2fe5219a28749805fb04904205266bb6.ppt
- Количество слайдов: 51
Comparison of Breathing Circuits of Modern Anesthesia Machines: A transitional graphic presentation Created by: Michael A. Olympio, MD Chairman, Committee on Technology Anesthesia Patient Safety Foundation Professor of Anesthesiology Vice Chairman for Education Department of Anesthesiology Wake Forest University School of Medicine December 2003, Version I

Disclaimer This educational tool is the sole creation of the author, who takes full responsibility for its accuracy and representation. Efforts have been taken to ensure this accuracy, even though the schematics are not intended to duplicate official drawings from the manufacturer. Indeed, the variation in manufacturer’ schematics makes it very difficult to compare one circuit to another. It has previously been impossible to overlay one schematic onto another to compare their differences. These circuits represent functional interpretations of the author, particularly with regard to the adjustable pressure limiting valve (A), and its analogous relationship to the ventilator relief valve (VR). Since these two valves serve analogous functions, they are drawn as one and the same, in context with the manual or mechanical circuit; The actual machines depicted do not share these valves. Similarly, the names that I have ascribed to these components do not always match the names given by the manufacturers, although there is general consistency. The drawings and components are not drawn to scale, but are rather sized to conveniently fit on the page. The author regrets that not all manufacturers and not all machines are represented, though they may share many similarities. The author invites those manufacturers to send me their schematic diagrams for potential future inclusion. Only USA FDA-approved machines are considered in this version. Since this original work has not been published elsewhere, the author retains all copyright privileges. This work may not be replicated or copied in any form, nor taken from this website without the express written permission of the author. It is a personally-funded, and unsupported contribution to the APSF web site, for the benefit of its readership, and to promote patient safety through the understanding of complex new breathing circuits. Michael A. Olympio, MD Professor of Anesthesiology Wake Forest University School of Medicine molympio@wfubmc. edu

Acknowledgement The author would like to thank the Datex-Ohmeda Corporation, Madison, WI, and the Dräger Medical Corporation, Telford, PA, and its many educators and engineers who have graciously helped to educate me over the past 20 years. These individuals have been most influential in my career. They have supplied educational materials in both hard and electronic form, have welcomed me and/or supported my travel to their educational courses and factories, have voluntarily joined me at Wake Forest University, in the conduct of our anesthesia machine workshops, and they have consistently answered my questions, challenges, and criticisms regarding their products. The clarity, precision, and thoroughness of their two educational books, Explore!, from Datex. Ohmeda, Inc. , and the ‘green book’, “Operating Principles of Narkomed Anesthesia Systems” from Dräger Medical, Inc. , have profoundly structured my own educational foundation. The creators of these two books have accomplished much more than they will ever know. While providing such an education, these companies have allowed me to remain objective and have encouraged comparisons between equipment. I am very appreciative of these rare opportunities, and hope that others may learn from my experience. I am also indebted to my physician mentors, Drs. James Eisenkraft, Sem Lampotang, Jan Ehrenwerth, Al Grogono, Jeff Andrews and Mike Good, who’ve given me a roadmap to learn, while placing mountains in my path. Thank you.
Instructions • You will view a series of 13 different circuits, beginning with the conventional, and transitioning to other modern circuits. • Be sure to view the full-screen, slide show images. • Each is preceded by an explanation of what you will see, followed by its image. • The comprehensive circuit will have a photograph of the machine from which it is derived. • Upcoming transitions will be explained. The reader is encouraged to page up and down between images, in order to appreciate the transition. Power. Point should already be set to slide transition/fade smoothly/slow • Subsequent slides may repeat a previous circuit, followed by the transition to a new circuit, so that you might appreciate other changes in configuration.
Conventional Manual Circuit: Fig. 1 There are specific reasons for the location of the various components of the breathing circuit within a gas machine. The circuit contains two unidirectional valves, I (inspiratory) and E (expiratory), which allow the inspired and expired gas to flow in one direction, thus preventing the rebreathing of carbon dioxide. Fig. 1 demonstrates inspiration during manual ventilation, by squeezing the reservoir bag, B. Note how the location of the fresh gas inflow, F, just proximal to I, allows the patient to first receive fresh gas during inspiration. The location of the absorber (CO 2) is such that it does not absorb carbon dioxide during exhalation, but rather when the expired gas is “pushed” through it during inspiration. When F is higher than inspiratory flow, it will create a reversal of flow through the absorber during inspiration, and a redirection of the expired gas within the reservoir bag towards the APL (adjustable pressure-limiting), A, valve. In this manner of high F, the absorber will be spared from absorption of carbon dioxide, but it might become dehydrated; the patient will only receive the desired fresh gas components. Note that B is not placed between the absorber and the inspiratory valve, because of the absorber would be less efficient during controlled ventilation. Some of the patient’s gas that is “scrubbed” of carbon dioxide during exhalation would then be scavenged, S, during flow reversal as the bag is squeezed for inspiration. (Read: Dorsch. Understanding Anesthesia Equipment 4 th Ed. 1999: pp 244 -5. Williams and Wilkins)

Conventional manual breathing circuit.

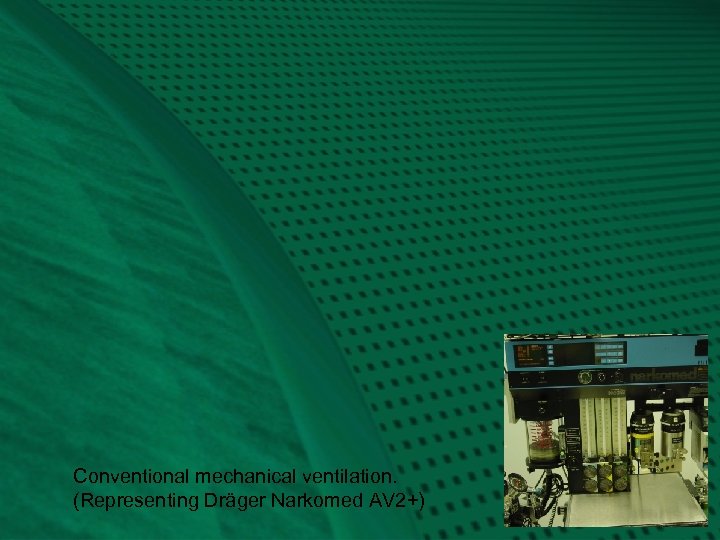
Conventional Mechanical Ventilation: Fig. 2 Conventional anesthesia machines provide mechanical ventilators to “squeeze the bag” for us. The ventilator bellows, VB, should be placed in the same relative location as the previous reservoir bag, to provide efficiency of absorbent use. Conventional ventilators also contain their own “APL” valve, which serves an analogous function. It may be called a ventilator relief valve, VR, as it relieves excess gas from the patient breathing circuit at the end of exhalation, contrary to manual ventilation, which relieves excess patient gas during inspiration. The VR also seals the circuit during inspiration, as shown diagrammatically. Note how I used the same APL valve for the ventilator, to emphasize functional similarities, even though conventional ventilators depicted in this series have their own separate valve. For either manual or mechanical ventilation, the APL or VR should be located between the expiratory valve and the absorber, such that exhaled gas with carbon dioxide is preferentially vented to the scavenger, as it is “chased” by fresh gas coming from the other direction. Next, you will review the preceding Fig. 1, and then transition into a conventional mechanical ventilator circuit, Fig. 2, representing the Dräger Narkomed AV 2+.

Conventional manual breathing circuit.
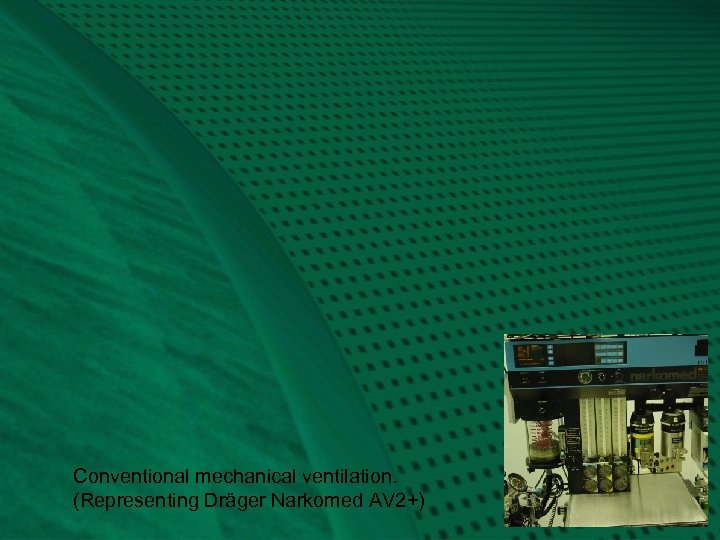
Conventional mechanical ventilation. (Representing Dräger Narkomed AV 2+)

Representing Narkomed AV-2+: Fig. 2 The hanging reservoir bag was replaced by a standing bellows, VB, which makes it easy to detect a disconnection or leak in the circuit, through collapse of the bellows. The bellows is “squeezed” (all the way down!) by pressure-regulated ventilator drive gas, VD, entraining room air through the venturi, VV. The ventilator exhaust valve, VE, must close during inspiration, to allow compression of VB. To prevent escape of patient gas during inspiration, a pilot line must transmit the drive gas pressure to the ventilator relief valve, VR, holding it closed. Ventilator drive gas is released directly into the room, via VE, during end-inspiratory pause and during exhalation. Note the location of the PEEP valve, P, which would serve to pressurize all of the breathing circuit except the bellows, VB, at the end of exhalation. The amount of PEEP may reduce delivered tidal volume according to its location, circuit design, and total compliance of the patient and circuit. (Elliott. J. Clin. Mon. 1989; 5: 100 -4) Carefully note how the fresh gas, F, continues to flow into the patient during the inspiratory phase, contributing to tidal volume and airway pressure. Not shown is a mechanical pressure limiter on top of the ventilator bellows canister.
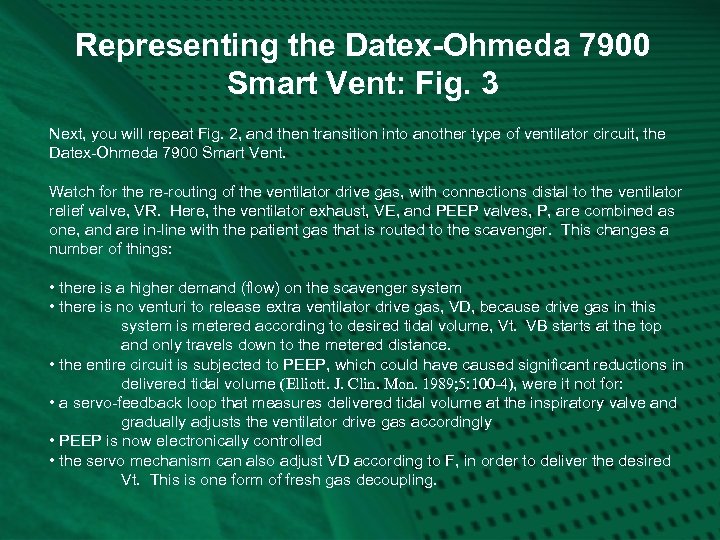
Representing the Datex-Ohmeda 7900 Smart Vent: Fig. 3 Next, you will repeat Fig. 2, and then transition into another type of ventilator circuit, the Datex-Ohmeda 7900 Smart Vent. Watch for the re-routing of the ventilator drive gas, with connections distal to the ventilator relief valve, VR. Here, the ventilator exhaust, VE, and PEEP valves, P, are combined as one, and are in-line with the patient gas that is routed to the scavenger. This changes a number of things: • there is a higher demand (flow) on the scavenger system • there is no venturi to release extra ventilator drive gas, VD, because drive gas in this system is metered according to desired tidal volume, Vt. VB starts at the top and only travels down to the metered distance. • the entire circuit is subjected to PEEP, which could have caused significant reductions in delivered tidal volume (Elliott. J. Clin. Mon. 1989; 5: 100 -4), were it not for: • a servo-feedback loop that measures delivered tidal volume at the inspiratory valve and gradually adjusts the ventilator drive gas accordingly • PEEP is now electronically controlled • the servo mechanism can also adjust VD according to F, in order to deliver the desired Vt. This is one form of fresh gas decoupling.
Conventional mechanical ventilation. (Representing Dräger Narkomed AV 2+)
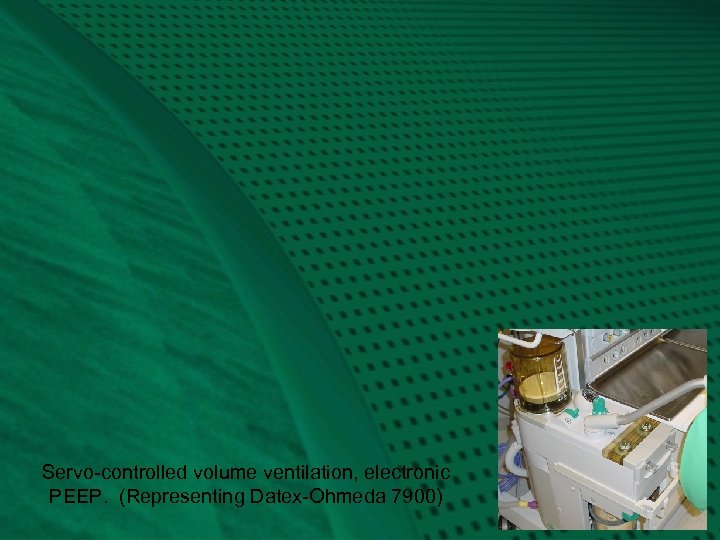
Servo-controlled volume ventilation, electronic PEEP. (Representing Datex-Ohmeda 7900)

Relocation of Fresh Gas Flow: Fig. 4 Now let’s explore some new (probably old!) circuit designs. What would happen if we relocate the fresh gas flow, F, distal to the inspiratory, I, valve? First of all, F would still flow continuously, but now it would continuously flow forward, instead of reversing during exhalation. Combined with a conventional mechanical ventilation system (as in Fig. 2), the patient would still receive extra tidal volume during I, but a respirometer on the exhalation limb would inappropriately measure exhaled gas combined with continuously flowing F. Also during exhalation, this location of F would create inefficient mixing of fresh gas with exhaled gas, such that gas exiting the scavenger would also contain fresh gas. Any gas going through the absorber would always contain carbon dioxide. In other words, the concentration of carbon dioxide exiting the scavenger would be reduced, thereby creating higher demand on the absorbent. (Read: Dorsch. Understanding Anesthesia Equipment 4 th Ed. 1999: pp 244 -5. Williams and Wilkins) Watch what happens as we transition, first, from a conventional manual circuit during inspiration, Fig. 1, to a circuit with F distal to the I-valve, Fig. 4, during exhalation.

Conventional manual breathing circuit.

FIGURE 4 Representing the Datex-Ohmeda S/5 ADU Relocation of fresh gas, F, distal to I valve.

Datex-Ohmeda S/5 ADU: Fig. 4 The “new” location of fresh gas inflow in the ADU provides and/or demands several advantages: 1. It purges and primes the inspiratory limb of the breathing circuit with the desired fresh gas prior to onset of the inspiratory phase, and then continues to deliver the fresh gas flow during the remainder of the I-phase. This may cause faster equilibration of inspired gas with fresh gas, and may be an advantage during low flow anesthesia. Notice in a conventional circuit that at end-inspiration, the inspiratory limb of the breathing circuit contains the least amount of fresh gas (as appreciated in Fig. 1). 2. The respirometer (Vt) must now be placed at the endotracheal tube for accurate measurement. This allows both inspired and expired volume measurements, and can therefore measure an endotracheal tube cuff leak, partial disconnection at the tube, or broncho-pleural fistula, for example. 3. The relocation of the respirometer also allows airway pressure monitoring at the patient, where it is most representative of actual airway pressure. 4. The combination of volume and pressure measurement allows for the creation and analysis of spirometry.

ADU A-EV 1 Ventilator: Fig. 5 Having explored the ADU manual circuit, we can now compare its mechanical ventilator circuit to Fig. 2, the Dräger Narkomed AV 2+. The next frame will repeat the AV 2+ and then transition into the ADU mechanical ventilation circuit (A-EV 1), shown during inspiration. Unlike the AV 2+, there is no venturi, because VD is a metered amount of gas. There is now, however, a PEEP valve in a similar location. You will again see the relocation of the fresh gas inflow, as it was in Fig. 4. Notice in Fig. 5 how the fresh gas, F, continues to flow into the patient during inspiration. However, F is measured electronically and sent to the ventilator computer, which in turn regulates the output of VD, the drive gas, in order to maintain the desired tidal volume, Vt. Vt is measured and reported to the clinician from the spirometry sensor, but is not used in any way by the ventilator control, as it was in Fig. 3 for the Aestiva/5 7900. Another new feature is compliance compensation of Vt, as shown by the electronic arrow. The measured compliance of the system and hoses will subsequently adjust the VD to maintain desired Vt. This is also a feature of the Dräger 6400, Fabius, and Julian.
Conventional mechanical ventilation. (Representing Dräger Narkomed AV 2+)

FIGURE 5 Compliance compensation Representing the Datex-Ohmeda S/5 ADU with A-EV 1 ventilator.

Relocation of the reservoir bag: Fig. 6 What if we started with the conventional manual circuit, Fig. 1 in the next slide, and then transitioned to Fig. 6, which shows the reservoir bag now distal to the absorber, and proximal to the fresh gas inflow? A number of things would change: • During inspiration, as shown, a combination of reservoir gas and fresh gas would still go to the patient, as in Fig. 1. • Now, however, some of the gas from the reservoir bag will go backwards across the absorbent and out the APL valve. This would mean that some of the exhaled gas that has already been scrubbed of carbon dioxide, would then be dumped to the scavenger. This is inefficient use of the absorbent. • Since the forced inspiration of gas now goes backwards across the absorbent, it could be an advantage in preventing any absorbent dust from getting blown into the patient. • During exhalation (not shown), it provides a preferential route for fresh gas flow into the reservoir bag. At higher fresh gas flow, however, the bag will fill unmixed with exhaled gas. In Fig. 1 you can imagine how fresh gas flows through the absorber and always combines with exhaled gas before it reaches the bag.

Conventional manual breathing circuit.

FIGURE 6 Relocation of the reservoir bag. This transition does NOT represent any USA machine circuit.

Piston Ventilator: Fig 7 For reasons mentioned, the previous circuit (Fig. 6) is not suitable for manual ventilation, but could it be adapted to mechanical ventilation? Considering this new reservoir of fresh gas, and the manner in which it fills during exhalation, several new concepts merged into the creation of another modern anesthesia machine circuit: First, instead of isolating the reservoir bag from the circuit during use of the mechanical ventilator, as in all the previous circuits, why not leave it there as a collection reservoir of fresh gas during inspiration? In this manner, the fresh gas could be re-routed to the bag, and away from the patient during inspiration. This would represent another form of (true) fresh gas decoupling whereby the gas does not flow to the patient at all during inspiration. Second, instead of using the conventional bellows-in-a-bottle ventilator, controlled by electronic-pneumatic circuits, why not switch to an electronic-mechanical piston ventilator reminiscent of those used in ICU’s some 40 years ago? Watch how the next transition from Fig. 6 to Fig. 7 incorporates these two changes.

FIGURE 6 Relocation of the reservoir bag. This transition does NOT represent any USA machine circuit.

FIGURE 7 Addition of a piston ventilator. This transition does NOT represent any USA machine circuit.

Piston Ventilator: Fig 7 Now there might appear to be some major problems with this new circuit: First, as the ventilator piston rises on the upstroke (inspiration, as shown), the gas is going to go straight into the reservoir bag instead of into the patient. Second, at high fresh gas flow (not shown), the bag could be distended, fresh gas would reverse flow upwards and then again, contribute to tidal volume as it joined the gas coming from the piston. This would defeat the goal of fresh gas decoupling, and would be unlikely to inflate the lungs because of the bag compliance. Third, the circuit may share some of the disadvantages of Fig. 6, in terms of economy of absorbent use. Fourth, the APL valve must be converted into an automated ventilator relief valve, to prevent the escape of gas during inspiration and: Fifth, the piston has to know when and how to retract, since it is mechanically driven and electronically controlled.

Control Valves: Fig. 8 The modern age of computer circuits and electronically controlled, pneumatically operated valves has allowed us to solve most of these problems. Watch the transition to Fig. 8, after repeating Fig. 7, and look for the appearance of three new valves: 1. A decoupling valve, DV, at the fresh gas inlet to the reservoir bag 2. A manual/spontaneous-exhaust, M/S-E, valve at the inlet of the APL valve 3. Another ventilator relief valve, VR, that provides a direct route to a new scavenger valve, SV.

FIGURE 7 Addition of a piston ventilator. This transition does NOT represent any USA machine circuit.

FIGURE 8 Insertion of control valves. A M/S-E Almost a representation of the Dräger Narkomed 6400.

Piston Ventilation Possible: Fig. 8 Now we can solve the problems listed in the previous slide, because we have added 4 valves to this circuit. Let’s see how they function. First, the decoupling valve closes during inspiration, such that the gas from the piston will be directed into the patient and not into the reservoir bag. Second, the decoupling valve forces fresh gas to flow only into the reservoir bag, even at high flow, and not contribute to tidal volume during inspiration. Third, the economy of absorbent was not improved because exhaled gas will still traverse the absorbent as it flows to the reservoir bag during exhalation (not shown). Fourth, during mechanical ventilation, the manual/spontaneous - exhaust valve, M/S-E, remains closed in order to cyclicly route all gas through the ventilator relief valve, VR, on to the scavenger valve, SV. The VR closes during inspiration, and opens at the end of exhalation, after the DV has opened, and then after the piston has retracted. Note that during manual ventilation (not shown), the VR remains closed, with M/S-E open. During spontaneous ventilation, functionally, M/S would be closed while VR and A are opened. Fifth, the basic piston movement has been described, but its operation and control is complex, and determined by the mode of ventilation.

Dräger 6400 Divan Ventilator: Fig. 9 Watch next as we repeat the previous image of Figure 8 and transition into the Dräger 6400 Divan Ventilator circuit in Figure 9 demonstrates the status of the circuit during exhalation, whereas Figure 8 was drawn in inspiration. We have also added a 5 th valve, for PEEP, and it is located in the expiratory limb of the breathing circuit, proximal to the expiratory valve. Next, a word about the M/S-E valve. Although the circuit is drawn functionally equivalent to the Narkomed 6400, there is a manually operated lever, physically located on top of the APL valve, A, which Dräger calls a “Man/Spont” valve. The actual M/S-E valve, represented in these figures, is hidden within the valve block assembly of the breathing circuit, and it is electronically regulated to close during mechanical ventilation (E-function). Obviously, the APL must be isolated from the mechanical circuit during mechanical ventilation, and that is how it is accomplished.

FIGURE 8 Insertion of control valves. A M/S-E Almost a representation of the Dräger Narkomed 6400.

FIGURE 9 Representation of the Dräger Narkomed 6400 with Divan ventilator. M/S-E A

Dräger Narkomed 6400: Fig. 9 In addition to that described above, realize the following points about this circuit: • With the initial opening of the decoupling valve, exhaled gas is initially scrubbed of carbon dioxide, and dilutes the fresh gas that has accumulated in the reservoir bag. • Regulation of the resistor valve, P, provides PEEP during exhalation. • Then, as the piston retracts, it draws the previously scrubbed gas backwards over the absorbent and into the piston cylinder. • Next, the ventilator relief valve opens, and: • If fresh gas flow is high enough, excess gas, under slight pressure, will passively open the scavenger valve and vent to the scavenger suction canister (not shown).

Time to Re-think Everything Wow, that seemed like it was getting complicated. Four electronically regulated valves, a passive scavenger valve and a newly designed APL valve that allows quick-opening to atmosphere without unscrewing the APL. And what about all that new gas mixing? How readily can I change the concentration of the inspired mixture? We’ll have to wait on some more bench research before the mixing effects are clear. Can we make this piston circuit any simpler? Well, lets go back to Fig. 6, when all we did was to place the reservoir bag next to the fresh gas flow. Then, we are going to maintain the same concept of fresh gas decoupling into the bag, while another piston is used to mechanically ventilate the patient. This time, however, let’s place the piston in the same area, distal to the fresh gas flow, instead of the conventional location in the Narkomed 6400. Watch this transition from Figure 6 to Figure 10.

FIGURE 6 Relocation of the reservoir bag. This transition does NOT represent any USA machine circuit.

FIGURE 10 New location of a ventilator piston. This figure does NOT represent any USAFDA circuit.

New Location for Ventilator Piston: Fig. 10 With the piston in this location, as shown during inspiration, we can see that the ventilator gas will follow the path of least resistance, and probably end up in the reservoir bag. This system, therefore, would not function during mechanical ventilation. An ingenious mind, however, might suddenly see a simple solution to the problem. Let’s watch what happens as we transition from the previous Fig. 10 to the creation of another new machine, the Dräger Fabius GS, in Fig. 11.

FIGURE 10 New location of a ventilator piston. This figure does NOT represent any USAFDA circuit.

FIGURE 11 Representing the Dräger Fabius GS in mechanical ventilation mode.

Dräger Fabius GS: Fig. 11 Did you notice the simple solution to the problem of backflow? Dräger simply added a mechanical one-way check valve, the decoupling valve, DV, which closes automatically as the piston travels upwards during inspiration. Now the gas will flow forward to the patient, but there will not be any back pressure on the expiratory valve, E, to close it. Therefore, the engineers had to add the exhalation valve, EV/P, in the exhalation limb, just proximal to the E valve. This valve is electronically regulated and pneumatically controlled, and is shown in the closed EV position during inspiration. (Referred to as PEEP/PMAX valve by Dräger. ) Notice how the M/S valve must be automatically opened by the ventilator computer during mechanical ventilation. This bypasses the APL (Dräger calls it the “APL bypass valve”) and allows excess gas to escape from the system, after lifting the low pressure scavenger valve, SV. If this valve did not open, and the APL valve was closed, pressure would build in the system, dependent upon fresh gas flow. In spontaneous ventilation mode, the M/S valve in this diagram is functionally similar to the “spont” setting within the APL, because, when open, it routes the excess gas directly to the SV. The M/S valve in the diagram is seen in the photo as the silver dome beneath the grey APL on top of the black breathing circuit manifold. Realize that during exhalation (not shown), the EV/P functions as a PEEP valve.

Isn’t there a simpler way? Fresh Gas Flow Interruption: Fig. 12 Since we have computer controlled ventilators, why not have computer controlled fresh gas flow? Everything to this point has had needle-valve control of fresh gas, even though your ADU and Fabius GS measure and report the flow digitally. If we can control, measure, and report flow electronically, then we can also interrupt the flow during inspiration and provide another form of fresh gas decoupling. Note how the next transition from the conventional circuit, Fig. 1, to this new circuit, Fig. 12, also re-routes the fresh gas into the center of the absorber. This was done to provide a convenient entry port for the return of sampled gas from the gas analyzer, should that design become FDA approved. Note the obvious addition of the decoupling valve, DV, as described.

Conventional manual breathing circuit.

FIGURE 12 Fresh gas flow interruption during inspiration. This will become the Dräger Julian.

The Dräger Julian: Fig. 13 The previous circuit, as shown during manual inspiration, would not require a decoupling valve, nor does it use one during inspiration, because we rely upon the APL valve to adjust the amount of gas in the circuit for a given tidal volume. What we will see next, is the transition from this manual circuit to the Julian mechanical ventilation circuit. You will see the reservoir bag replaced by the bellows, but notice something different: • The bellows is now ‘hanging’ instead of ‘standing’ • The APL, as depicted, functionally ‘becomes’ a ventilator relief valve, as before • We still need a ventilator exhaust valve, VE, as in Figs. 2 and 3, and: • The VE can also serve as a PEEP valve as it does with the Aestiva 7900 smart vent.

FIGURE 12 Fresh gas flow interruption during inspiration. This will become the Dräger Julian.

FIGURE 13 The Dräger Julian

The Dräger Julian: Fig. 13 Here we have the Dräger Julian, with its hanging bellows, VB. How do we prevent an undetected disconnection of the breathing circuit? Recall that hanging ventilators of the 1970’s would continue to cycle because they entrained room air as the bellows descended, and they delivered an ‘inspiratory’ volume as the bellows cycled during inspiration. Notice how the electric eye ‘looks for’ the descent of the bellows. With a partial disconnect, and at low flow, and with a light-weight bellows, one could imagine a slow bellows descent to the bottom of the canister during exhalation. This might trigger an alarm. If one added PEEP to the canister, and surrounded the entire bellows with endexpiratory pressure, then the bellows would not fall, but might actually stay at the top during exhalation. Thus, the placement and use of PEEP could facilitate detection of a disconnect. As always, however, we rely on multiple other monitors of ventilation.

Summary of New Safety Features • • More accurate and/or corrected tidal volume through compliance and fresh gas compensation Potential return of sampled gas to facilitate low-flow Fresh gas decoupling may prevent hyperinflation of the lung Some forms of decoupling will even reroute the high flow of oxygen flush if it is depressed during mechanical inspiration Incorporation of electronic PEEP may prevent inaccurate, improper, or unintended PEEP Electronic selection of ventilation parameters might prevent improper setup of a mechanical ventilator Automated checkout procedures (presumably) will detect a problem that clinicians may not, particularly in these complex systems New disconnect alarm system for hanging bellows might reduce the incidence of inadequate ventilation
Conclusion I hope this transitional graphic presentation of modern breathing circuits has helped you to understand the function of modern anesthesia machines. To look at them, they are quite ominous and frightening, because we can no longer see the ‘pipes’ nor even imagine the direction of gas flow within the system. They are complicated, indeed, and require a multitude of sensors that are also used in conjunction with automated electronic checkout procedures. The computer must sequentially and intelligently test the valves with and without gas flow, and during synchronized compression of the ventilator. We must try to understand these systems, so that we will understand the importance, significance, and safety of auto-testing, and the meaning of various warnings and alarms. Manufacturers are developing safer and smarter equipment, and have provided machines that do what we want, and exactly what we expect them to do. Good luck and enjoy the new technology! Please send feedback to molympio@wfubmc. edu
2fe5219a28749805fb04904205266bb6.ppt