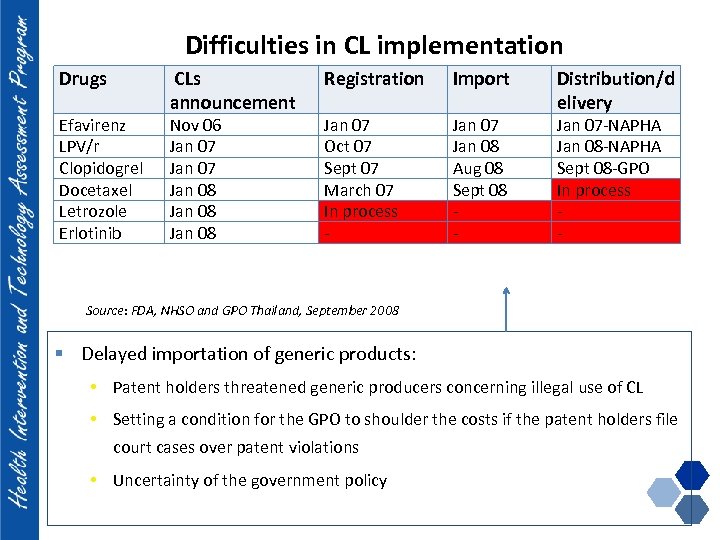
Difficulties in CL implementation Drugs Efavirenz LPV/r Clopidogrel Docetaxel Letrozole Erlotinib CLs announcement Nov 06 Jan 07 Jan 08 Registration Import Jan 07 Oct 07 Sept 07 March 07 In process - Jan 07 Jan 08 Aug 08 Sept 08 - Distribution/d elivery Jan 07 -NAPHA Jan 08 -NAPHA Sept 08 -GPO In process - Source: FDA, NHSO and GPO Thailand, September 2008 § Delayed importation of generic products: • Patent holders threatened generic producers concerning illegal use of CL • Setting a condition for the GPO to shoulder the costs if the patent holders file court cases over patent violations • Uncertainty of the government policy
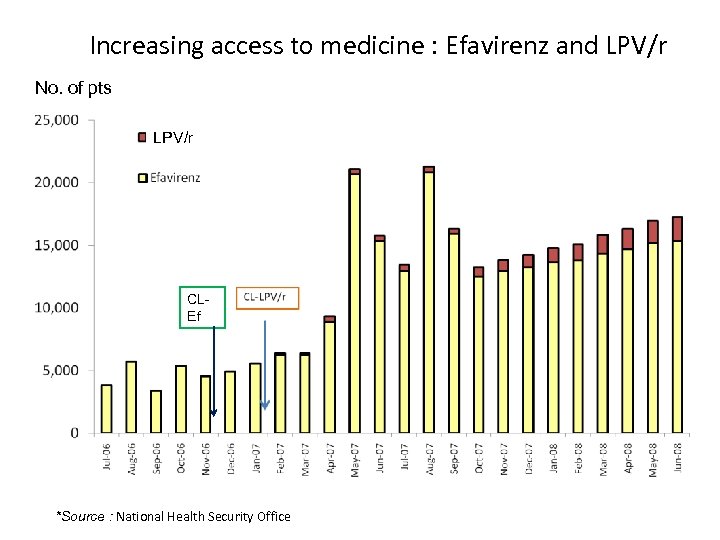
Increasing access to medicine : Efavirenz and LPV/r No. of pts LPV/r CLEf *Source : National Health Security Office

Negative responses § Priority Watch List (PWL) status and trade retaliation • GSP cut on three exports (flat-screen TV, gold jewelry, ethylene terephthalate) § Threaten to withdraw foreign investments § Threaten to file cases to the Administration and IP Courts § Withdrawal of new medicine registration application § Propaganda to undermine the country’s image

The current and future movements § § In February 2008, the new minister announced reconsideration of the CL. The SG of the FDA was moved. The GPO board was revised. Strong public reactions brought back the CL implementation and the minister is now out of office. The GPO board was brought back. The National Health Security Board re-establishes the Committee to improve the access to essential medicines. This is the committee that works on the CL proposal. Establishing improving access to medicine committee which consist of MOPH, MOF, MFA, MOC, MOI, PREMA, Patient network.

What have we learned? § TRIPs flexibilities are possible with sufficient knowledge and skills and social and political support. § TRIPs flexibilities did bring the prices down and improve access to essential medicines. § Logistics management
CL studies in Thailand • Introducing government use of patents on essential medicines in Thailand, 2006 -2007 http: //ihppthaigov. net/index. php? option=com_content&task=view&id=138&Itemid=1 42 • The implications of CL on essential medicines in Thailand Assessment framework Economics Health Psychosocial Health/drug expenditure Access to medicines (+/-) Country image Export values Quality of generic drugs under CL Public awareness on IP and human rights issues Health gains Long-term effects: Foreign direct investments Productivity • innovations • confidence in investments 13

Thank you for your attention For more information, please contact : inthira@ihpp. thaigov. net
b0c7895c3c83bc93d065dbfd91697462.ppt