6b8bce9e2e341abc150558c1f167f71d.ppt
- Количество слайдов: 84

Complex Coronary Cases Supported by: • Abbott Vascular • Boston Scientific Corporation • Medtronic, Inc. • Astra. Zeneca • St Jude’s Medical • Abiomed • Vascular solution • CSI Inc.

Disclosures Samin K. Sharma, MBBS, FACC Speaker’s Bureau – Boston Scientific Corporation, Abbott Vascular Inc, Angio. Score, The Medicines Company, Daiichi Sankyo Inc, Abiomed Annapoorna S. Kini, MBBS, FACC Nothing to disclose Sameer Mehta, MBBS, FACC Consulting Fees – The Medicines Company American College of Cardiology Foundation staff involved with this case have nothing to disclose
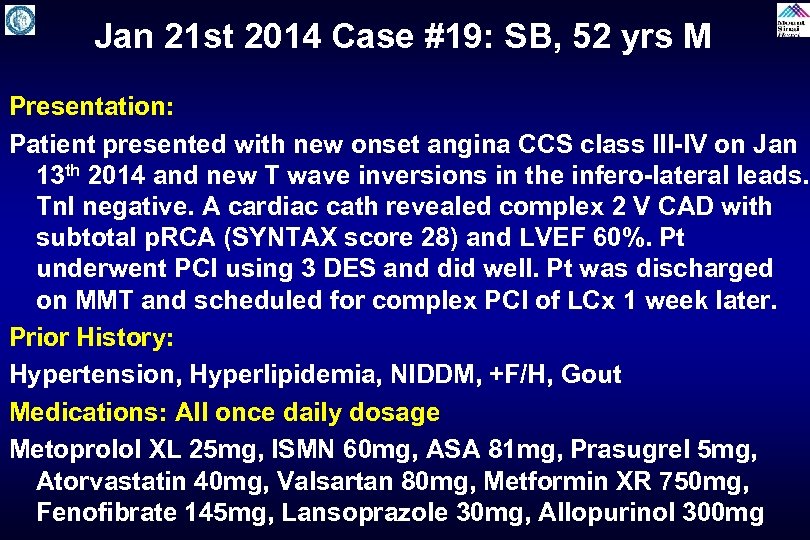
Jan 21 st 2014 Case #19: SB, 52 yrs M Presentation: Patient presented with new onset angina CCS class III-IV on Jan 13 th 2014 and new T wave inversions in the infero-lateral leads. Tn. I negative. A cardiac cath revealed complex 2 V CAD with subtotal p. RCA (SYNTAX score 28) and LVEF 60%. Pt underwent PCI using 3 DES and did well. Pt was discharged on MMT and scheduled for complex PCI of LCx 1 week later. Prior History: Hypertension, Hyperlipidemia, NIDDM, +F/H, Gout Medications: All once daily dosage Metoprolol XL 25 mg, ISMN 60 mg, ASA 81 mg, Prasugrel 5 mg, Atorvastatin 40 mg, Valsartan 80 mg, Metformin XR 750 mg, Fenofibrate 145 mg, Lansoprazole 30 mg, Allopurinol 300 mg
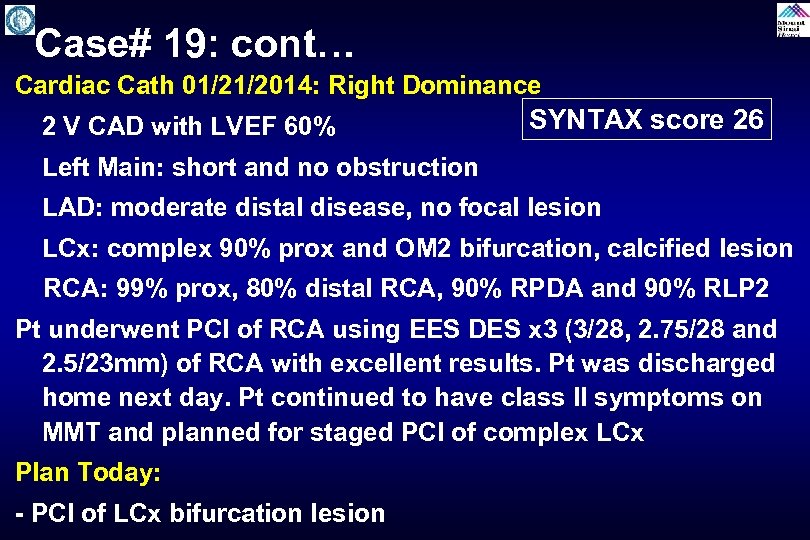
Case# 19: cont… Cardiac Cath 01/21/2014: Right Dominance 2 V CAD with LVEF 60% SYNTAX score 26 Left Main: short and no obstruction LAD: moderate distal disease, no focal lesion LCx: complex 90% prox and OM 2 bifurcation, calcified lesion RCA: 99% prox, 80% distal RCA, 90% RPDA and 90% RLP 2 Pt underwent PCI of RCA using EES DES x 3 (3/28, 2. 75/28 and 2. 5/23 mm) of RCA with excellent results. Pt was discharged home next day. Pt continued to have class II symptoms on MMT and planned for staged PCI of complex LCx Plan Today: - PCI of LCx bifurcation lesion
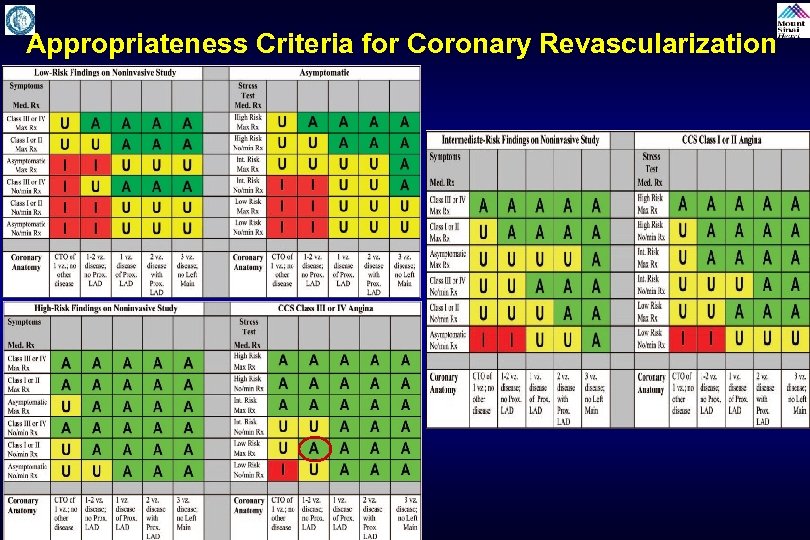
Appropriateness Criteria for Coronary Revascularization

Issues Involving The Case • Update on platelet responsiveness in PCI • Update in therapy to prevent CIN/AKI

Issues Involving The Case • Update on platelet responsiveness in PCI • Update in therapy to prevent CIN/AKI
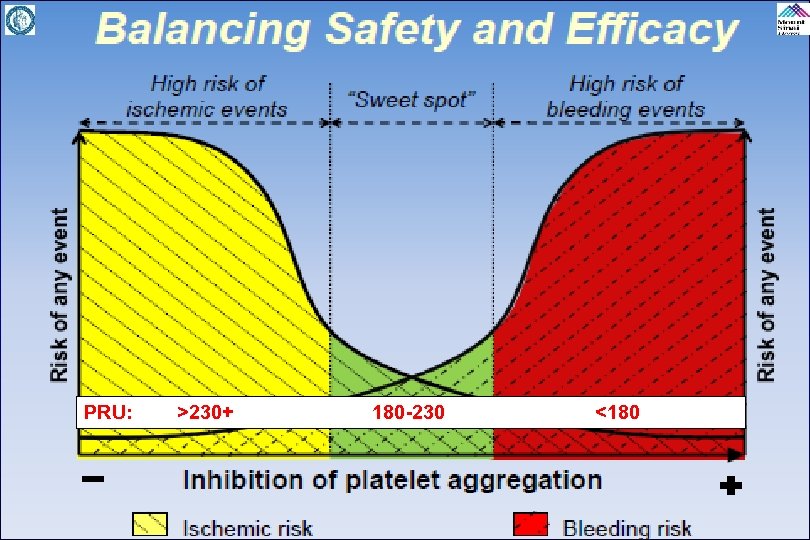
PRU: >230+ 180 -230 <180

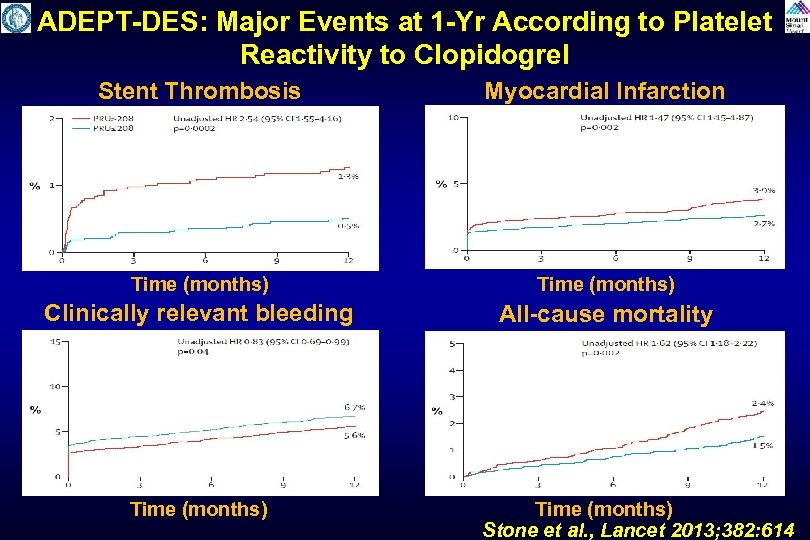
ADEPT-DES: Major Events at 1 -Yr According to Platelet Reactivity to Clopidogrel Stent Thrombosis Myocardial Infarction Time (months) Clinically relevant bleeding All-cause mortality Time (months) Stone et al. , Lancet 2013; 382: 614
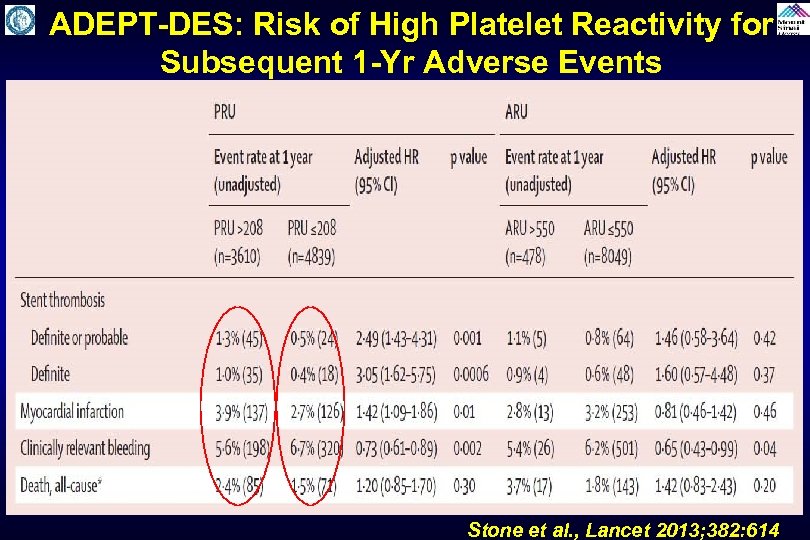
ADEPT-DES: Risk of High Platelet Reactivity for Subsequent 1 -Yr Adverse Events Stone et al. , Lancet 2013; 382: 614
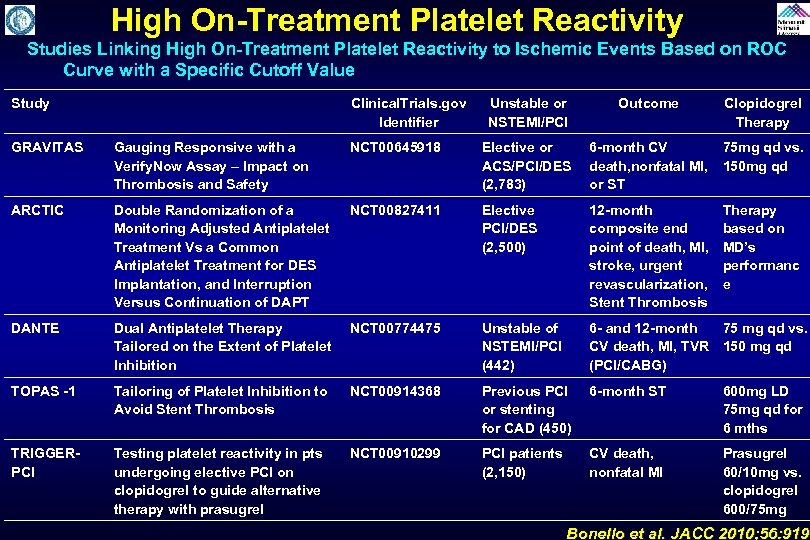
High On-Treatment Platelet Reactivity Studies Linking High On-Treatment Platelet Reactivity to Ischemic Events Based on ROC Curve with a Specific Cutoff Value Study Clinical. Trials. gov Identifier Unstable or NSTEMI/PCI Outcome Clopidogrel Therapy GRAVITAS Gauging Responsive with a Verify. Now Assay – Impact on Thrombosis and Safety NCT 00645918 Elective or ACS/PCI/DES (2, 783) 6 -month CV 75 mg qd vs. death, nonfatal MI, 150 mg qd or ST ARCTIC Double Randomization of a Monitoring Adjusted Antiplatelet Treatment Vs a Common Antiplatelet Treatment for DES Implantation, and Interruption Versus Continuation of DAPT NCT 00827411 Elective PCI/DES (2, 500) 12 -month composite end point of death, MI, stroke, urgent revascularization, Stent Thrombosis DANTE Dual Antiplatelet Therapy Tailored on the Extent of Platelet Inhibition NCT 00774475 Unstable of NSTEMI/PCI (442) 6 - and 12 -month 75 mg qd vs. CV death, MI, TVR 150 mg qd (PCI/CABG) TOPAS -1 Tailoring of Platelet Inhibition to Avoid Stent Thrombosis NCT 00914368 Previous PCI or stenting for CAD (450) 6 -month ST 600 mg LD 75 mg qd for 6 mths TRIGGERPCI Testing platelet reactivity in pts undergoing elective PCI on clopidogrel to guide alternative therapy with prasugrel NCT 00910299 PCI patients (2, 150) CV death, nonfatal MI Prasugrel 60/10 mg vs. clopidogrel 600/75 mg Therapy based on MD’s performanc e Bonello et al. JACC 2010; 56: 919
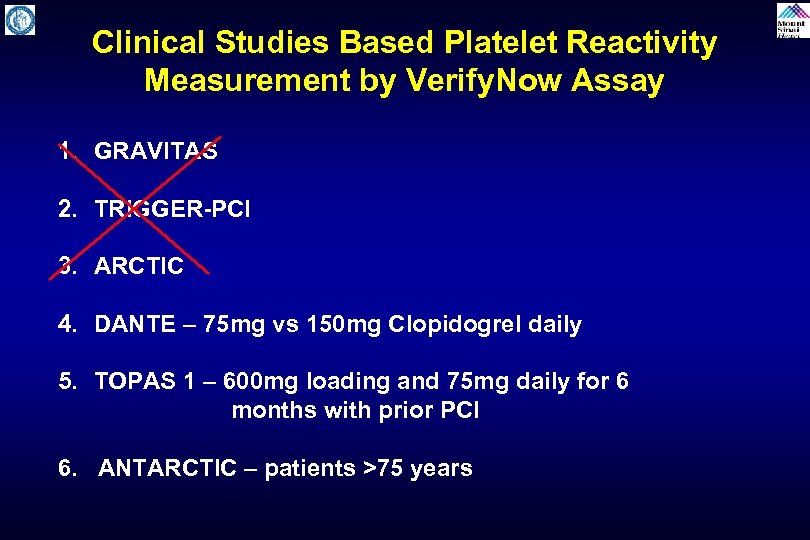
Clinical Studies Based Platelet Reactivity Measurement by Verify. Now Assay 1. GRAVITAS 2. TRIGGER-PCI 3. ARCTIC 4. DANTE – 75 mg vs 150 mg Clopidogrel daily 5. TOPAS 1 – 600 mg loading and 75 mg daily for 6 months with prior PCI 6. ANTARCTIC – patients >75 years
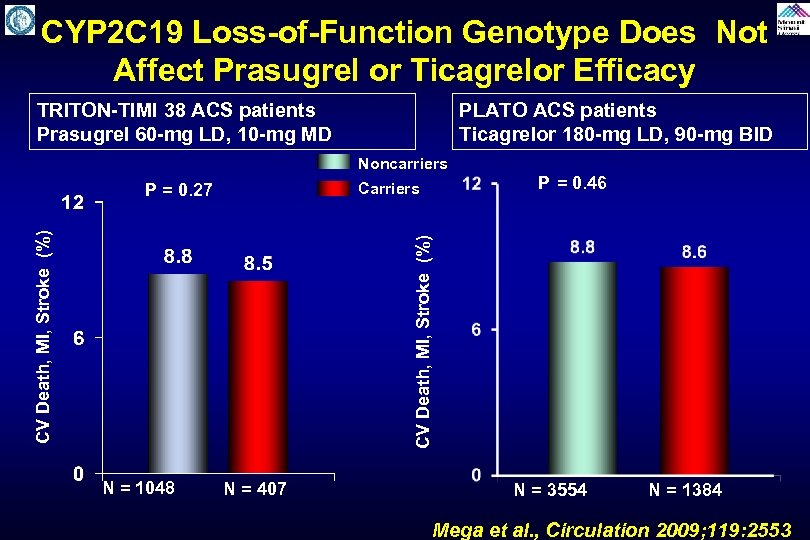
CYP 2 C 19 Loss-of-Function Genotype Does Not Affect Prasugrel or Ticagrelor Efficacy TRITON-TIMI 38 ACS patients Prasugrel 60 -mg LD, 10 -mg MD PLATO ACS patients Ticagrelor 180 -mg LD, 90 -mg BID Noncarriers Carriers P = 0. 46 CV Death, MI, Stroke (%) P = 0. 27 N = 1048 N = 407 N = 3554 N = 1384 Mega et al. , Circulation 2009; 119: 2553
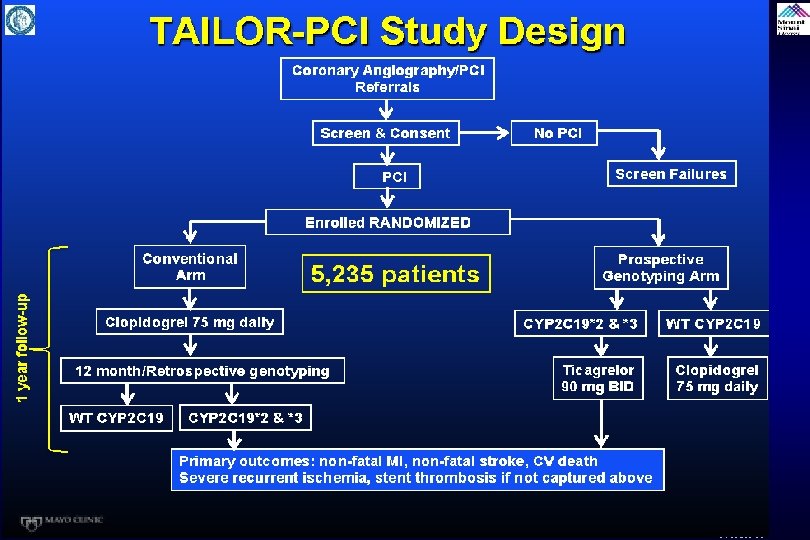

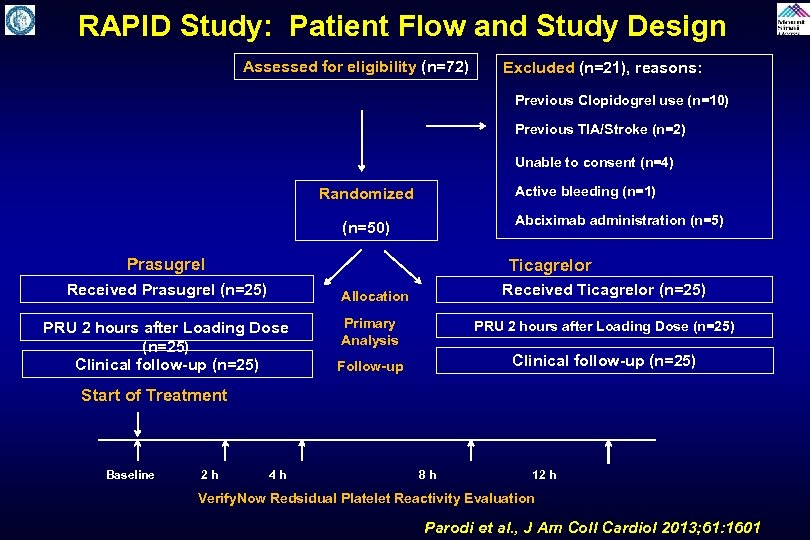
RAPID Study: Patient Flow and Study Design Assessed for eligibility (n=72) Excluded (n=21), reasons: Previous Clopidogrel use (n=10) Previous TIA/Stroke (n=2) Unable to consent (n=4) Randomized (n=50) Prasugrel Received Prasugrel (n=25) PRU 2 hours after Loading Dose (n=25) Clinical follow-up (n=25) Active bleeding (n=1) Abciximab administration (n=5) Ticagrelor Allocation Received Ticagrelor (n=25) Primary Analysis PRU 2 hours after Loading Dose (n=25) Follow-up Clinical follow-up (n=25) Start of Treatment Baseline 2 h 4 h 8 h 12 h Verify. Now Redsidual Platelet Reactivity Evaluation Parodi et al. , J Am Coll Cardiol 2013; 61: 1601
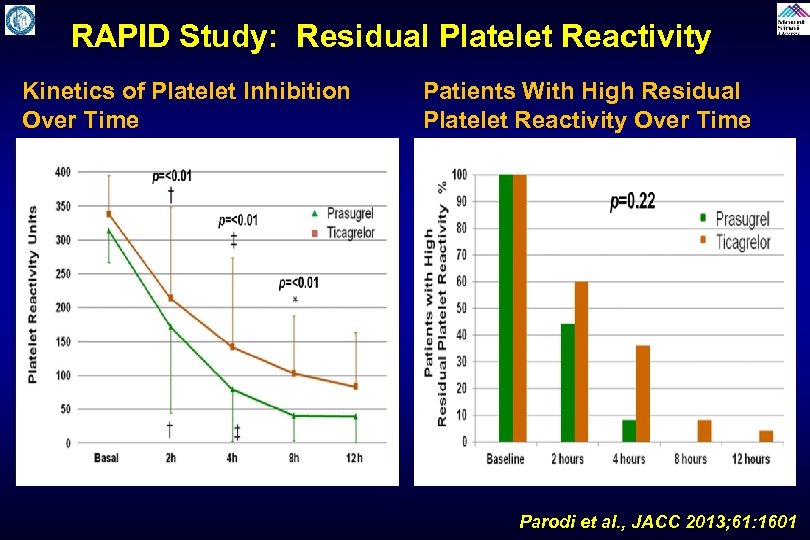
RAPID Study: Residual Platelet Reactivity Kinetics of Platelet Inhibition Over Time Patients With High Residual Platelet Reactivity Over Time Parodi et al. , JACC 2013; 61: 1601
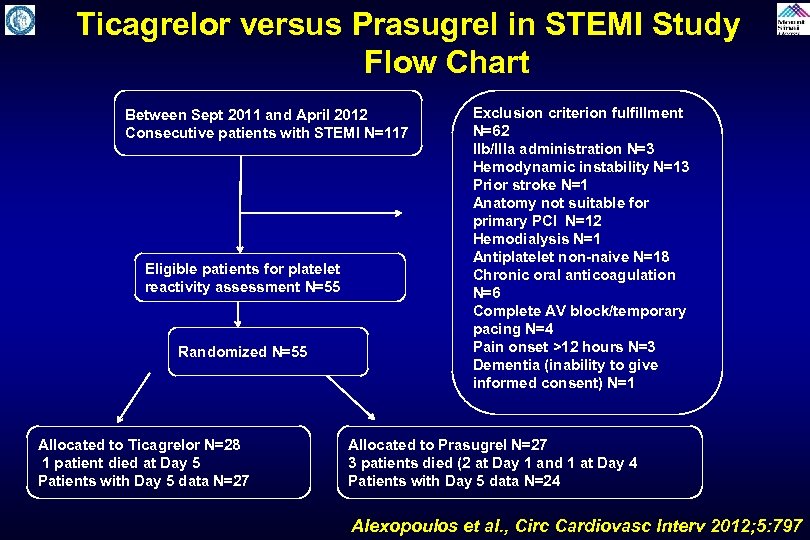
Ticagrelor versus Prasugrel in STEMI Study Flow Chart Between Sept 2011 and April 2012 Consecutive patients with STEMI N=117 Eligible patients for platelet reactivity assessment N=55 Randomized N=55 Allocated to Ticagrelor N=28 1 patient died at Day 5 Patients with Day 5 data N=27 Exclusion criterion fulfillment N=62 Ilb/Illa administration N=3 Hemodynamic instability N=13 Prior stroke N=1 Anatomy not suitable for primary PCI N=12 Hemodialysis N=1 Antiplatelet non-naive N=18 Chronic oral anticoagulation N=6 Complete AV block/temporary pacing N=4 Pain onset >12 hours N=3 Dementia (inability to give informed consent) N=1 Allocated to Prasugrel N=27 3 patients died (2 at Day 1 and 1 at Day 4 Patients with Day 5 data N=24 Alexopoulos et al. , Circ Cardiovasc Interv 2012; 5: 797
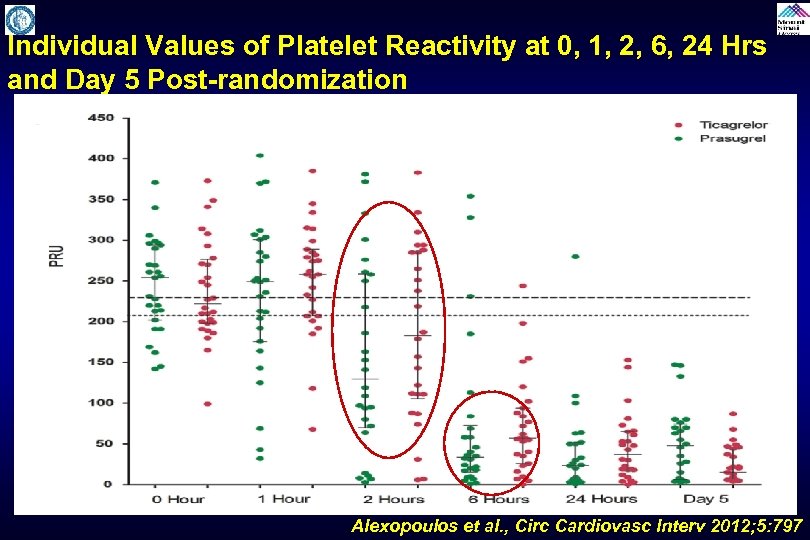
Individual Values of Platelet Reactivity at 0, 1, 2, 6, 24 Hrs and Day 5 Post-randomization Alexopoulos et al. , Circ Cardiovasc Interv 2012; 5: 797

Impact of Prasugrel Reload Dosing Regimens on High On-Treatment Platelet Reactivity Rates in Patients on Maintenance Prasugrel Therapy Ferreiro et al. , JACC Cardio Interv 2013; 6: 182
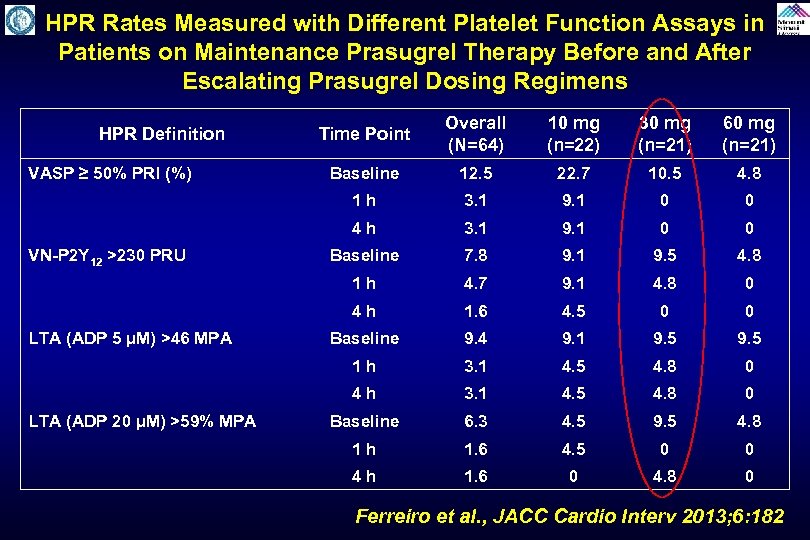
HPR Rates Measured with Different Platelet Function Assays in Patients on Maintenance Prasugrel Therapy Before and After Escalating Prasugrel Dosing Regimens 60 mg (n=21) Baseline 12. 5 22. 7 10. 5 4. 8 3. 1 9. 1 0 0 Baseline 7. 8 9. 1 9. 5 4. 8 4. 7 9. 1 4. 8 0 1. 6 4. 5 0 0 Baseline 9. 4 9. 1 9. 5 1 h 3. 1 4. 5 4. 8 0 4 h LTA (ADP 20 µM) >59% MPA 30 mg (n=21) 4 h LTA (ADP 5 µM) >46 MPA 10 mg (n=22) 1 h VN-P 2 Y 12 >230 PRU Overall (N=64) 4 h VASP ≥ 50% PRI (%) Time Point 1 h HPR Definition 3. 1 4. 5 4. 8 0 Baseline 6. 3 4. 5 9. 5 4. 8 1 h 1. 6 4. 5 0 0 4 h 1. 6 0 4. 8 0 Ferreiro et al. , JACC Cardio Interv 2013; 6: 182
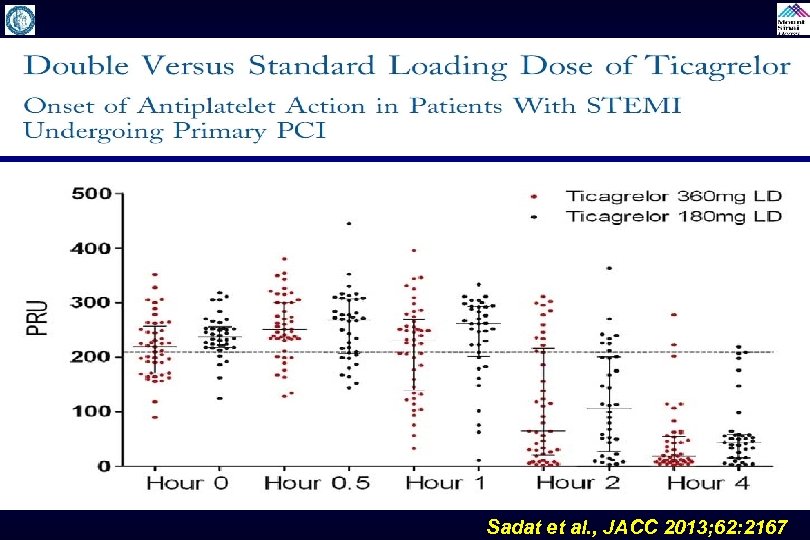
Sadat et al. , JACC 2013; 62: 2167

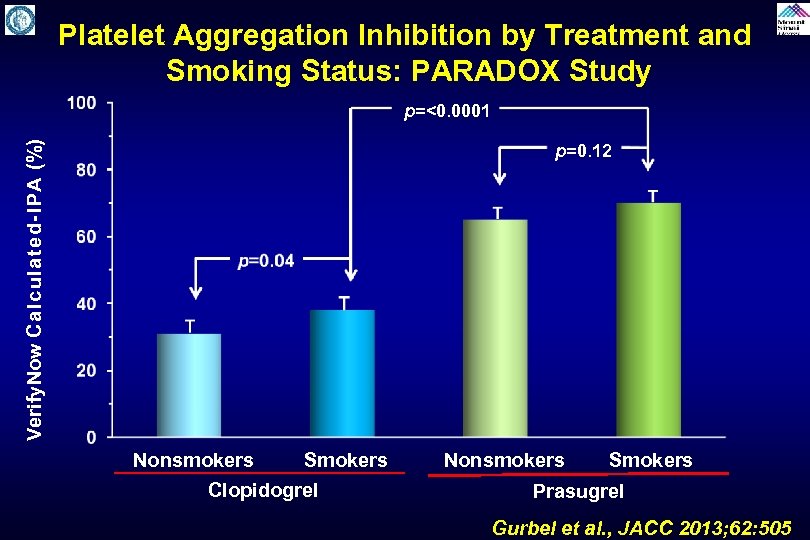
Platelet Aggregation Inhibition by Treatment and Smoking Status: PARADOX Study Verify. Now Calculated-IPA (%) p=<0. 0001 p=0. 12 Nonsmokers Smokers Nonsmokers Smokers Clopidogrel Prasugrel Gurbel et al. , JACC 2013; 62: 505
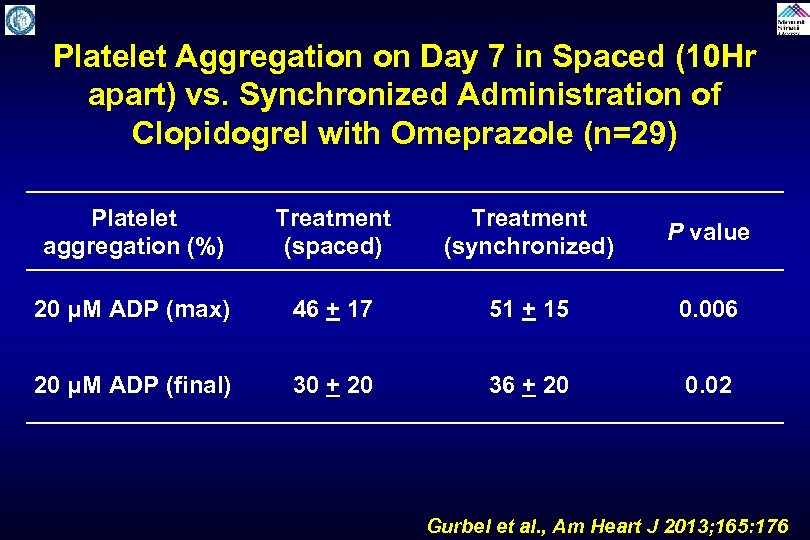
Platelet Aggregation on Day 7 in Spaced (10 Hr apart) vs. Synchronized Administration of Clopidogrel with Omeprazole (n=29) Platelet aggregation (%) Treatment (spaced) Treatment (synchronized) P value 20 μM ADP (max) 46 + 17 51 + 15 0. 006 20 μM ADP (final) 30 + 20 36 + 20 0. 02 Gurbel et al. , Am Heart J 2013; 165: 176
Am Heart J 2013; 165: 515
Steg et al. , Lancet, September 3, 2013
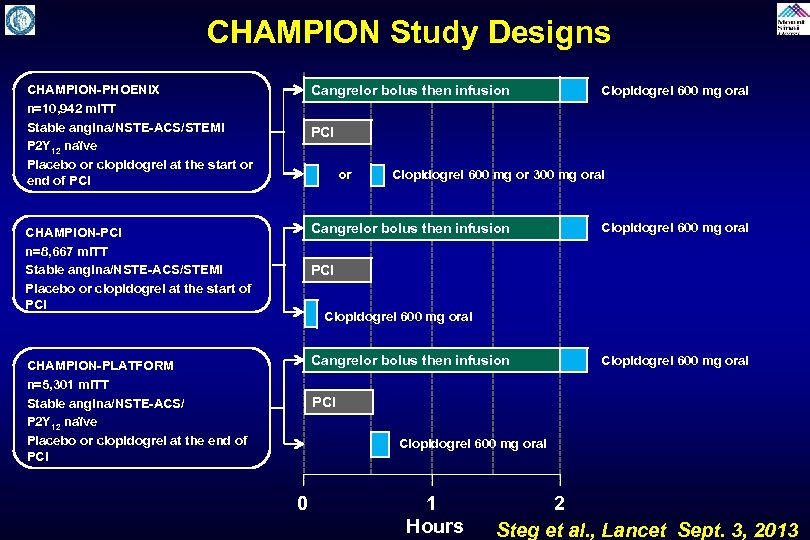
CHAMPION Study Designs CHAMPION-PHOENIX n=10, 942 m. ITT Stable angina/NSTE-ACS/STEMI P 2 Y 12 naïve Placebo or clopidogrel at the start or end of PCI Cangrelor bolus then infusion CHAMPION-PCI n=8, 667 m. ITT Stable angina/NSTE-ACS/STEMI Placebo or clopidogrel at the start of PCI Cangrelor bolus then infusion CHAMPION-PLATFORM n=5, 301 m. ITT Stable angina/NSTE-ACS/ P 2 Y 12 naïve Placebo or clopidogrel at the end of PCI Cangrelor bolus then infusion Clopidogrel 600 mg oral PCI or Clopidogrel 600 mg or 300 mg oral Clopidogrel 600 mg oral PCI Clopidogrel 600 mg oral 0 1 Hours 2 Steg et al. , Lancet Sept. 3, 2013
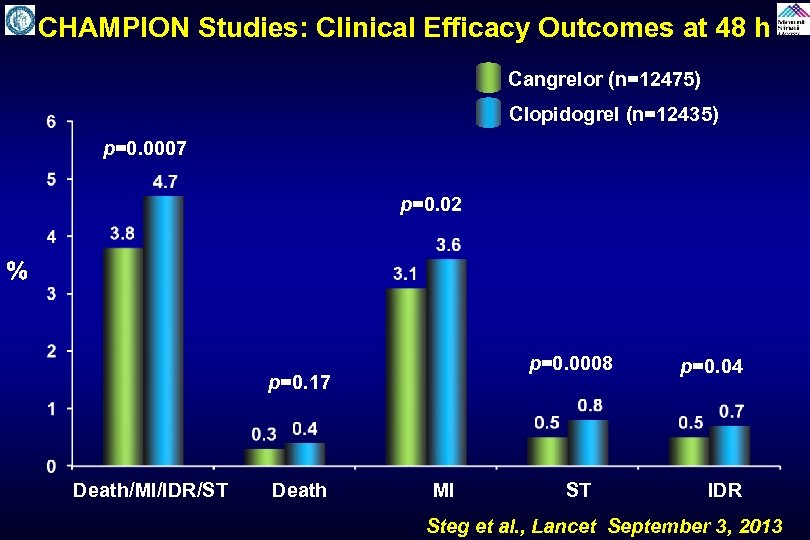
CHAMPION Studies: Clinical Efficacy Outcomes at 48 h Cangrelor (n=12475) Clopidogrel (n=12435) p=0. 0007 p=0. 02 % p=0. 17 p=0. 0008 p=0. 04 Death/MI/IDR/ST Death MI ST IDR Steg et al. , Lancet September 3, 2013
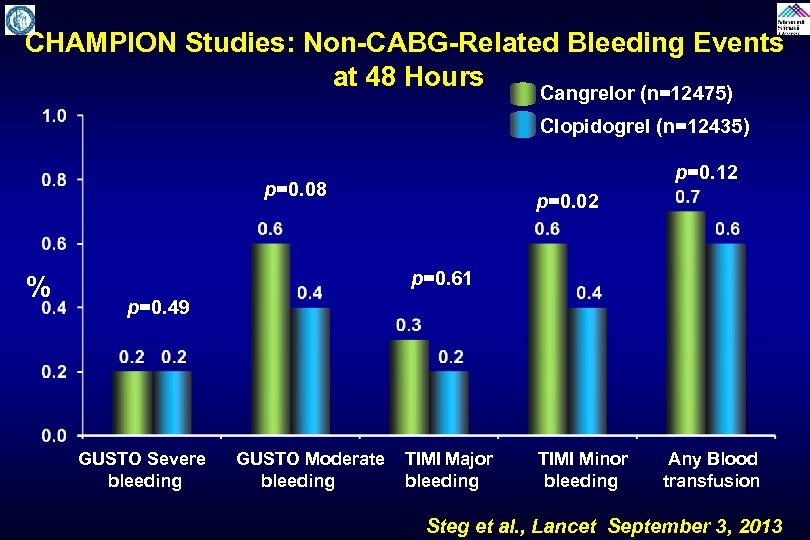
CHAMPION Studies: Non-CABG-Related Bleeding Events at 48 Hours Cangrelor (n=12475) Clopidogrel (n=12435) p=0. 12 p=0. 08 % p=0. 02 p=0. 61 p=0. 49 GUSTO Severe GUSTO Moderate TIMI Major TIMI Minor Any Blood bleeding transfusion Steg et al. , Lancet September 3, 2013
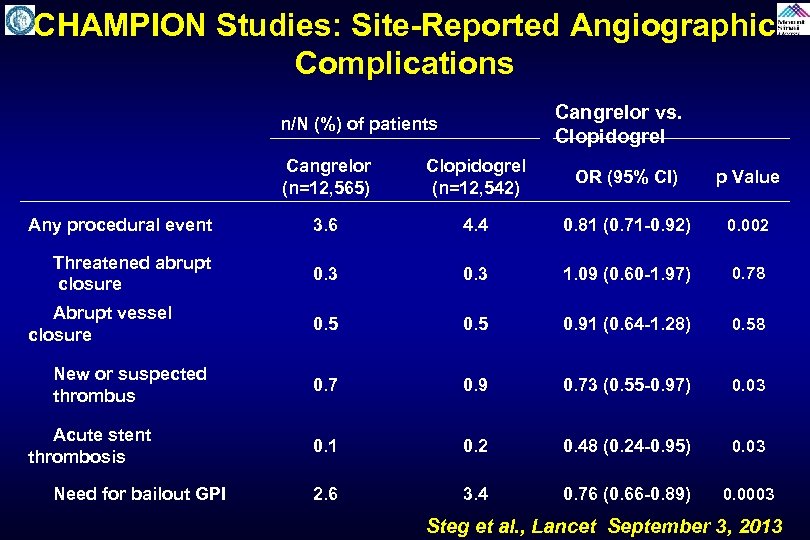
CHAMPION Studies: Site-Reported Angiographic Complications Cangrelor vs. Clopidogrel n/N (%) of patients Cangrelor (n=12, 565) Clopidogrel (n=12, 542) OR (95% CI) p Value Any procedural event 3. 6 4. 4 0. 81 (0. 71 -0. 92) 0. 002 Threatened abrupt closure 0. 3 1. 09 (0. 60 -1. 97) 0. 78 Abrupt vessel closure 0. 5 0. 91 (0. 64 -1. 28) 0. 58 New or suspected thrombus 0. 7 0. 9 0. 73 (0. 55 -0. 97) 0. 03 Acute stent thrombosis 0. 1 0. 2 0. 48 (0. 24 -0. 95) 0. 03 Need for bailout GPI 2. 6 3. 4 0. 76 (0. 66 -0. 89) 0. 0003 Steg et al. , Lancet September 3, 2013

Issues Involving The Case • Update on platelet responsiveness in PCI • Update in therapy to prevent CIN/AKI
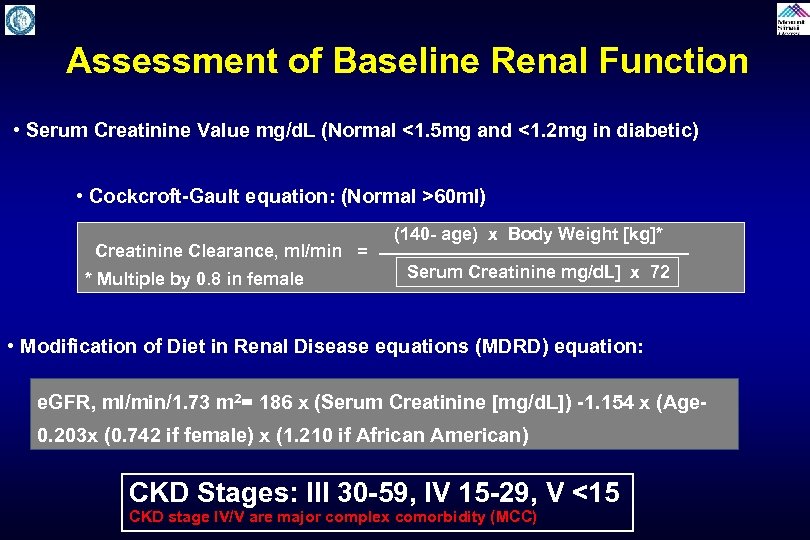
Assessment of Baseline Renal Function • Serum Creatinine Value mg/d. L (Normal <1. 5 mg and <1. 2 mg in diabetic) • Cockcroft-Gault equation: (Normal >60 ml) (140 - age) x Body Weight [kg]* Creatinine Clearance, ml/min = Serum Creatinine mg/d. L] x 72 * Multiple by 0. 8 in female • Modification of Diet in Renal Disease equations (MDRD) equation: e. GFR, ml/min/1. 73 m 2= 186 x (Serum Creatinine [mg/d. L]) -1. 154 x (Age 0. 203 x (0. 742 if female) x (1. 210 if African American) CKD Stages: III 30 -59, IV 15 -29, V <15 CKD stage IV/V are major complex comorbidity (MCC)
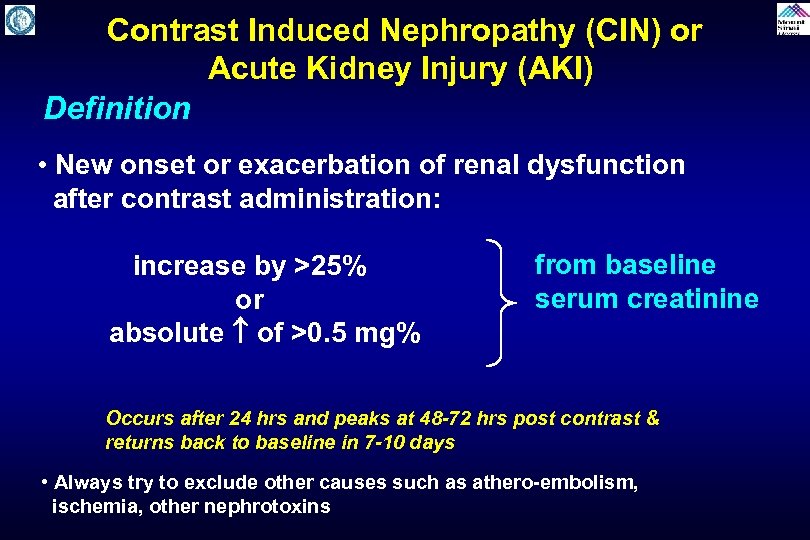
Contrast Induced Nephropathy (CIN) or Acute Kidney Injury (AKI) Definition • New onset or exacerbation of renal dysfunction after contrast administration: increase by >25% or absolute of >0. 5 mg% from baseline serum creatinine Occurs after 24 hrs and peaks at 48 -72 hrs post contrast & returns back to baseline in 7 -10 days • Always try to exclude other causes such as athero-embolism, ischemia, other nephrotoxins
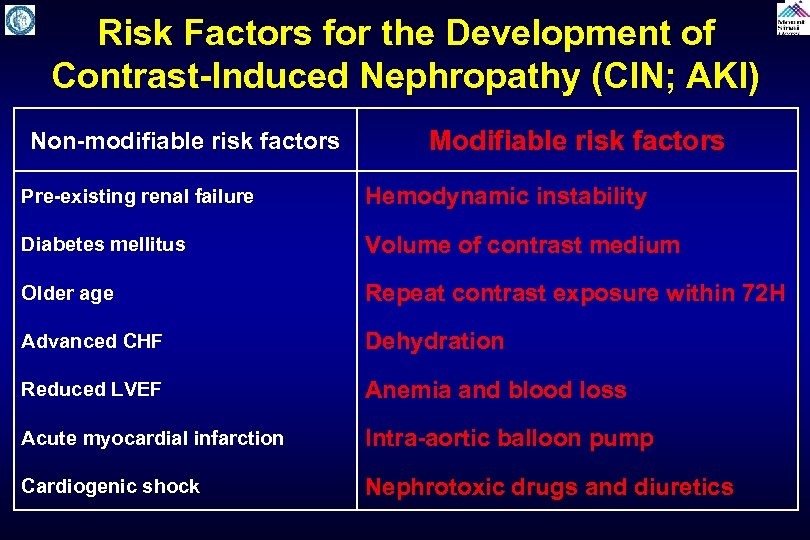
Risk Factors for the Development of Contrast-Induced Nephropathy (CIN; AKI) Non-modifiable risk factors Modifiable risk factors Pre-existing renal failure Hemodynamic instability Diabetes mellitus Volume of contrast medium Older age Repeat contrast exposure within 72 H Advanced CHF Dehydration Reduced LVEF Anemia and blood loss Acute myocardial infarction Intra-aortic balloon pump Cardiogenic shock Nephrotoxic drugs and diuretics

CIN Risk Assessment • Many individual risk factors for the development of CIN have been reported. • Although the combination of two or more risk factors is rather common in daily practice, the cumulative risk of several variables on the renal function is not well defined. • This dictates the need for global assessment of the impact of these variables on the development of CIN. • Prospectively identifying patients at risk of CIN would be of immense value in targeting prophylactic therapy to those at high risk.
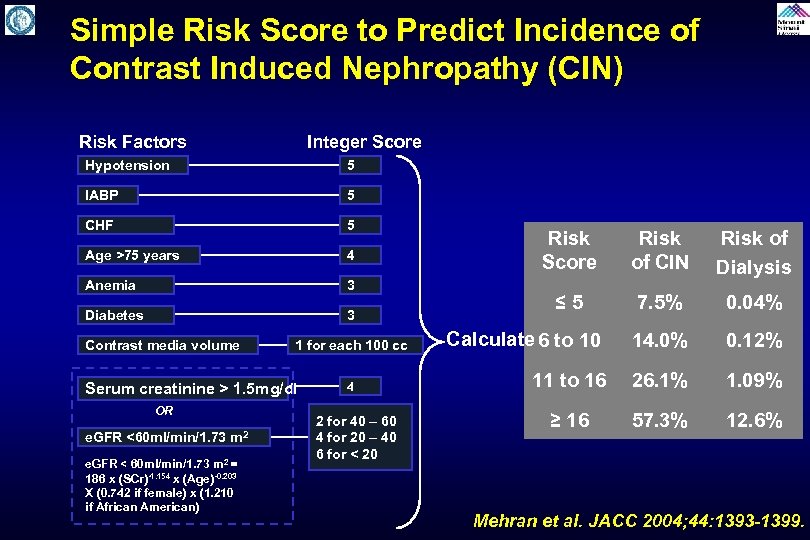
Simple Risk Score to Predict Incidence of Contrast Induced Nephropathy (CIN) Risk Factors Integer Score Hypotension 5 IABP 5 CHF 5 Age >75 years 4 Anemia 3 Diabetes 3 Contrast media volume 1 for each 100 cc Serum creatinine > 1. 5 mg/dl OR e. GFR <60 ml/min/1. 73 m 2 e. GFR < 60 ml/min/1. 73 m 2 = 186 x (SCr)-1. 154 x (Age)-0. 203 X (0. 742 if female) x (1. 210 if African American) 4 2 for 40 – 60 4 for 20 – 40 6 for < 20 Risk Score Risk of CIN Risk of Dialysis ≤ 5 7. 5% 0. 04% Calculate 6 to 10 14. 0% 0. 12% 11 to 16 26. 1% 1. 09% ≥ 16 57. 3% 12. 6% Mehran et al. JACC 2004; 44: 1393 -1399.
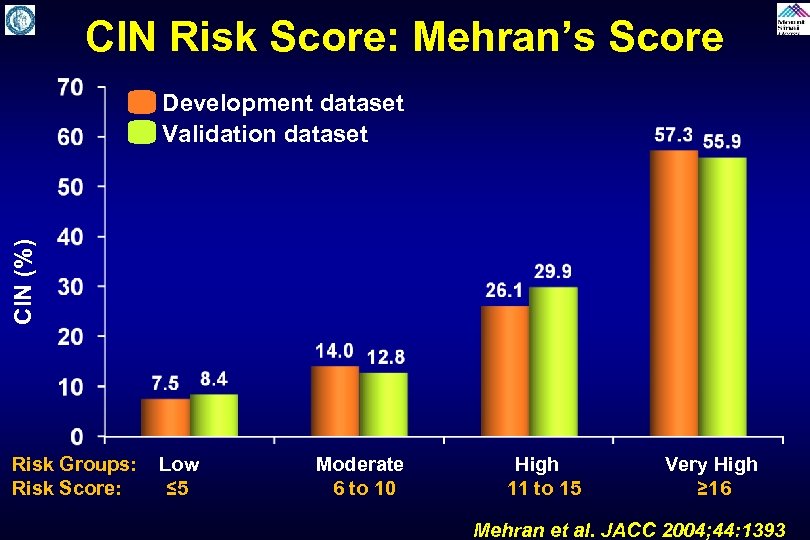
CIN Risk Score: Mehran’s Score CIN (%) Development dataset Validation dataset Risk Groups: Low Moderate High Very High Risk Score: ≤ 5 6 to 10 11 to 15 ≥ 16 Mehran et al. JACC 2004; 44: 1393

§ Data derived from the Blue Cross Blue Shield of Michigan Cardiovascular Consortium (BMC 2) § 68, 573 procedures performed across Michigan between January 2010 through June 2012 § Training set: 48, 001 procedures; CIN rate 2. 6%, dialysis rate 0. 35% § Validation set: 20, 572 procedures; CIN rate 2. 5%, dialysis rate 0. 32% Gurm et al. , JACC 2013; 61: 2242
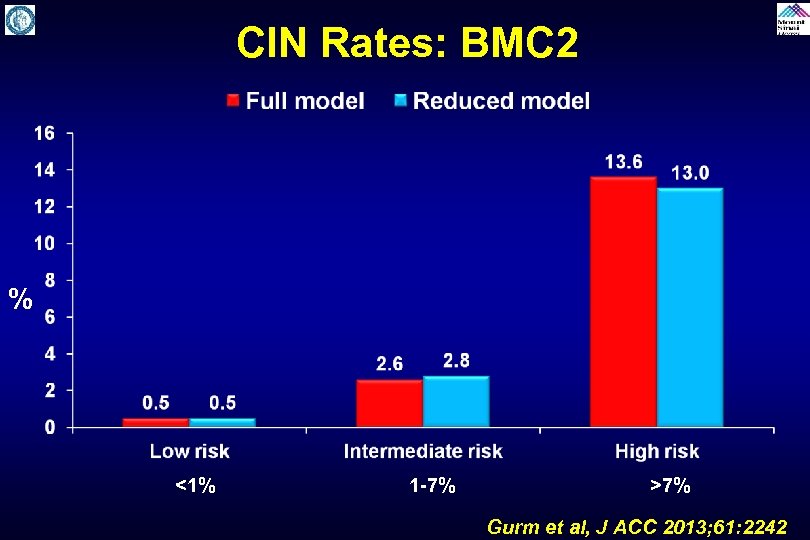
CIN Rates: BMC 2 % <1% 1 -7% >7% Gurm et al, J ACC 2013; 61: 2242
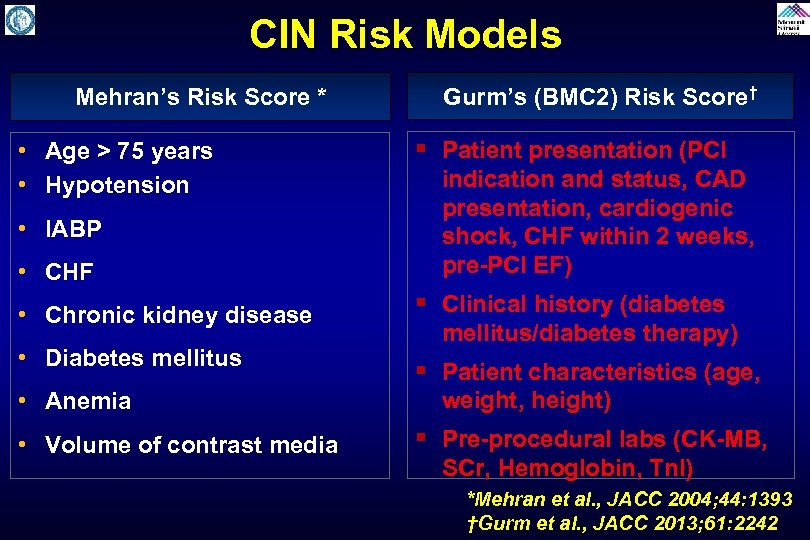
CIN Risk Models Mehran’s Risk Score * • Age > 75 years • Hypotension • IABP • CHF • Chronic kidney disease • Diabetes mellitus • Anemia • Volume of contrast media Gurm’s (BMC 2) Risk Score† § Patient presentation (PCI indication and status, CAD presentation, cardiogenic shock, CHF within 2 weeks, pre-PCI EF) § Clinical history (diabetes mellitus/diabetes therapy) § Patient characteristics (age, weight, height) § Pre-procedural labs (CK-MB, SCr, Hemoglobin, Tn. I) *Mehran et al. , JACC 2004; 44: 1393 †Gurm et al. , JACC 2013; 61: 2242

Mehran risk score § http: //www. qxmd. com/calculateonline/nephrology/contrast-nephropathypost-pci BMC 2 risk score § https: //bmc 2. org/calculators/cin

The Reasons to Use Risk Calculators • Risk stratification for patient-level decision making • Optimization of patient care • Risk adjustment for the assessment of quality of care • Research purposes
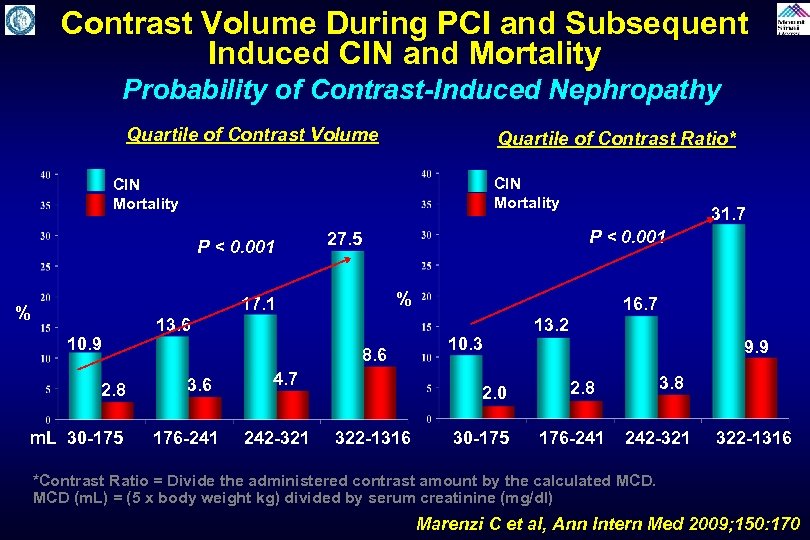
Contrast Volume During PCI and Subsequent Induced CIN and Mortality Probability of Contrast-Induced Nephropathy Quartile of Contrast Volume Quartile of Contrast Ratio* CIN Mortality P < 0. 001 10. 9 2. 8 P < 0. 001 27. 5 % 17. 1 % 13. 6 8. 6 31. 7 4. 7 m. L 30 -175 176 -241 242 -321 322 -1316 16. 7 10. 3 2. 0 13. 2 9. 9 2. 8 30 -175 176 -241 242 -321 322 -1316 *Contrast Ratio = Divide the administered contrast amount by the calculated MCD (m. L) = (5 x body weight kg) divided by serum creatinine (mg/dl) Marenzi C et al, Ann Intern Med 2009; 150: 170
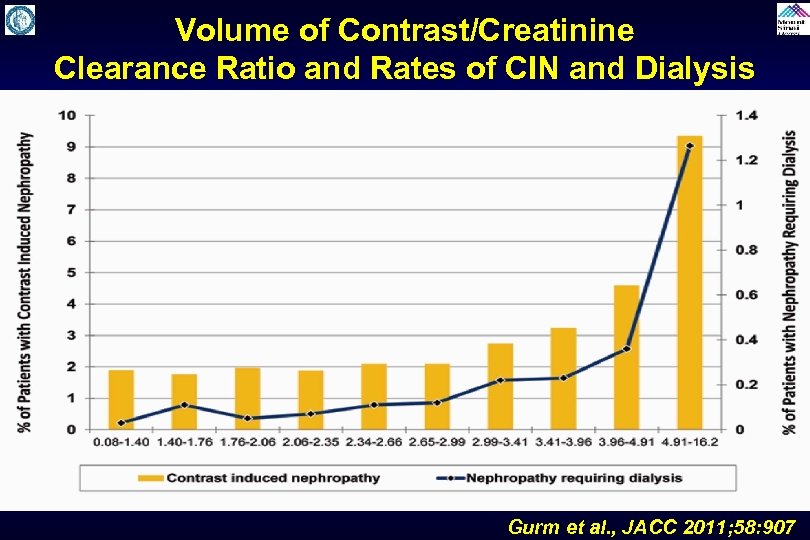
Volume of Contrast/Creatinine Clearance Ratio and Rates of CIN and Dialysis Gurm et al. , JACC 2011; 58: 907
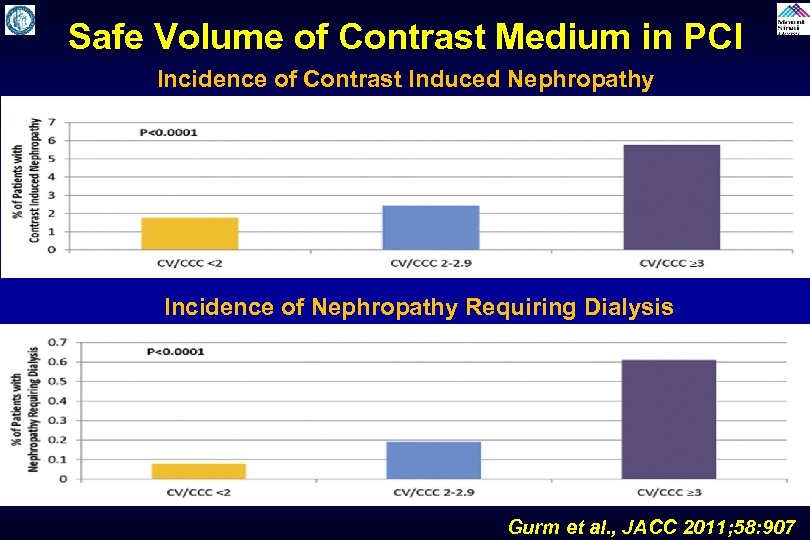
Safe Volume of Contrast Medium in PCI Incidence of Contrast Induced Nephropathy Incidence of Nephropathy Requiring Dialysis Gurm et al. , JACC 2011; 58: 907

Maioli et al. , Circulation 2012; 125: 3099
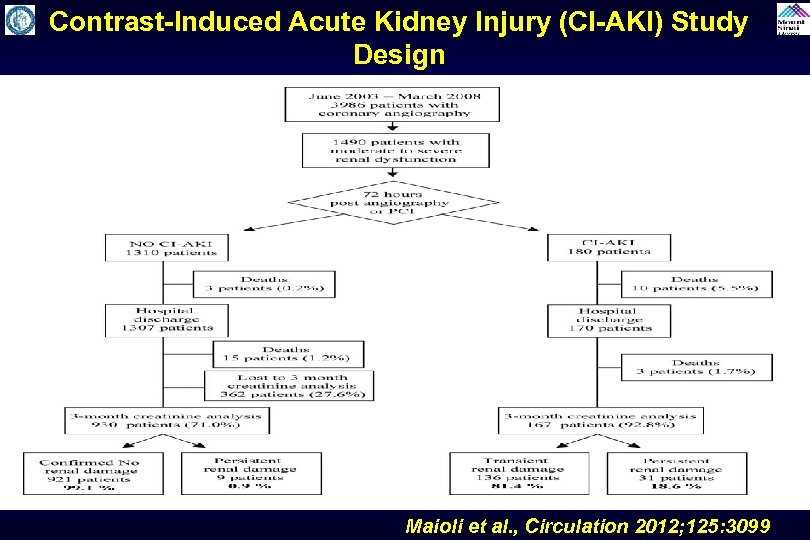
Contrast-Induced Acute Kidney Injury (CI-AKI) Study Design Maioli et al. , Circulation 2012; 125: 3099
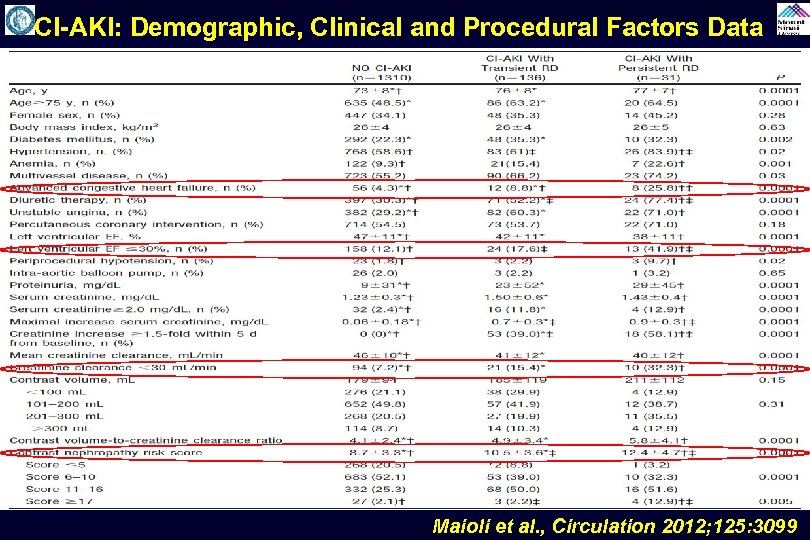
CI-AKI: Demographic, Clinical and Procedural Factors Data Maioli et al. , Circulation 2012; 125: 3099
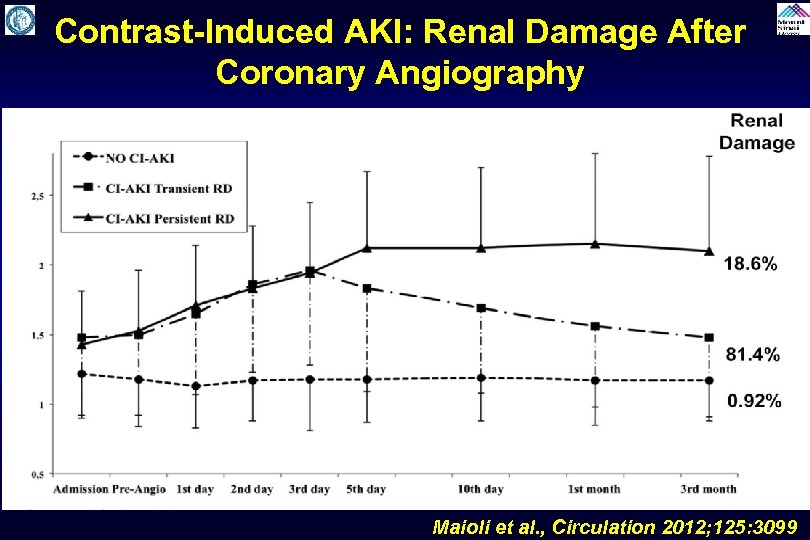
Contrast-Induced AKI: Renal Damage After Coronary Angiography Maioli et al. , Circulation 2012; 125: 3099
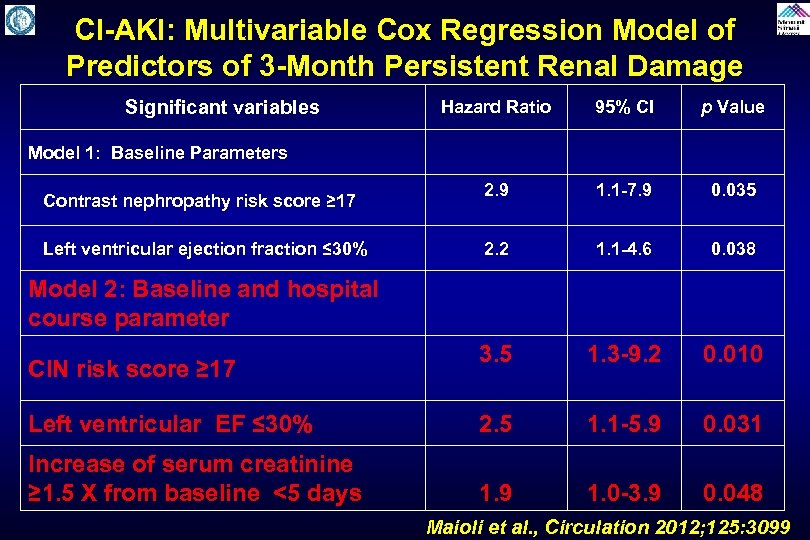
CI-AKI: Multivariable Cox Regression Model of Predictors of 3 -Month Persistent Renal Damage Significant variables Hazard Ratio 95% CI p Value 2. 9 1. 1 -7. 9 0. 035 2. 2 1. 1 -4. 6 0. 038 3. 5 1. 3 -9. 2 0. 010 Left ventricular EF ≤ 30% 2. 5 1. 1 -5. 9 0. 031 Increase of serum creatinine ≥ 1. 5 X from baseline <5 days 1. 9 1. 0 -3. 9 0. 048 Model 1: Baseline Parameters Contrast nephropathy risk score ≥ 17 Left ventricular ejection fraction ≤ 30% Model 2: Baseline and hospital course parameter CIN risk score ≥ 17 Maioli et al. , Circulation 2012; 125: 3099
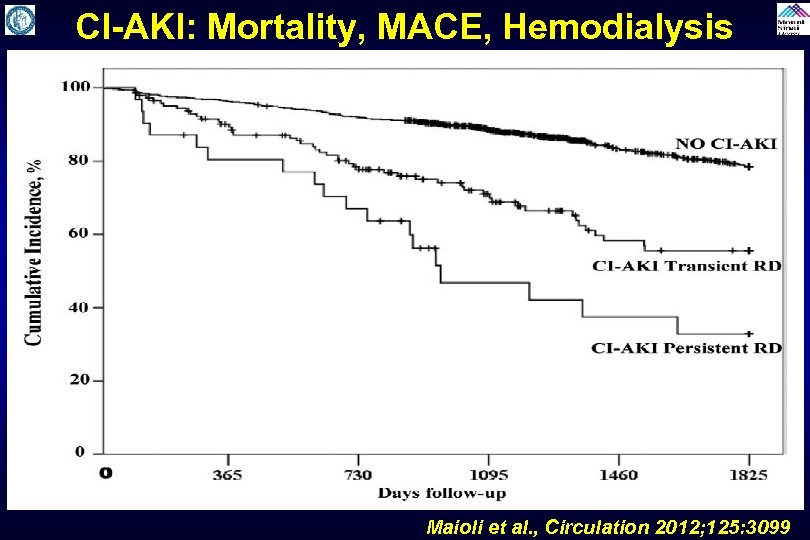
CI-AKI: Mortality, MACE, Hemodialysis Maioli et al. , Circulation 2012; 125: 3099
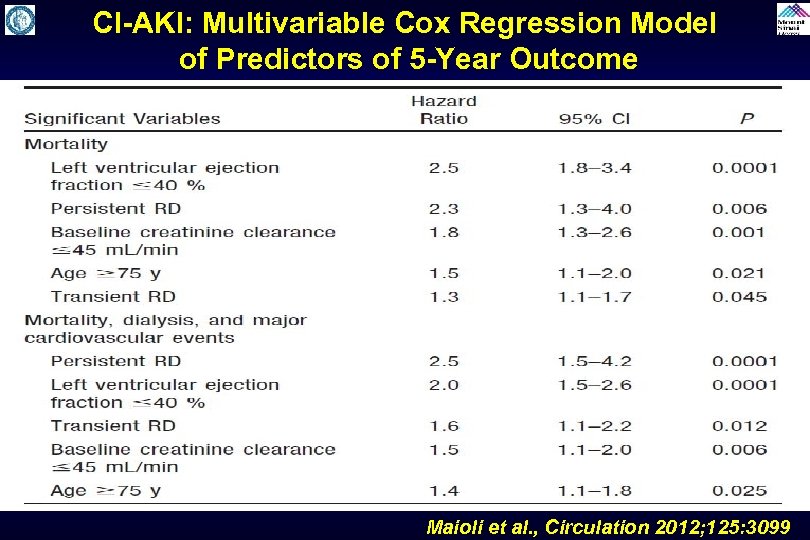
CI-AKI: Multivariable Cox Regression Model of Predictors of 5 -Year Outcome Maioli et al. , Circulation 2012; 125: 3099
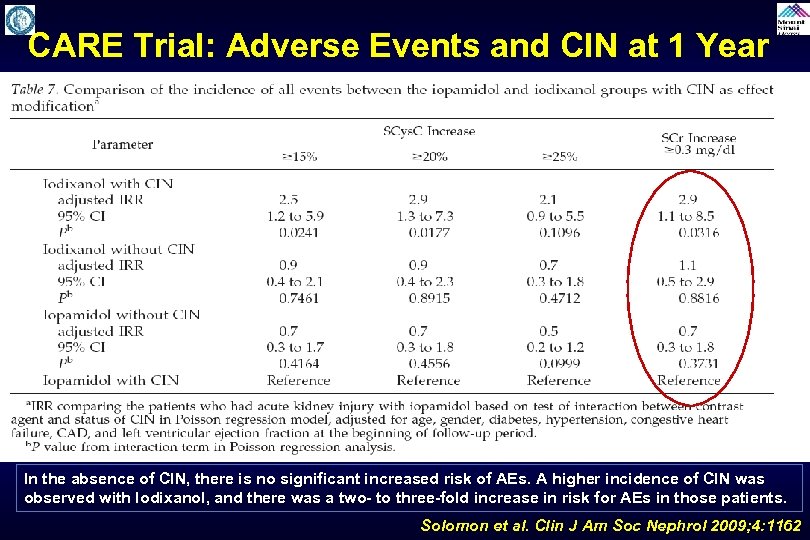
CARE Trial: Adverse Events and CIN at 1 Year In the absence of CIN, there is no significant increased risk of AEs. A higher incidence of CIN was observed with Iodixanol, and there was a two- to three-fold increase in risk for AEs in those patients. Solomon et al. Clin J Am Soc Nephrol 2009; 4: 1162

Tips to Limit the Contrast Load to Reduce CIN • 4 or 5 Fr Diagnostic catheter • Keep holding the catheter with forward push while injecting • 1: 2 contrast dilution in some injections • 5 or 6 Fr Guide catheters • Avoid Guide catheters with side holes • Avoid flush shots and use fixed landmarks for balloon and stent positioning • Perform PCI of the simple lesion with diagnostic cath and stage the complex lesion after >1 -4 weeks

Contrast Induced Nephropathy (CIN-AKI) Agents Studied for Prevention of CIN Beneficial Not effective • Saline hydration • Dopamine • Low-/iso-osmolar • Aminophylline non-ionic contrast • ANP • Sodium bicarbonate • Endothelin receptor antag • Ascorbic acid • Mannitol • Statins • Furosemide • Remote Ischemic • Fenoldopam preconditioning • NAC
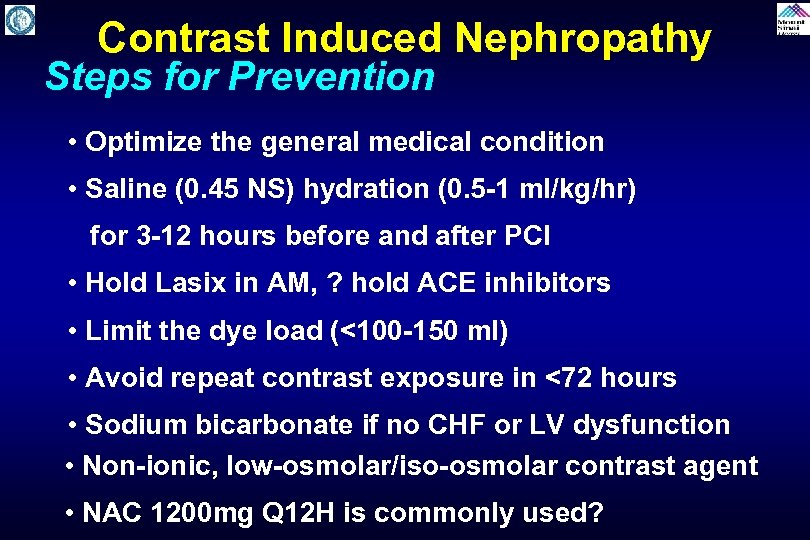
Contrast Induced Nephropathy Steps for Prevention • Optimize the general medical condition • Saline (0. 45 NS) hydration (0. 5 -1 ml/kg/hr) for 3 -12 hours before and after PCI • Hold Lasix in AM, ? hold ACE inhibitors • Limit the dye load (<100 -150 ml) • Avoid repeat contrast exposure in <72 hours • Sodium bicarbonate if no CHF or LV dysfunction • Non-ionic, low-osmolar/iso-osmolar contrast agent • NAC 1200 mg Q 12 H is commonly used?

Renal. Guard. TM for CI-AKI Prevention Is Designed To: § Create and maintain high urine output § Prevent contrast agents from clogging tubules § Limit toxin exposure in kidneys § Automated matched fluid replacement in real-time to reduce side effects associated with over- or under-hydration US: Investigational device. Limited by Federal Law to investigational use.
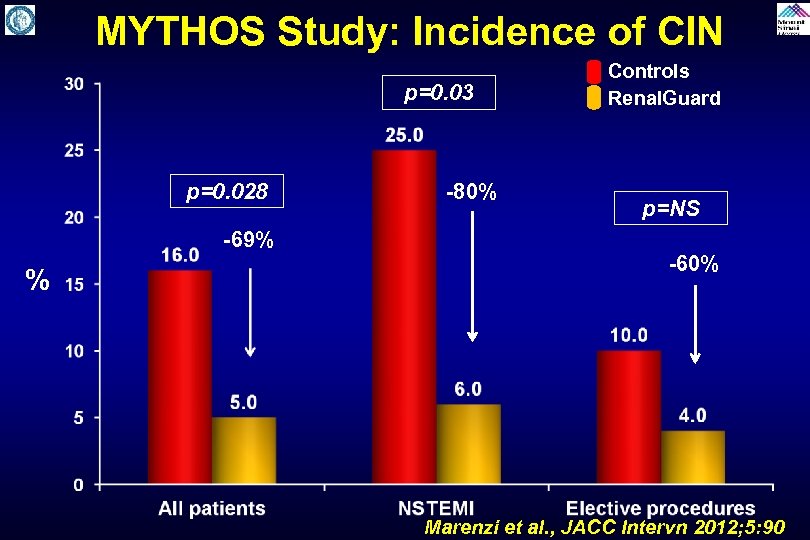
MYTHOS Study: Incidence of CIN p=0. 03 p=0. 028 -69% % -80% Controls Renal. Guard p=NS -60% Marenzi et al. , JACC Intervn 2012; 5: 90

REMEDIAL II REnal Insufficiency Following Contrast MEDIA Administration II Tria. L Renal. Guard system in high risk patients for contrast induced acute kidney injury Carlo Briguori, MD, Ph. D Laboratoy of Interventional Cardiology Clinica Mediterranea, Naples - Italy
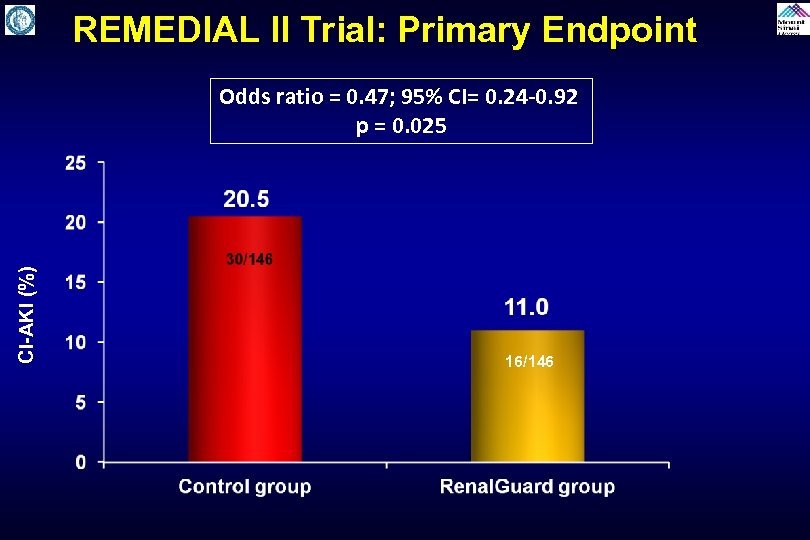
REMEDIAL II Trial: Primary Endpoint CI-AKI (%) Odds ratio = 0. 47; 95% CI= 0. 24 -0. 92 p = 0. 025 16/146

RENALGUARD Trial A study to evaluate the safety and efficacy of the Renal. Guard System when compared with standard care in the prevention of contrast induced nephropathy in the catheterization laboratory N=326 RGS 001 D Version 4. 1 – 14 April 2011

Remote Ischemic Preconditioning Trials Study to evaluate the effectiveness of remote ischemic preconditioning by causing upper arm ischemia by through 4 cycles of 5 -minute inflation and 5 -minute deflation of a blood pressure cuff at 200 mm. Hg. It is done few minutes before contrast administration and may protect against CIN by causing reperfusion to the remote area (kidney) and has shown to be beneficial in experimental model to reduce AKI. Trials: RIPC, Ren. Pro, ERICCIN (ongoing)
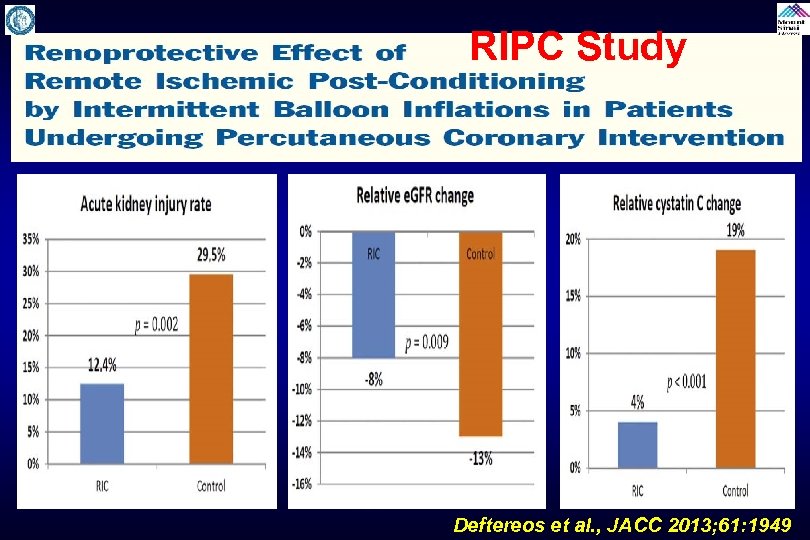
RIPC Study Deftereos et al. , JACC 2013; 61: 1949
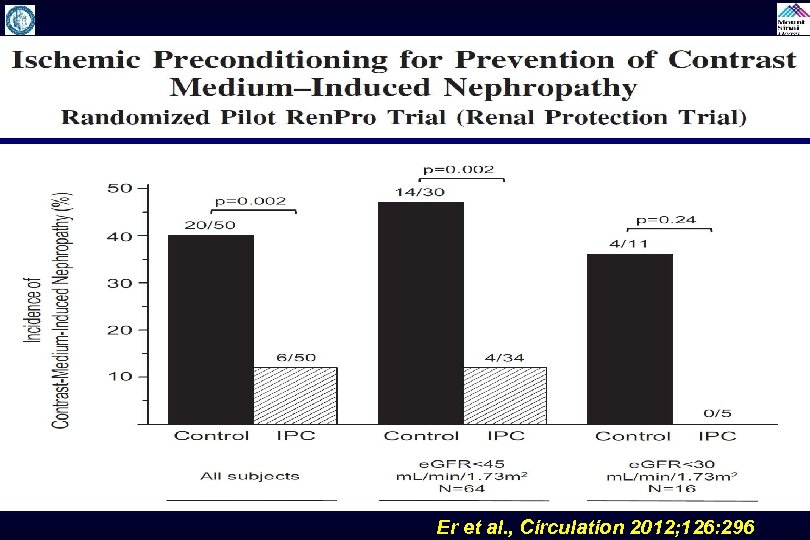
Er et al. , Circulation 2012; 126: 296

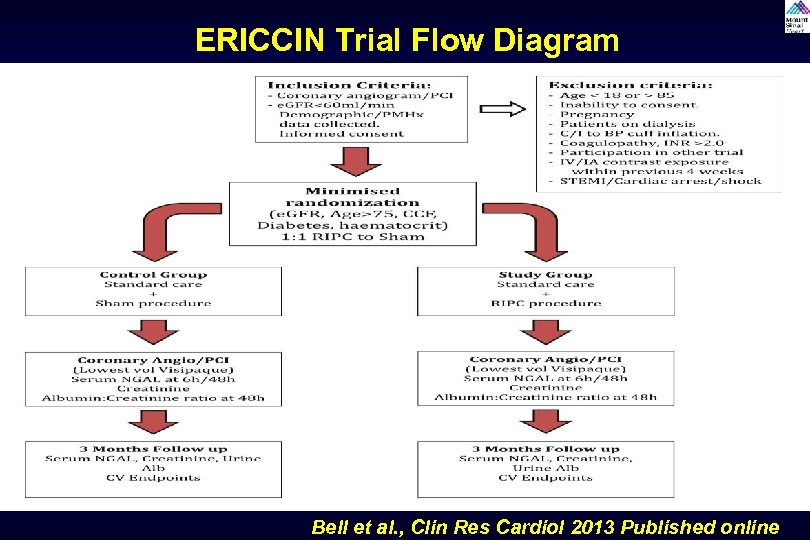
ERICCIN Trial Flow Diagram Bell et al. , Clin Res Cardiol 2013 Published online

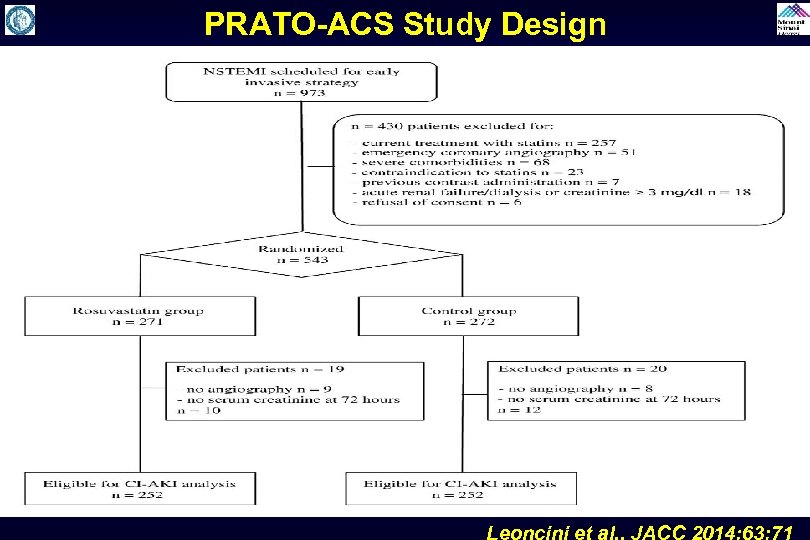
PRATO-ACS Study Design Leoncini et al. , JACC 2014; 63: 71
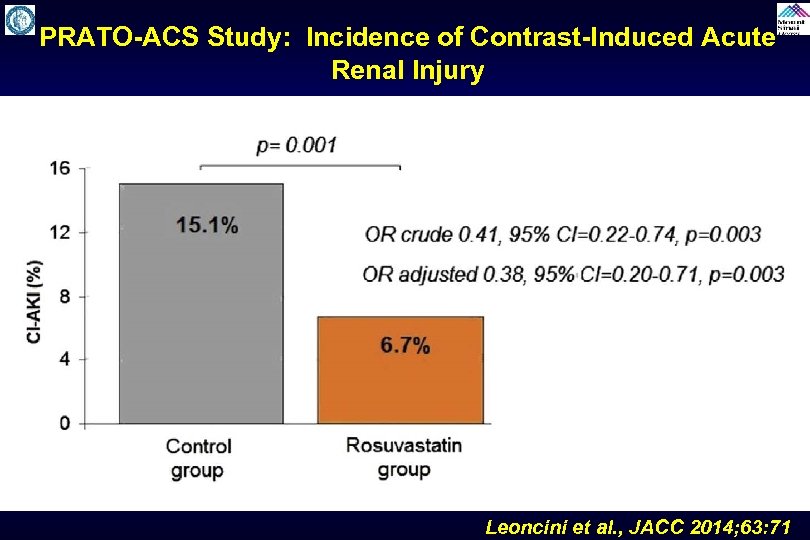
PRATO-ACS Study: Incidence of Contrast-Induced Acute Renal Injury Leoncini et al. , JACC 2014; 63: 71

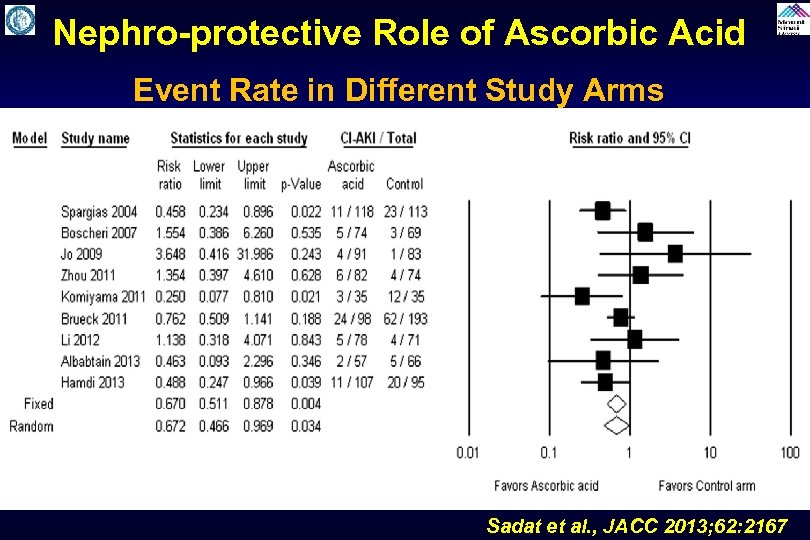
Nephro-protective Role of Ascorbic Acid Event Rate in Different Study Arms Sadat et al. , JACC 2013; 62: 2167
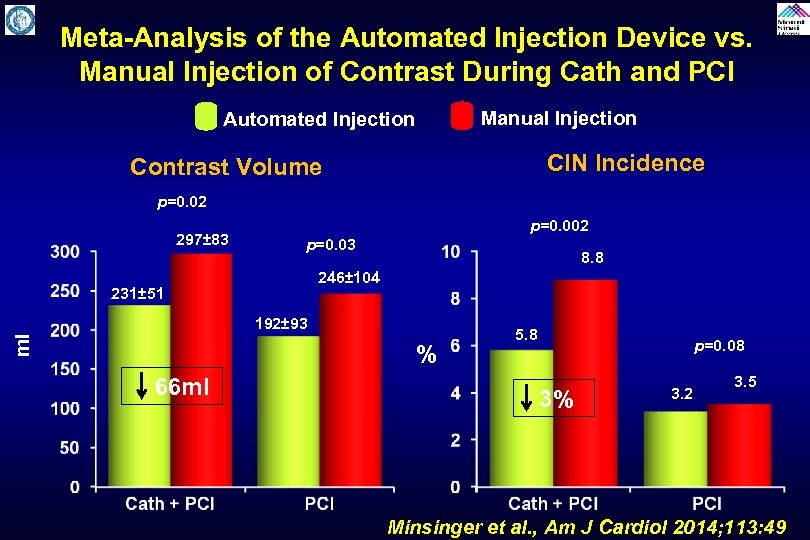
Meta-Analysis of the Automated Injection Device vs. Manual Injection of Contrast During Cath and PCI Manual Injection Automated Injection CIN Incidence Contrast Volume p=0. 02 297± 83 p=0. 002 p=0. 03 8. 8 246± 104 231± 51 ml 192± 93 % 66 ml 5. 8 3% p=0. 08 3. 2 3. 5 Minsinger et al. , Am J Cardiol 2014; 113: 49
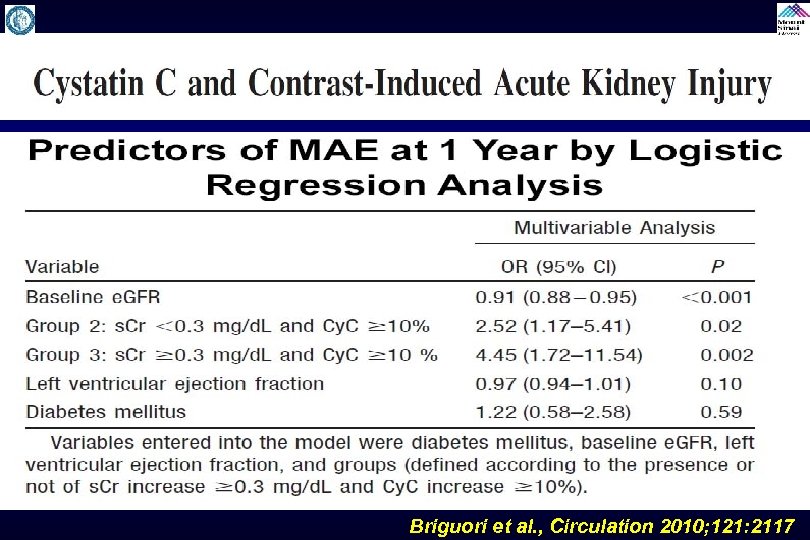
Briguori et al. , Circulation 2010; 121: 2117
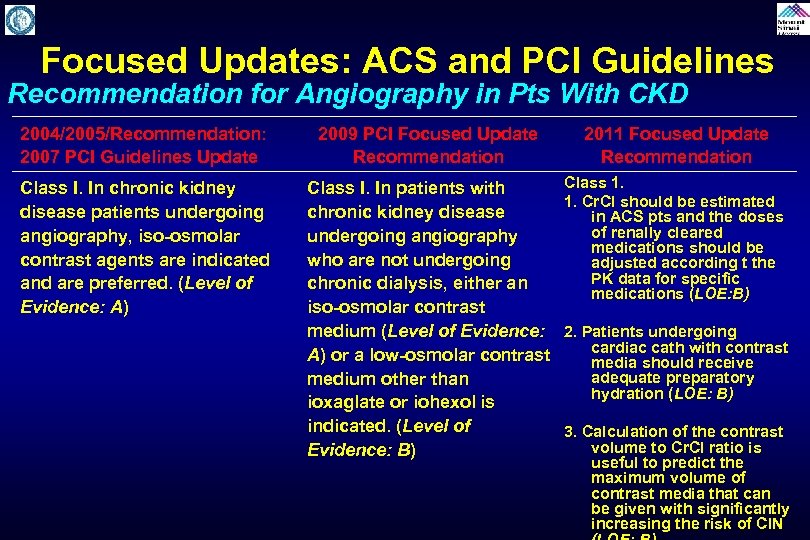
Focused Updates: ACS and PCI Guidelines Recommendation for Angiography in Pts With CKD 2004/2005/Recommendation: 2007 PCI Guidelines Update 2009 PCI Focused Update Recommendation 2011 Focused Update Recommendation Class I. In chronic kidney disease patients undergoing angiography, iso-osmolar contrast agents are indicated and are preferred. (Level of Evidence: A) Class I. In patients with chronic kidney disease undergoing angiography who are not undergoing chronic dialysis, either an iso-osmolar contrast medium (Level of Evidence: A) or a low-osmolar contrast medium other than ioxaglate or iohexol is indicated. (Level of Evidence: B) Class 1. 1. Cr. CI should be estimated in ACS pts and the doses of renally cleared medications should be adjusted according t the PK data for specific medications (LOE: B) 2. Patients undergoing cardiac cath with contrast media should receive adequate preparatory hydration (LOE: B) 3. Calculation of the contrast volume to Cr. CI ratio is useful to predict the maximum volume of contrast media that can be given with significantly increasing the risk of CIN

Take Home Message Update in Platelet Responsiveness and Prevention of CIN-AKI during PCI ü High on treatment platelet reactivity is associated with adverse outcomes post PCI. Use of newer agents of Prasugrel and Ticagrelor in ACS has been effective with studies showing a trend towards rapid maximum PI with Prasugrel vs. Ticagrelor. Routine testing for PI is not warranted. ü Development of CIN-AKI during PCI is dependent on multiple factors with baseline SCr and contrast volume playing a major role. Persistent CIN is associated with higher long-term MACE. Amongst the various strategies to reduce CIN, aggressive forced hydration is most promising.

Question # 1 Which trial of platelet aggregation inhibition has resulted in positive outcome: A. GRAVITAS B. ARCTIC C. TRIGGER PCI D. ANTARTIC E. None yet

Question # 2 Following are the trial of CIN prevention using Renal Guard system except : A. MYTHOS B. REMEDIAL II C. CARE D. RENAL GUARD

Question # 3 Following agents or strategies have consistently shown to reduce CIN except : A. Aggressive hydration B. Ischemic preconditioning C. Automated contrast injection D. N-acetylcysteine E. Statins

Question # 1 Which trial of platelet aggregation inhibition has resulted in positive outcome: A. GRAVITAS B. ARCTIC C. TRIGGER PCI D. ANTARTIC E. None yet The correct answer is E as trials A-C have been negative and ANTARTIC trial is still ongoing

Question # 2 Following are the trial of CIN prevention using Renal Guard system except : A. MYTHOS B. REMEDIAL II C. CARE D. RENAL GUARD The correct answer is C which compared low osmolar vs. iso-osmolar contrast without use of Renal Guard system

Question # 3 Following agents or strategies have consistently shown to reduce CIN except : A. Aggressive hydration B. Ischemic preconditioning C. Automated contrast injection D. N-acetylcysteine (NAC) E. Statins The correct answer is D as randomized trial of NAC has been negative
6b8bce9e2e341abc150558c1f167f71d.ppt