a5e0462f3d352f89b288b592647e9d0a.ppt
- Количество слайдов: 44

Complex Coronary Cases Supported by: • Abbott Vascular • Boston Scientific Corporation • Medtronic, Inc. • Daiichi Sankyo, Inc. and Lilly USA, LLC

Disclosures Samin K. Sharma, MBBS, FACC Speaker’s Bureau – Boston Scientific Corporation, Abbott, The Medicines Company, Daiichi Sankyo, Inc. and Lilly USA, LLC Annapoorna S. Kini, MBBS, FACC Nothing to disclose Sameer Mehta, MBBS, FACC Consulting Fees – The Medicines Company Valentin Fuster, MD, Ph. D, MACC Nothing to disclose American College of Cardiology Foundation staff involved with this case have nothing to disclose

December 18 th 2012 Case #6: DE, 78 yr F Presentation: Presented on 9/7/2012 with NSTEMI and cath revealed 2 V CAD and normal LV function. Patient underwent Rota and DES of calcific RCA lesions. Pt continued to have CCS class II angina on MMT and has calcified LAD/D 1 bifurcation lesion. Prior History: Hyperlipidemia, Hypertension, IDDM, Dextrocardia with SI, Obesity, Thrombocytopenia, Ex-smoker Medications: All once daily dosage Candesartan/HCTZ 32/12. 5 mg, ISMN 60 mg, Rosuvastatin 20 mg, Aspirin 81 mg, Clopidogrel 75 mg, Amlodipine 10 mg, Insulin Metformin XL 1000 mg
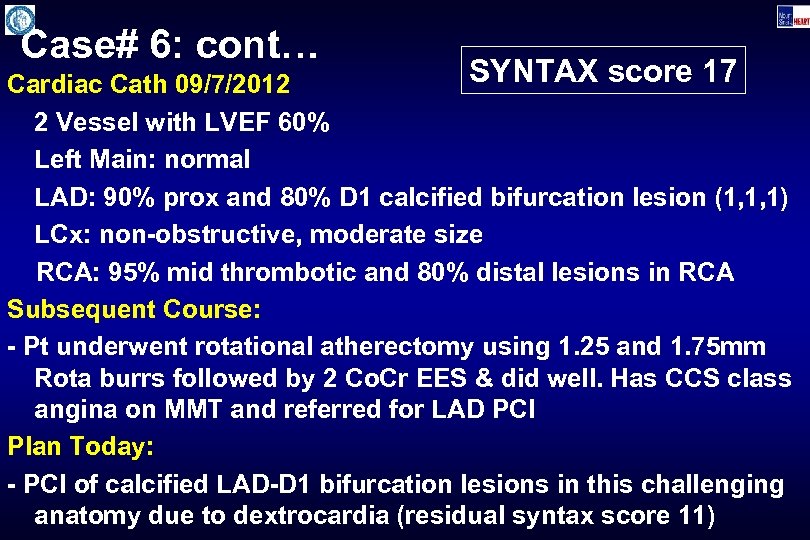
Case# 6: cont… SYNTAX score 17 Cardiac Cath 09/7/2012 2 Vessel with LVEF 60% Left Main: normal LAD: 90% prox and 80% D 1 calcified bifurcation lesion (1, 1, 1) LCx: non-obstructive, moderate size RCA: 95% mid thrombotic and 80% distal lesions in RCA Subsequent Course: - Pt underwent rotational atherectomy using 1. 25 and 1. 75 mm Rota burrs followed by 2 Co. Cr EES & did well. Has CCS class angina on MMT and referred for LAD PCI Plan Today: - PCI of calcified LAD-D 1 bifurcation lesions in this challenging anatomy due to dextrocardia (residual syntax score 11)
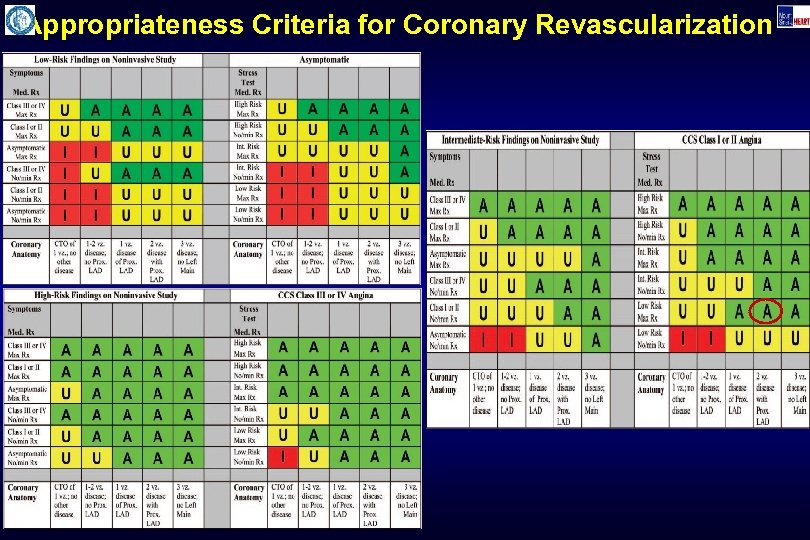
Appropriateness Criteria for Coronary Revascularization
Issues Involving The Case • Multi-vessel CAD: PCI vs. CABG; FREEDOM Trial • Sidebranch intervention in bifurcation lesions
Issues Involving The Case • Multi-vessel CAD: PCI vs. CABG; FREEDOM Trial • Sidebranch intervention in bifurcation lesions
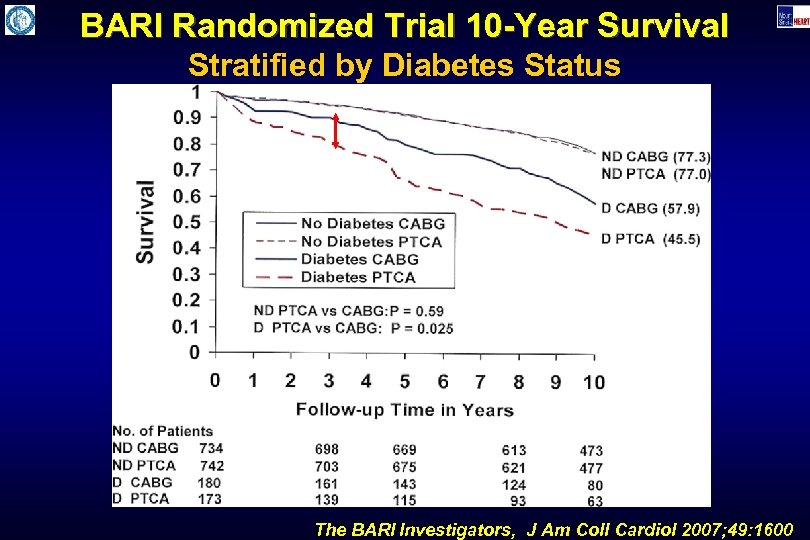
BARI Randomized Trial 10 -Year Survival Stratified by Diabetes Status The BARI Investigators, J Am Coll Cardiol 2007; 49: 1600
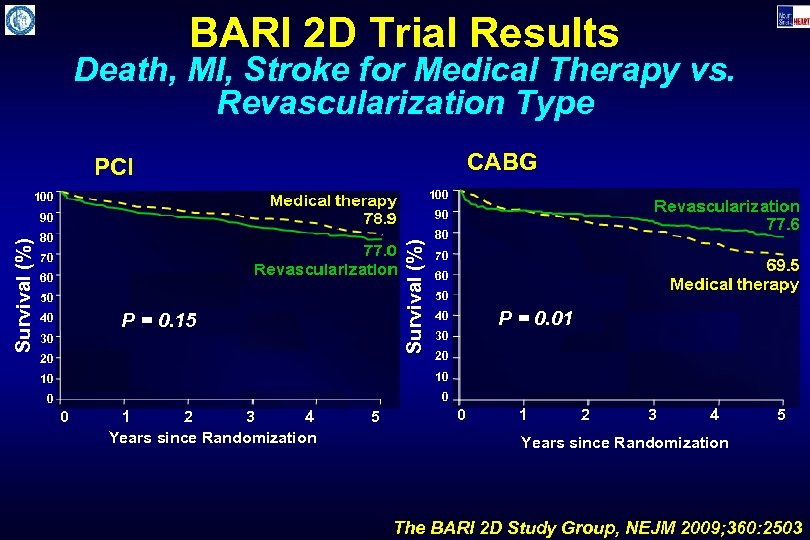
BARI 2 D Trial Results Death, MI, Stroke for Medical Therapy vs. Revascularization Type CABG PCI 90 90 80 80 Survival (%) 100 70 60 50 P = 0. 15 40 30 20 70 60 50 P = 0. 01 40 30 20 10 10 0 1 2 3 4 Years since Randomization 5 0 1 2 3 4 5 Years since Randomization The BARI 2 D Study Group, NEJM 2009; 360: 2503
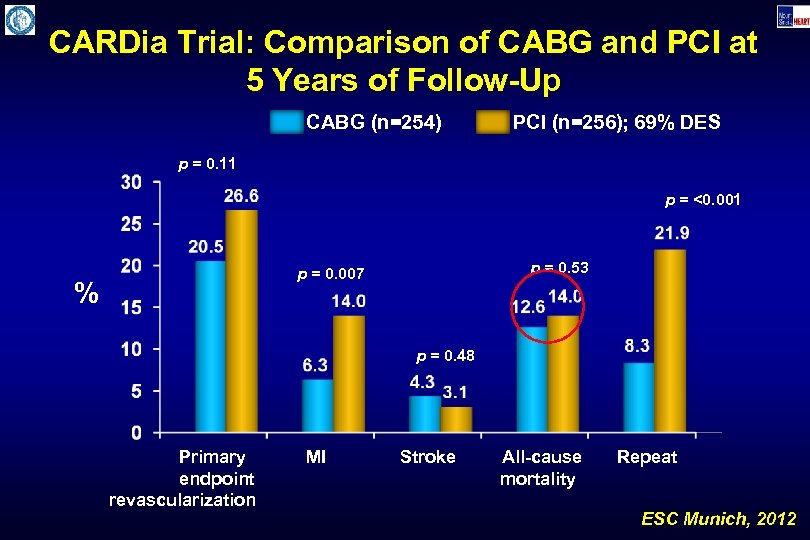
CARDia Trial: Comparison of CABG and PCI at 5 Years of Follow-Up CABG (n=254) PCI (n=256); 69% DES p = 0. 11 p = <0. 001 p = 0. 53 p = 0. 007 % p = 0. 48 Primary endpoint revascularization MI Stroke All-cause mortality Repeat ESC Munich, 2012
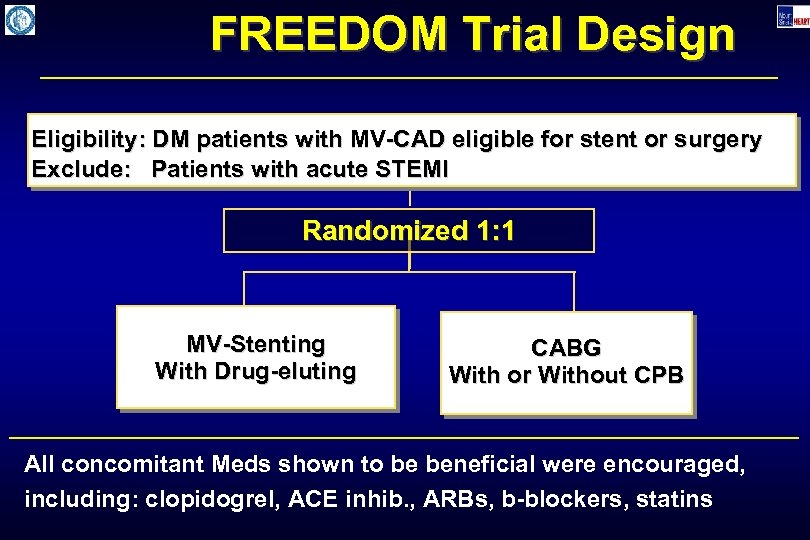
FREEDOM Trial Design Eligibility: DM patients with MV-CAD eligible for stent or surgery Exclude: Patients with acute STEMI Randomized 1: 1 MV-Stenting With Drug-eluting CABG With or Without CPB All concomitant Meds shown to be beneficial were encouraged, including: clopidogrel, ACE inhib. , ARBs, b-blockers, statins
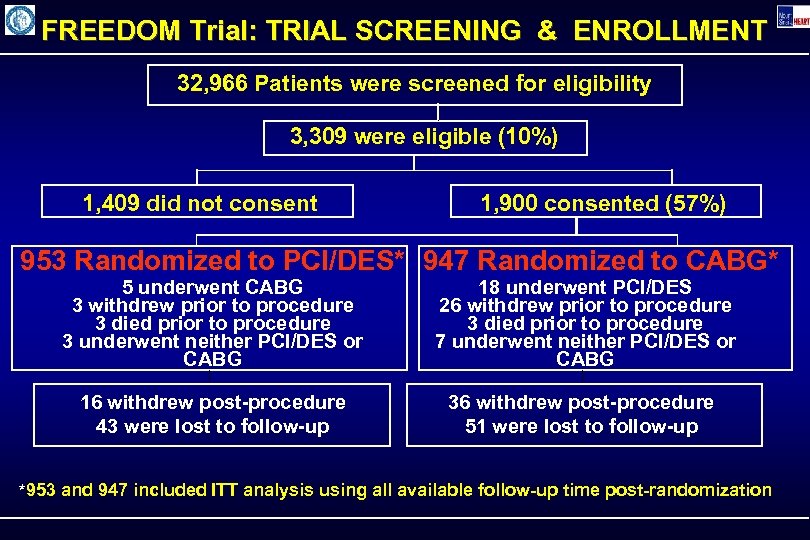
FREEDOM Trial: TRIAL SCREENING & ENROLLMENT 32, 966 Patients were screened for eligibility 3, 309 were eligible (10%) 1, 409 did not consent 1, 900 consented (57%) 953 Randomized to PCI/DES* 947 Randomized to CABG* 5 underwent CABG 3 withdrew prior to procedure 3 died prior to procedure 3 underwent neither PCI/DES or CABG 18 underwent PCI/DES 26 withdrew prior to procedure 3 died prior to procedure 7 underwent neither PCI/DES or CABG 16 withdrew post-procedure 43 were lost to follow-up 36 withdrew post-procedure 51 were lost to follow-up *953 and 947 included ITT analysis using all available follow-up time post-randomization
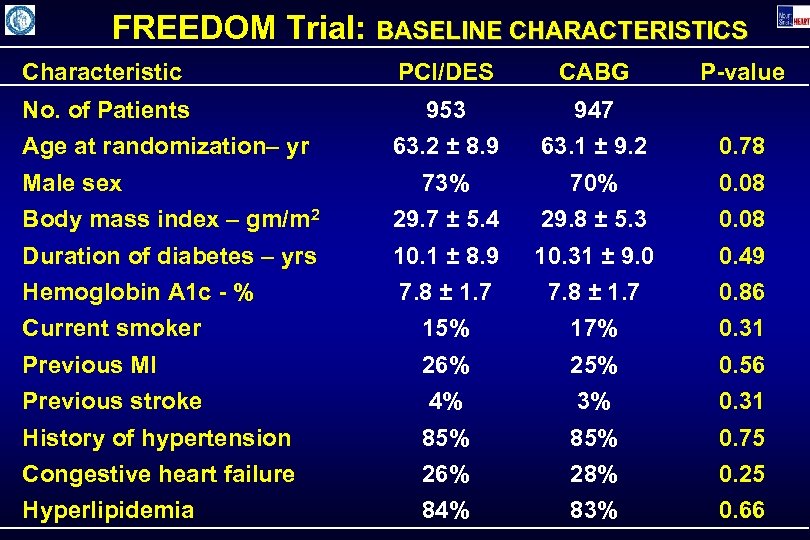
FREEDOM Trial: BASELINE CHARACTERISTICS Characteristic PCI/DES CABG No. of Patients 953 947 63. 2 ± 8. 9 63. 1 ± 9. 2 0. 78 73% 70% 0. 08 Body mass index – gm/m 2 29. 7 ± 5. 4 29. 8 ± 5. 3 0. 08 Duration of diabetes – yrs 10. 1 ± 8. 9 10. 31 ± 9. 0 0. 49 Hemoglobin A 1 c - % 7. 8 ± 1. 7 0. 86 Current smoker 15% 17% 0. 31 Previous MI 26% 25% 0. 56 Previous stroke 4% 3% 0. 31 History of hypertension 85% 0. 75 Congestive heart failure 26% 28% 0. 25 Hyperlipidemia 84% 83% 0. 66 Age at randomization– yr Male sex P-value
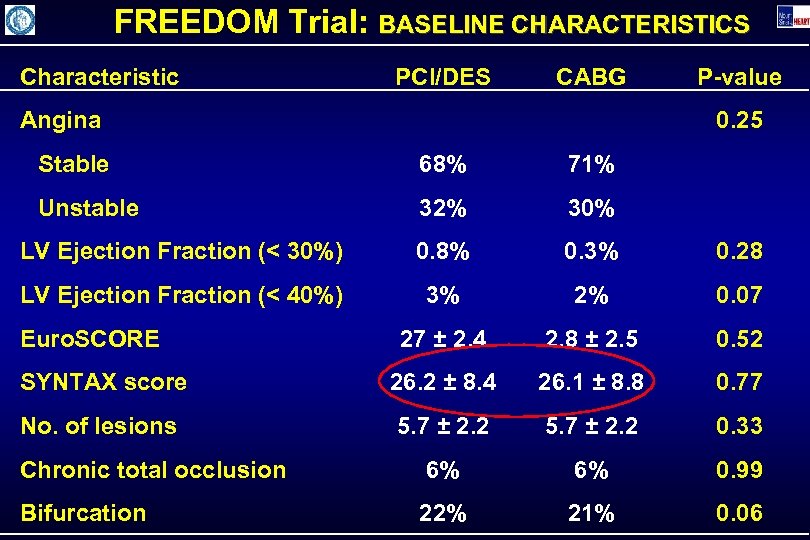
FREEDOM Trial: BASELINE CHARACTERISTICS Characteristic PCI/DES CABG Angina P-value 0. 25 Stable 68% 71% Unstable 32% 30% LV Ejection Fraction (< 30%) 0. 8% 0. 3% 0. 28 LV Ejection Fraction (< 40%) 3% 2% 0. 07 27 ± 2. 4 2. 8 ± 2. 5 0. 52 SYNTAX score 26. 2 ± 8. 4 26. 1 ± 8. 8 0. 77 No. of lesions 5. 7 ± 2. 2 0. 33 Chronic total occlusion 6% 6% 0. 99 Bifurcation 22% 21% 0. 06 Euro. SCORE
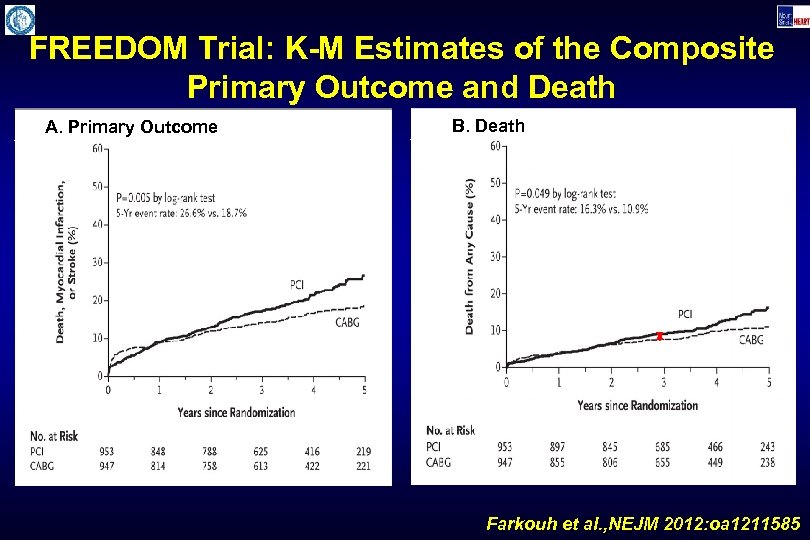
FREEDOM Trial: K-M Estimates of the Composite Primary Outcome and Death A. Primary Outcome B. Death Farkouh et al. , NEJM 2012: oa 1211585
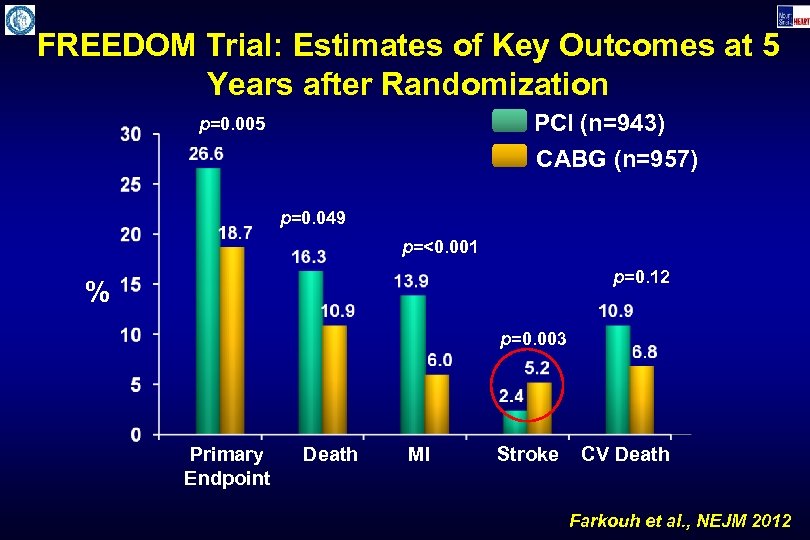
FREEDOM Trial: Estimates of Key Outcomes at 5 Years after Randomization PCI (n=943) p=0. 005 CABG (n=957) p=0. 049 p=<0. 001 p=0. 12 % p=0. 003 Primary Endpoint Death MI Stroke CV Death Farkouh et al. , NEJM 2012
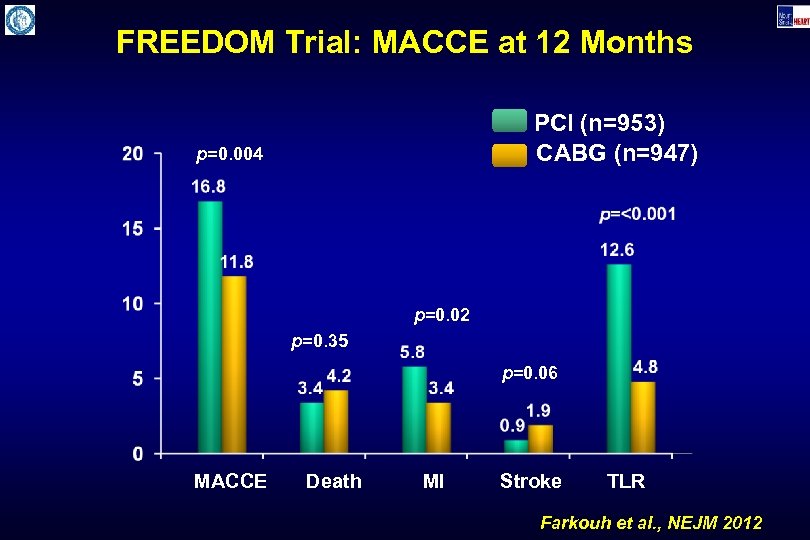
FREEDOM Trial: MACCE at 12 Months PCI (n=953) CABG (n=947) p=0. 004 p=0. 02 p=0. 35 p=0. 06 MACCE Death MI Stroke TLR Farkouh et al. , NEJM 2012
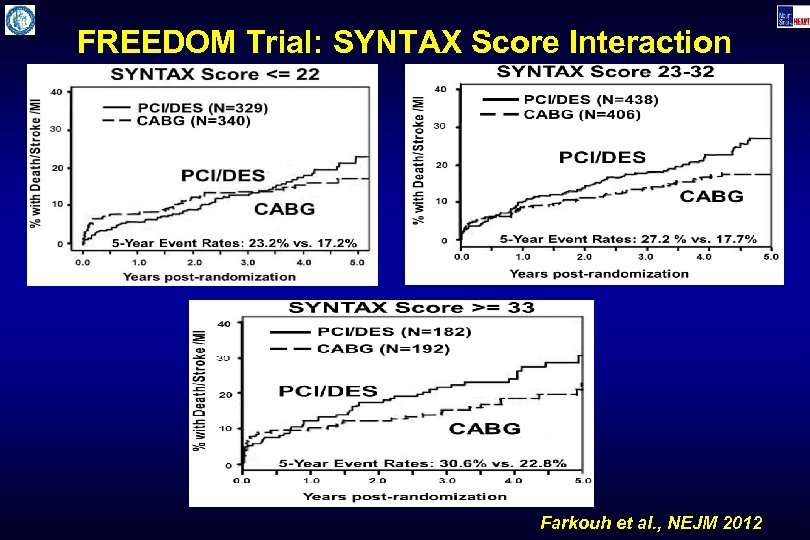
FREEDOM Trial: SYNTAX Score Interaction Farkouh et al. , NEJM 2012
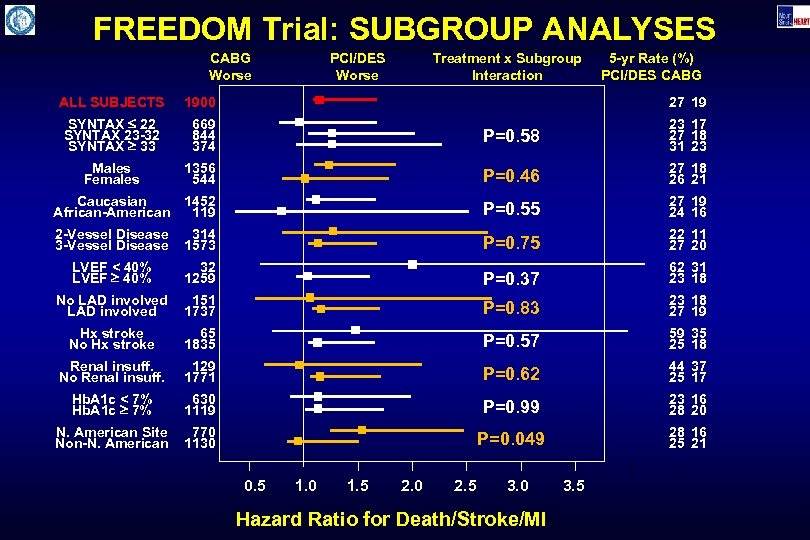
FREEDOM Trial: SUBGROUP ANALYSES CABG Worse PCI/DES Worse Treatment x Subgroup Interaction 5 -yr Rate (%) PCI/DES CABG ALL SUBJECTS 1900 27 19 SYNTAX 22 SYNTAX 23 -32 SYNTAX 33 669 844 374 P=0. 58 23 17 27 18 31 23 Males Females 1356 544 P=0. 46 27 18 26 21 Caucasian African-American 1452 119 P=0. 55 27 19 24 16 2 -Vessel Disease 314 1573 P=0. 75 22 11 27 20 LVEF < 40% LVEF 40% 32 1259 P=0. 37 62 31 23 18 No LAD involved 151 1737 P=0. 83 23 18 27 19 Hx stroke No Hx stroke 65 1835 P=0. 57 59 35 25 18 Renal insuff. No Renal insuff. 129 1771 P=0. 62 44 37 25 17 Hb. A 1 c < 7% Hb. A 1 c 7% 630 1119 P=0. 99 23 16 28 20 N. American Site Non-N. American 770 1130 P=0. 049 28 16 25 21 0. 5 1. 0 1. 5 2. 0 2. 5 3. 0 Hazard Ratio for Death/Stroke/MI 3. 5

Conclusions For patients with diabetes and advanced coronary artery disease, CABG was superior to PCI in that it significantly reduced rates of death and myocardial infraction, with a higher rate of stroke. (Funded by the National Heart, Lung, and Blood Institute and others; FREEDOM Clinincal. Trials. gov number, NCT 00086450. )

FREEDOM Trial Hlatky, NEJM 2012

The results of FREEDOM trial suggests that patients with diabetes ought to be informed about the potential survival benefit from CABG for the treatment of multi-vessel disease. These discussions should begin before coronary angiography in order to provide enough time for the patients to digest the information, discuss it with family members and members of the heart team, and come to an informed decision. Hlatky, NEJM 2012

Many PCIs today are ad hoc procedures, performed at the time of diagnostic coronary angiography, with the same physician making the diagnosis, recommending the treatment, and performing the procedure. There is little time for informed discussion about alternative treatment options, either medical therapy on the one hand or CABG on the other. Well-informed patients might choose any of those options on the basis of their concerns about the various outcomes of treatment, such as survival, stroke, myocardial infarction, angina, and recovery time. This is a complicated decision, and clinical guidelines in the United States 9 and Europe 10 now emphasize the importance of more deliberate decision making about coronary revascularization, including discussions with a multidisciplinary heart team. Hillis et al. , J Am Coll Cardiol 2011: 58: e 123; Wijns, et al. , Eur Heart J 2010: 31: 2501
Issues Involving The Case • Multi-vessel CAD: PCI vs. CABG; FREEDOM Trial • Sidebranch intervention in bifurcation lesions
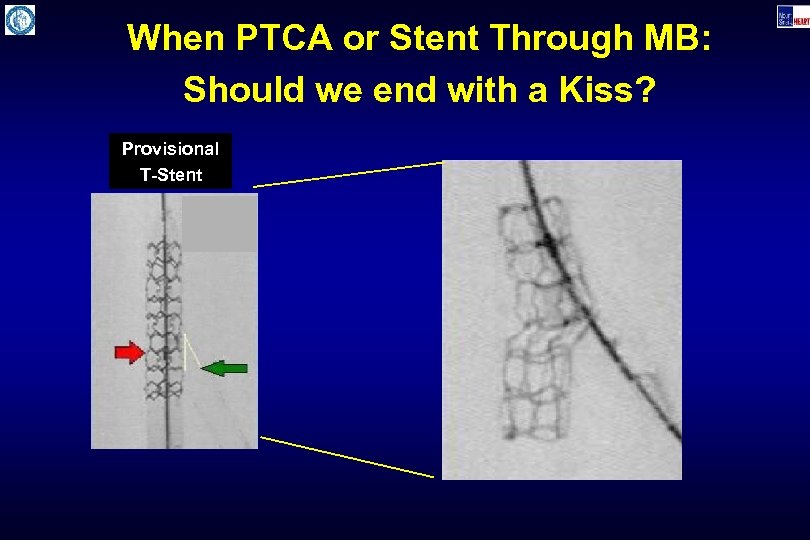
When PTCA or Stent Through MB: Should we end with a Kiss? Provisional T-Stent
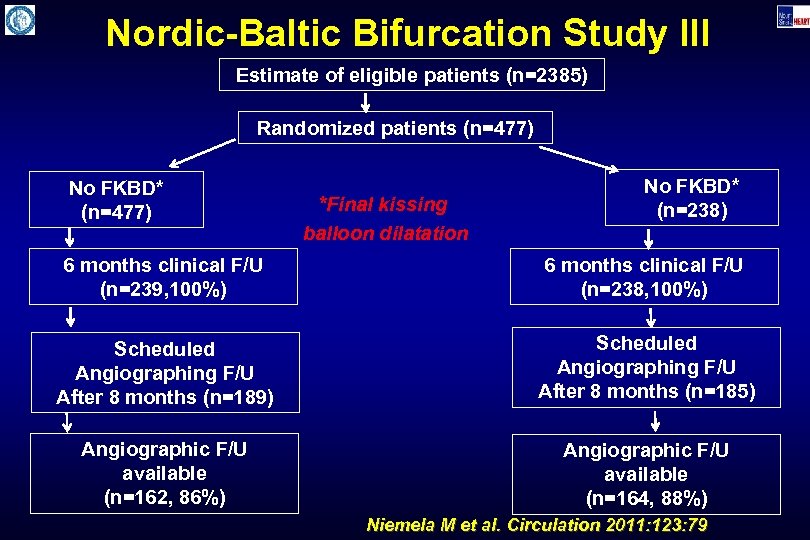
Nordic-Baltic Bifurcation Study III Estimate of eligible patients (n=2385) Randomized patients (n=477) No FKBD* (n=477) *Final kissing balloon dilatation No FKBD* (n=238) 6 months clinical F/U (n=239, 100%) 6 months clinical F/U (n=238, 100%) Scheduled Angiographing F/U After 8 months (n=189) Scheduled Angiographing F/U After 8 months (n=185) Angiographic F/U available (n=162, 86%) Angiographic F/U available (n=164, 88%) Niemela M et al. Circulation 2011: 123: 79
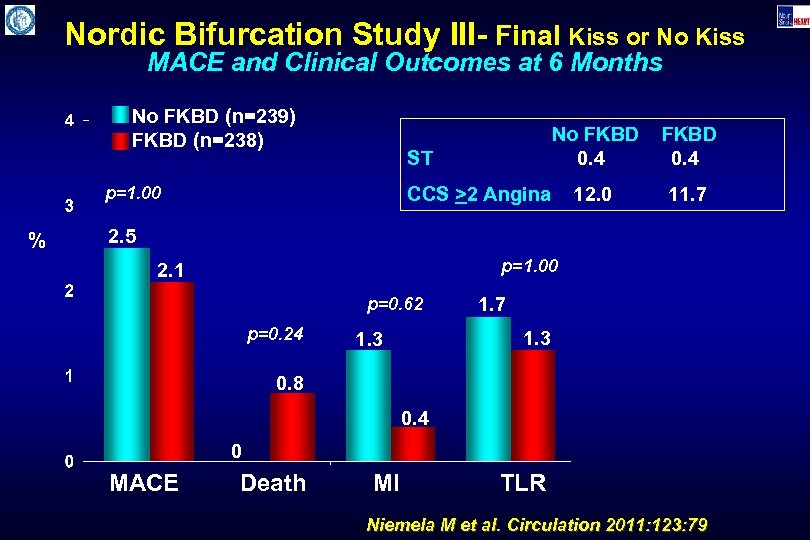
Nordic Bifurcation Study III- Final Kiss or No Kiss MACE and Clinical Outcomes at 6 Months No FKBD (n=239) FKBD (n=238) ST p=1. 00 % No FKBD 0. 4 12. 0 11. 7 CCS >2 Angina 2. 5 p=1. 00 2. 1 p=0. 62 p=0. 24 1. 7 1. 3 0. 8 0. 4 0 MACE Death MI TLR Niemela M et al. Circulation 2011: 123: 79
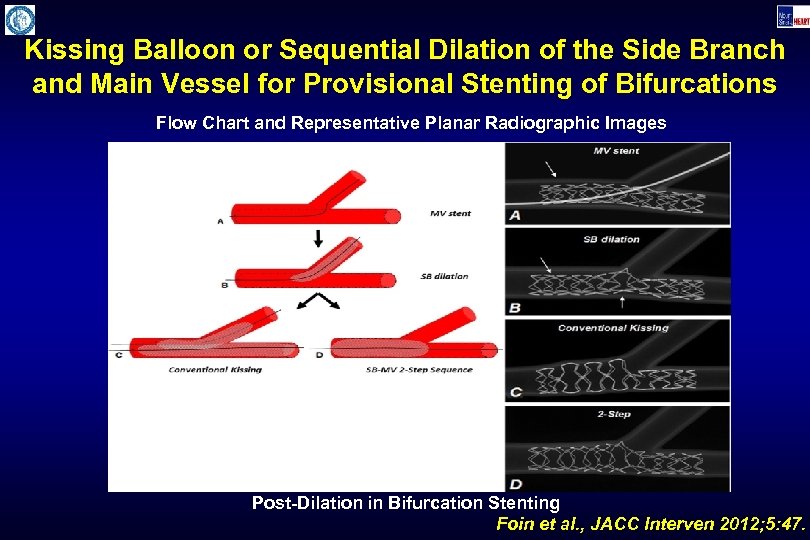
Kissing Balloon or Sequential Dilation of the Side Branch and Main Vessel for Provisional Stenting of Bifurcations Flow Chart and Representative Planar Radiographic Images Post-Dilation in Bifurcation Stenting Foin et al. , JACC Interven 2012; 5: 47.
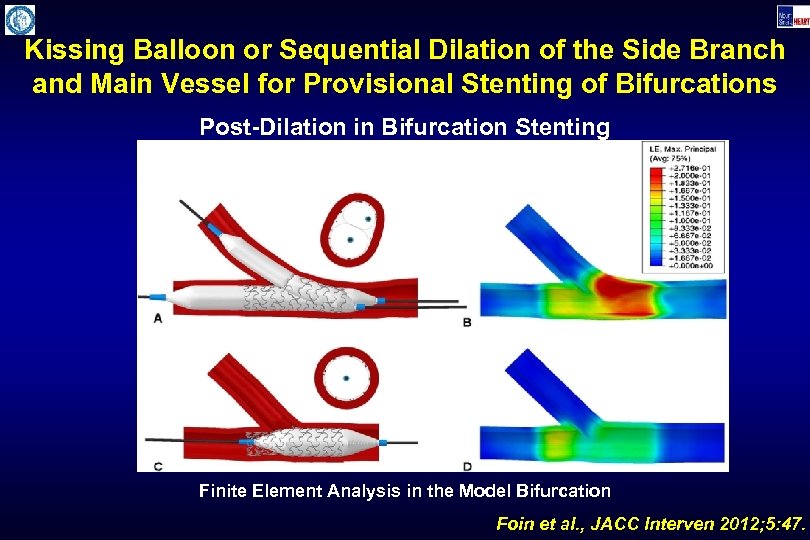
Kissing Balloon or Sequential Dilation of the Side Branch and Main Vessel for Provisional Stenting of Bifurcations Post-Dilation in Bifurcation Stenting Finite Element Analysis in the Model Bifurcation Foin et al. , JACC Interven 2012; 5: 47.
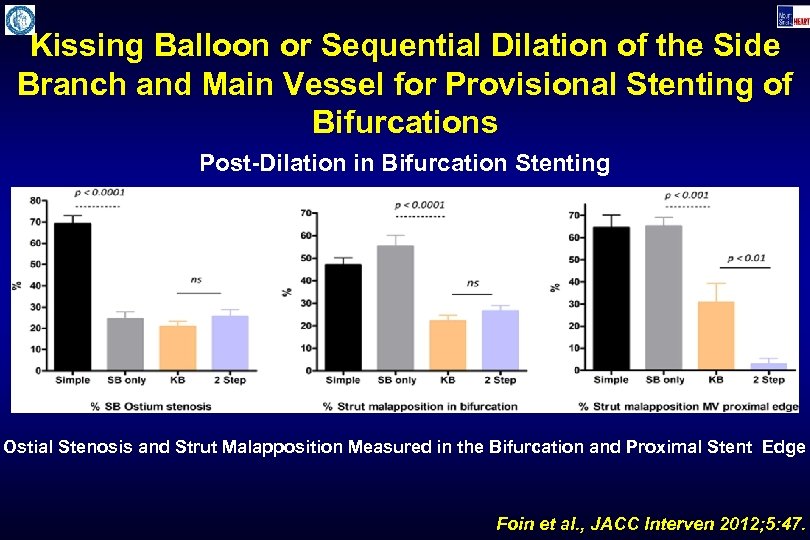
Kissing Balloon or Sequential Dilation of the Side Branch and Main Vessel for Provisional Stenting of Bifurcations Post-Dilation in Bifurcation Stenting Ostial Stenosis and Strut Malapposition Measured in the Bifurcation and Proximal Stent Edge Foin et al. , JACC Interven 2012; 5: 47.
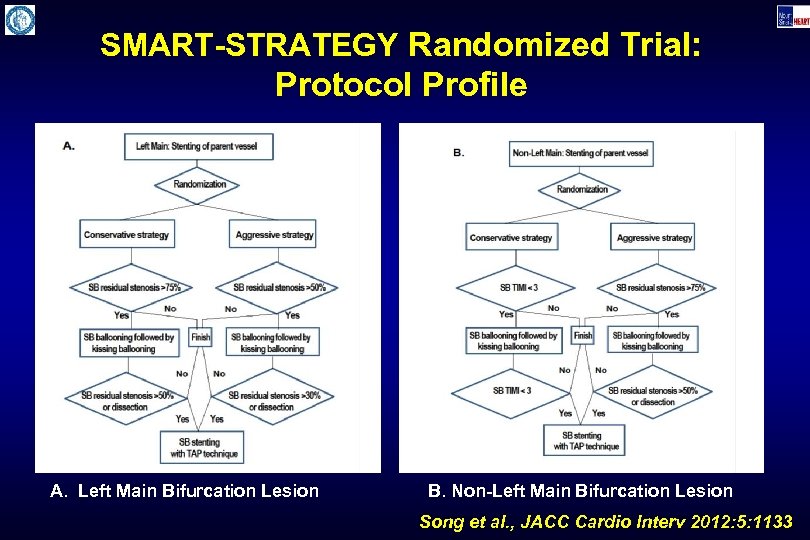
SMART-STRATEGY Randomized Trial: Protocol Profile A. Left Main Bifurcation Lesion B. Non-Left Main Bifurcation Lesion Song et al. , JACC Cardio Interv 2012: 5: 1133
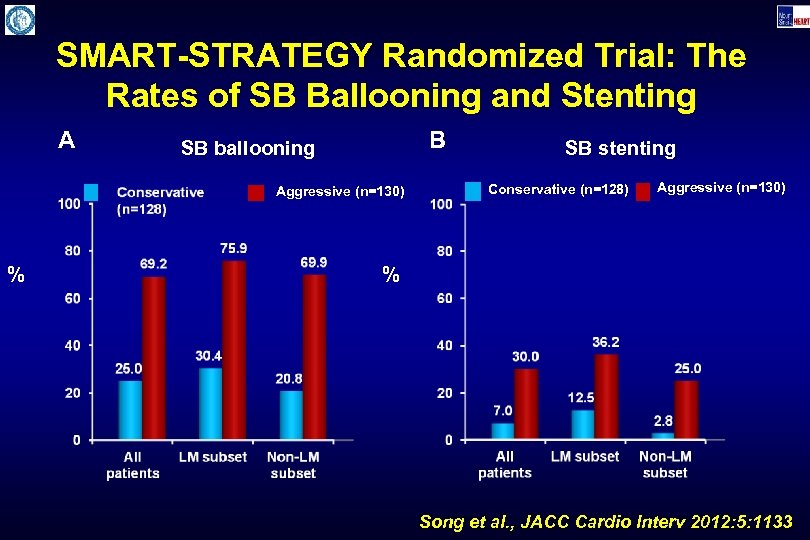
SMART-STRATEGY Randomized Trial: The Rates of SB Ballooning and Stenting A B SB ballooning Aggressive (n=130) % SB stenting Conservative (n=128) Aggressive (n=130) % Song et al. , JACC Cardio Interv 2012: 5: 1133
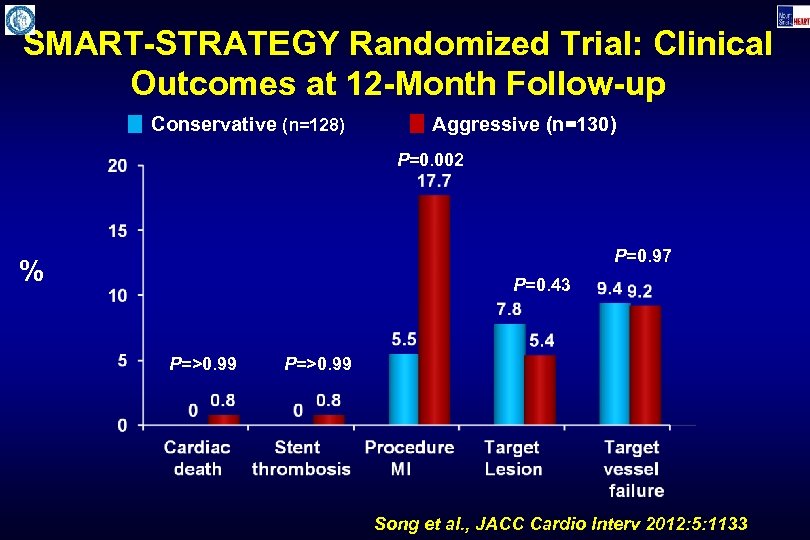
SMART-STRATEGY Randomized Trial: Clinical Outcomes at 12 -Month Follow-up Conservative (n=128) Aggressive (n=130) P=0. 002 P=0. 97 % P=0. 43 P=>0. 99 Song et al. , JACC Cardio Interv 2012: 5: 1133
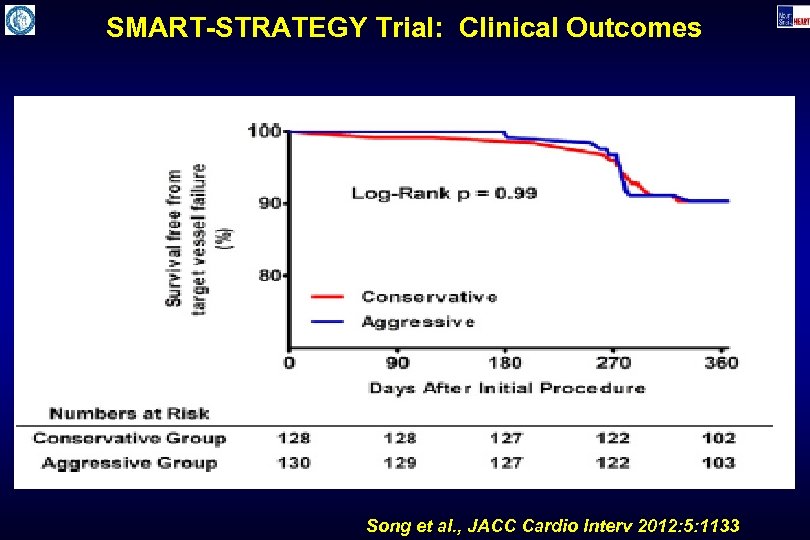
SMART-STRATEGY Trial: Clinical Outcomes Song et al. , JACC Cardio Interv 2012: 5: 1133
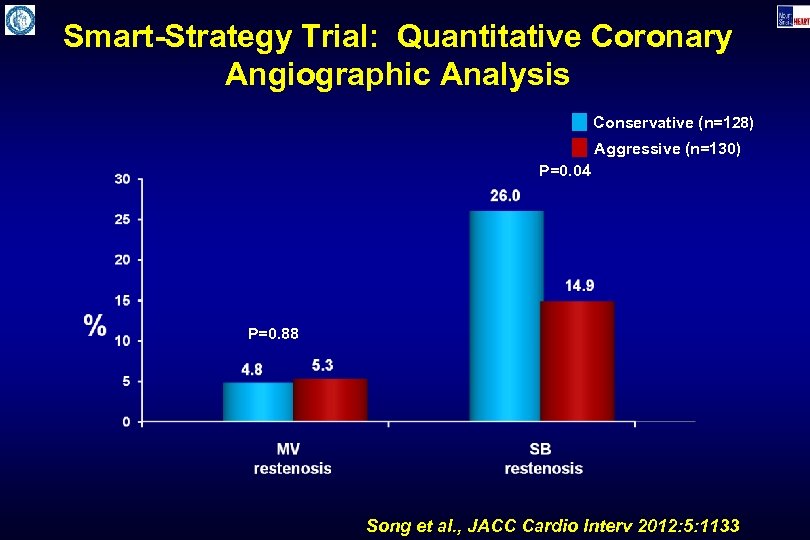
Smart-Strategy Trial: Quantitative Coronary Angiographic Analysis Conservative (n=128) Aggressive (n=130) P=0. 04 P=0. 88 Song et al. , JACC Cardio Interv 2012: 5: 1133
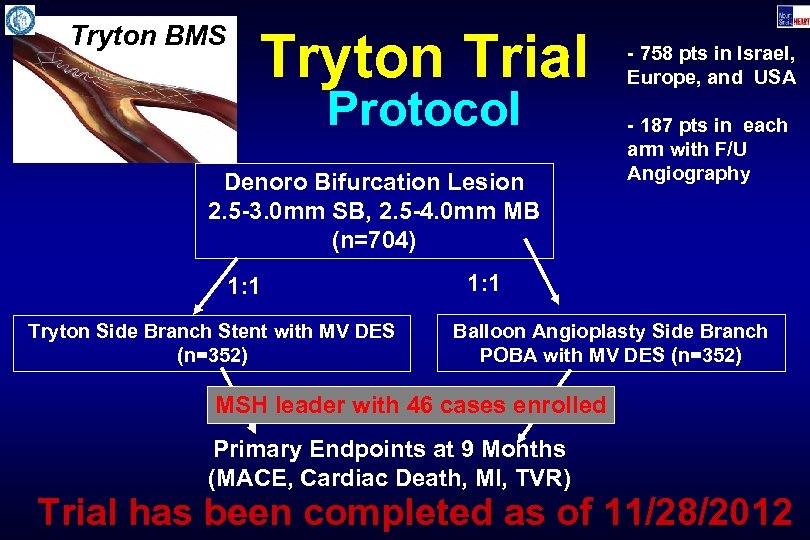
Tryton BMS Tryton Trial Protocol Denoro Bifurcation Lesion 2. 5 -3. 0 mm SB, 2. 5 -4. 0 mm MB (n=704) 1: 1 Tryton Side Branch Stent with MV DES (n=352) - 758 pts in Israel, Europe, and USA - 187 pts in each arm with F/U Angiography 1: 1 Balloon Angioplasty Side Branch POBA with MV DES (n=352) MSH leader with 46 cases enrolled Primary Endpoints at 9 Months (MACE, Cardiac Death, MI, TVR) Trial has been completed as of 11/28/2012
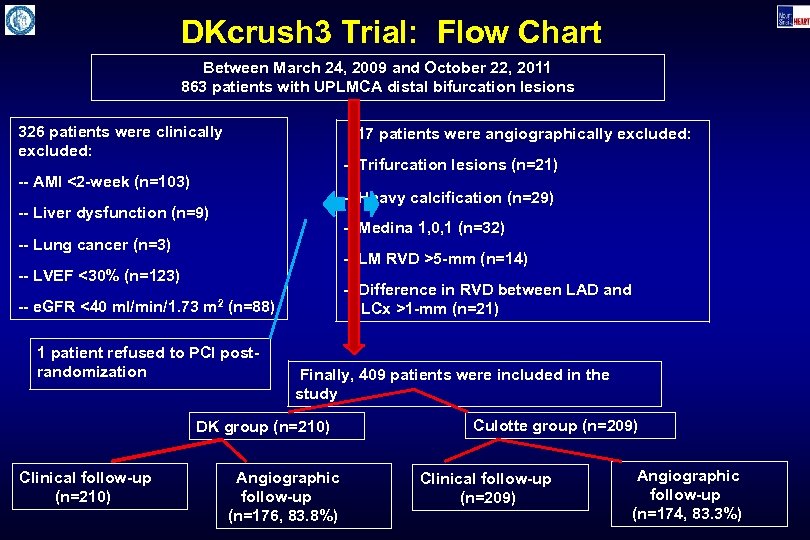
DKcrush 3 Trial: Flow Chart Between March 24, 2009 and October 22, 2011 863 patients with UPLMCA distal bifurcation lesions 326 patients were clinically excluded: 117 patients were angiographically excluded: -- Trifurcation lesions (n=21) -- AMI <2 -week (n=103) -- Heavy calcification (n=29) -- Liver dysfunction (n=9) -- Medina 1, 0, 1 (n=32) -- Lung cancer (n=3) -- LM RVD >5 -mm (n=14) -- LVEF <30% (n=123) -- e. GFR <40 ml/min/1. 73 m 2 -- Difference in RVD between LAD and LCx >1 -mm (n=21) (n=88) 1 patient refused to PCI postrandomization Finally, 409 patients were included in the study DK group (n=210) Clinical follow-up (n=210) Angiographic follow-up (n=176, 83. 8%) Culotte group (n=209) Clinical follow-up (n=209) Angiographic follow-up (n=174, 83. 3%)

Take Home Message: Incorporation of FREEDOM Trial and SB Intervention in Coronary Bifurcations ü Long-term data of coronary revascularization for complex MV CAD, clearly favors CABG over 1 st generation DES PCI and should become the default therapy by guidelines; FREEDOM Trial ü Data are emerging that SBr lesions unless has <TIMI III flow, can be left alone without any increase in cardiac events at follow-up; KIO (keep-it-open) concept

Question # 1 Following are the trials which included all patients with diabetes mellitus except: A. BARI B. BARI 2 D C. FREEDOM D. CARDia E. SYNTAX

Question # 2 In the FREEDOM Trial, following endpoints were lower in the CABG arm versus PCI arm except: A. All cause mortality B. TVR/TLR C. Stroke D. MI E. Death

Question # 3 The TRYTON Stent Trial includes which combination for bifurcation lesion stenting; A. DES in MV and DES in Sidebranch B. BMS in MV and DES in Sidebranch C. DES in MV and BMS in Sidebranch D. BMS in MV and BMS in Sidebranch

Question # 1 Following are the trials which included all patients with diabetes mellitus except: A. BARI B. BARI 2 D C. FREEDOM D. CARDia E. SYNTAX The correct answer is E. SNTAX Trial included all patients of whom about 25% were diabetics. BARI Trial randomized pts with diabetes to PTCA vs. CABG while BARI 2 D Trial randomized diabetic pts to immediate vs. delayed revascularization. Both FREEDOM and CARDia Trials randomized diabetic pts to PCI vs. CABG Serruys et al. NEJM 2009; 360: 961.

Question # 2 In the FREEDOM Trial, following endpoints were lower in the CABG arm versus PCI arm except: A. All cause mortality B. TVR/TLR C. Stroke D. MI E. Cardiac Death The correct answer is C. In the FREEDOM Trial all endpoints of death, MI and TLR were lower in the CABG group while only stroke was lower in the DES/PCI group FREEDOM Trial. NEJM Nov 4 th 2012 (pub ahead of print)

Question # 3 The TRYTON Stent Trial includes which combination for bifurcation lesion stenting; A. DES in MV and DES in Sidebranch B. BMS in MV and DES in Sidebranch C. DES in MV and BMS in Sidebranch D. BMS in MV and BMS in Sidebranch The correct answer is C. In the TRYTON Trial, Tryton sidebranch stent is a BMS while MV included EES DES. TRYTON Trial
a5e0462f3d352f89b288b592647e9d0a.ppt