9cec104eec5ae5ba8fb9b1e14c82475b.ppt
- Количество слайдов: 30
 Comparing Three Life Cycle Management Approaches Adalat ● Tiazac *Redacted for public/non-client distribution ● Cefadroxil
Comparing Three Life Cycle Management Approaches Adalat ● Tiazac *Redacted for public/non-client distribution ● Cefadroxil
 3 case studies to illustrate 3 Life Cycle Management principles The Path to Success ● Economic Biases ● Traps
3 case studies to illustrate 3 Life Cycle Management principles The Path to Success ● Economic Biases ● Traps
 How To Get A Formulation Patent 3 Key Steps: Formality Useful New
How To Get A Formulation Patent 3 Key Steps: Formality Useful New
 Formalities A patent application looks like a paper or thesis Abstract; Introduction Materials & Methods; Conclusions may be “speculative” Results, Data not required (cf. Crick & Watson) incorrect Conclusions = useless patent vague Methods = invalid patent legal Claims
Formalities A patent application looks like a paper or thesis Abstract; Introduction Materials & Methods; Conclusions may be “speculative” Results, Data not required (cf. Crick & Watson) incorrect Conclusions = useless patent vague Methods = invalid patent legal Claims
 Usefulness The patent application must assert a usefulness Assert : "I suspect this will work. " Clinical data not required. Exception : if government has data showing invention does not work cf. throwaway uses a : just one, not "all, " nor even "best" usefulness : "made or used in any industry” 2 e = mc
Usefulness The patent application must assert a usefulness Assert : "I suspect this will work. " Clinical data not required. Exception : if government has data showing invention does not work cf. throwaway uses a : just one, not "all, " nor even "best" usefulness : "made or used in any industry” 2 e = mc
 ADALAT® CC Bayer's sustained-release nifedipine insolubility impedes absorption into blood increased solubility makes blood level spike and decreases sustained bioavailability Alternative approaches: large-size tablets, expensive and light-sensitive liquid formulations
ADALAT® CC Bayer's sustained-release nifedipine insolubility impedes absorption into blood increased solubility makes blood level spike and decreases sustained bioavailability Alternative approaches: large-size tablets, expensive and light-sensitive liquid formulations
 Bayer's solution - increase the specific surface area (SSA). –Prior art teaches SSA less than 0. 5 square meters/gram –SSAs < 0. 5 : no special bioavailability advantages –Bayer patent : nifedipine with SSA > 1 –improved bioavailability –Patent Office disputed that this actually works –Decision: cooperate with government, or argue? –Cost to cooperate : a round-trip business-class ticket from San Diego to Paris –Cost to argue : maybe 12 -15 tickets –What is the value of patent on "nifedipine with SSA greater than 1” when the product is not yet even launched?
Bayer's solution - increase the specific surface area (SSA). –Prior art teaches SSA less than 0. 5 square meters/gram –SSAs < 0. 5 : no special bioavailability advantages –Bayer patent : nifedipine with SSA > 1 –improved bioavailability –Patent Office disputed that this actually works –Decision: cooperate with government, or argue? –Cost to cooperate : a round-trip business-class ticket from San Diego to Paris –Cost to argue : maybe 12 -15 tickets –What is the value of patent on "nifedipine with SSA greater than 1” when the product is not yet even launched?
 Bayer cooperated, filing clinical pharmacokinetic data –Dr. Eduard Porges: "plateau-like effect in dissolution testing of. . . crystals having a SSA of 1 to 4 m 2/g, " SSA below 1 or above 4 m 2/g has "a decreasing dissolution rate. " –Dr. Klemens Leopold Lustig: blood plasma level in dogs is higher "when using [] crystals between 0. 99 to 4. 05 m 2/g. “ Data shows: x < 1 does not work 1< x <4 works the best x >4 works, but not as well
Bayer cooperated, filing clinical pharmacokinetic data –Dr. Eduard Porges: "plateau-like effect in dissolution testing of. . . crystals having a SSA of 1 to 4 m 2/g, " SSA below 1 or above 4 m 2/g has "a decreasing dissolution rate. " –Dr. Klemens Leopold Lustig: blood plasma level in dogs is higher "when using [] crystals between 0. 99 to 4. 05 m 2/g. “ Data shows: x < 1 does not work 1< x <4 works the best x >4 works, but not as well
 ADALAT® CC Patent (continued) Patent Office concedes that Bayer high-SSA invention works, and grants patent to Bayer Patent covers “SSA > 1. 0” –Inexpensive & easy solution THE END
ADALAT® CC Patent (continued) Patent Office concedes that Bayer high-SSA invention works, and grants patent to Bayer Patent covers “SSA > 1. 0” –Inexpensive & easy solution THE END
 ADALAT® CC Patent (continued) Elan files a "Paragraph (iv)" ANDA on Adalat CC with API having an SSA of > 5. 0 m 2/g. Bayer sues. Prior art discloses SSA < 0. 5 –Bayer says patent covers any SSA > 1 –Elan says Bayer's researchers (Drs. Porges and Lustig) said that SSA over 4 does not work well Elan says it's not fair for the patent to cover versions which Bayer said do not work well Rule : The patent must assert a use, not “the best” use
ADALAT® CC Patent (continued) Elan files a "Paragraph (iv)" ANDA on Adalat CC with API having an SSA of > 5. 0 m 2/g. Bayer sues. Prior art discloses SSA < 0. 5 –Bayer says patent covers any SSA > 1 –Elan says Bayer's researchers (Drs. Porges and Lustig) said that SSA over 4 does not work well Elan says it's not fair for the patent to cover versions which Bayer said do not work well Rule : The patent must assert a use, not “the best” use
 Court concluded that Bayer data waived protection for any range > 4 –Cost to cooperate : one ticket –Cost to argue : maybe 15 tickets –Benefit to argue : tickets for everyone at this conference, plus tickets for the family, plus a house –Analysis : What will be the value of product?
Court concluded that Bayer data waived protection for any range > 4 –Cost to cooperate : one ticket –Cost to argue : maybe 15 tickets –Benefit to argue : tickets for everyone at this conference, plus tickets for the family, plus a house –Analysis : What will be the value of product?
 ADALAT® CC Patent (continued) Providing clinical data showing usefulness –government must prove that invention does not work –providing data to PTO may speed patent approval –providing data to PTO may damage patent "No good deed goes unpunished. ” Studied ignorance : What if Bayer neglected to test SSA > 2 at all? What happened to patent attorney? Dr. Porges?
ADALAT® CC Patent (continued) Providing clinical data showing usefulness –government must prove that invention does not work –providing data to PTO may speed patent approval –providing data to PTO may damage patent "No good deed goes unpunished. ” Studied ignorance : What if Bayer neglected to test SSA > 2 at all? What happened to patent attorney? Dr. Porges?
 How To Get A Formulation Patent Formalities Usefulness Newness
How To Get A Formulation Patent Formalities Usefulness Newness
 Newness Exactly or substantially the same Exactly the same : simple, but rare Your own work can damage your patent application cf. If Dr. Eduard Porges had published his data Informal disclosures are relevant Graduate theses, posters, academic department seminars
Newness Exactly or substantially the same Exactly the same : simple, but rare Your own work can damage your patent application cf. If Dr. Eduard Porges had published his data Informal disclosures are relevant Graduate theses, posters, academic department seminars
 Newness (continued) solution - file patent application on work you suspect might be commercially valuable, before public disclosure risk - waste money on dead-end filings What if Bayer had aggressively invested in a broad patent. . . and the product failed?
Newness (continued) solution - file patent application on work you suspect might be commercially valuable, before public disclosure risk - waste money on dead-end filings What if Bayer had aggressively invested in a broad patent. . . and the product failed?
 Newness (continued) "substantially" similar to How much is substantially? “It depends. ” e. g. , difference between <0. 5 m 2/g SSA and >1. 0 m 2/g SSA for nifedipine What effect does la difference have? lots of negotiation with Patent Office on this point Examples - Tiazac SR, cefadroxil, tacrine
Newness (continued) "substantially" similar to How much is substantially? “It depends. ” e. g. , difference between <0. 5 m 2/g SSA and >1. 0 m 2/g SSA for nifedipine What effect does la difference have? lots of negotiation with Patent Office on this point Examples - Tiazac SR, cefadroxil, tacrine
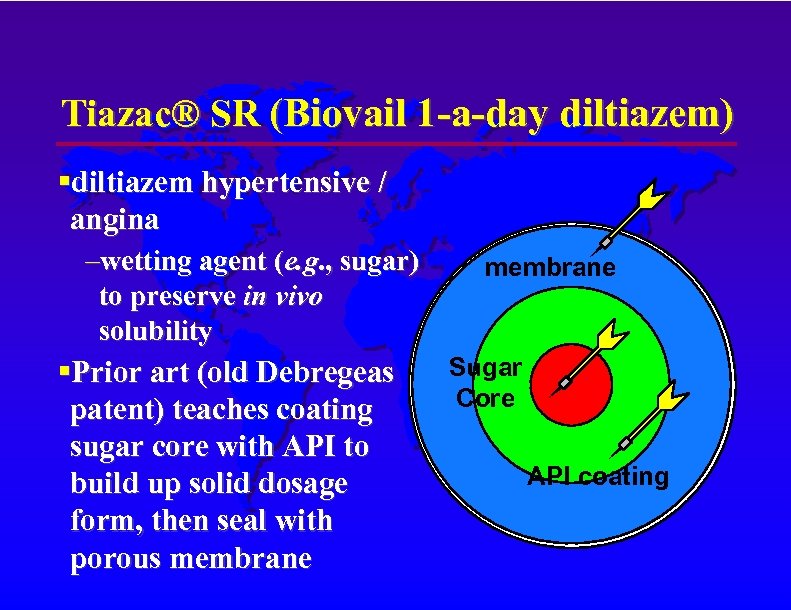 Tiazac® SR (Biovail 1 -a-day diltiazem) diltiazem hypertensive / angina –wetting agent (e. g. , sugar) to preserve in vivo solubility Prior art (old Debregeas patent) teaches coating sugar core with API to build up solid dosage form, then seal with porous membrane Sugar Core API coating
Tiazac® SR (Biovail 1 -a-day diltiazem) diltiazem hypertensive / angina –wetting agent (e. g. , sugar) to preserve in vivo solubility Prior art (old Debregeas patent) teaches coating sugar core with API to build up solid dosage form, then seal with porous membrane Sugar Core API coating
 Tiazac® SR Mixing wetting agent (e. g. , sugar) with diltiazem improves SR profile Biovail applies for patent on SR formulation Patent Office disputed this is new and demanded proof of newness Sugar + API homogenous blend
Tiazac® SR Mixing wetting agent (e. g. , sugar) with diltiazem improves SR profile Biovail applies for patent on SR formulation Patent Office disputed this is new and demanded proof of newness Sugar + API homogenous blend
 Tiazac SR Biovail filed a Declaration Biovail said they are different from earlier Debregeas patent. Patent Office agreed that a homogenous dosage form is substantially different from earlier Debregeas coated-core dosage form. Patent Office allowed the patent. –THE END
Tiazac SR Biovail filed a Declaration Biovail said they are different from earlier Debregeas patent. Patent Office agreed that a homogenous dosage form is substantially different from earlier Debregeas coated-core dosage form. Patent Office allowed the patent. –THE END
 Tiazac SR Andrx files ANDA for generic diltiazem SR, made per Debregeas coated-core patent: sugar core built up with API + ethylcel + polyvinylpyrrolidone; membrane jacket Biovail sues for patent infringement (Biovail v. Andrx, Federal Circuit (13 Feb 2001)) Patent covers homogenous sugar + API Andrx makes an old-fashioned coated core. How could there possibly be infringement? ? ?
Tiazac SR Andrx files ANDA for generic diltiazem SR, made per Debregeas coated-core patent: sugar core built up with API + ethylcel + polyvinylpyrrolidone; membrane jacket Biovail sues for patent infringement (Biovail v. Andrx, Federal Circuit (13 Feb 2001)) Patent covers homogenous sugar + API Andrx makes an old-fashioned coated core. How could there possibly be infringement? ? ?
 Tiazac SR Recall that in Patent Office, Biovail said its homogenous mix is different from earlier Debregeas coated-core patent. In court, however, Biovail says Andrx product dissolves in vivo to make homogenous sugar + API in stomach Court demanded proof of in vivo homogenous mixture
Tiazac SR Recall that in Patent Office, Biovail said its homogenous mix is different from earlier Debregeas coated-core patent. In court, however, Biovail says Andrx product dissolves in vivo to make homogenous sugar + API in stomach Court demanded proof of in vivo homogenous mixture
 Tiazac SR Biovail lacked any scientific data showing that Andrx's generic product in vivo makes the patented homogenous mixture. Court did not invalidate Biovail's patent as not new Court concluded that no data show that Andrx actually infringes patent.
Tiazac SR Biovail lacked any scientific data showing that Andrx's generic product in vivo makes the patented homogenous mixture. Court did not invalidate Biovail's patent as not new Court concluded that no data show that Andrx actually infringes patent.
 Cefadroxil polymorph cefadroxil monohydrate (bioactive form) - BMS monohydrate, Bouzard form - BMS : Patent Office disputed this actually works is new and demanded proof of efficacy newness BMS provided data showing the Bouzard form is easier to handle / fill capsules Patent Office agreed Bz monohydrate is new - it is not substantially similar to prior art monohydrate
Cefadroxil polymorph cefadroxil monohydrate (bioactive form) - BMS monohydrate, Bouzard form - BMS : Patent Office disputed this actually works is new and demanded proof of efficacy newness BMS provided data showing the Bouzard form is easier to handle / fill capsules Patent Office agreed Bz monohydrate is new - it is not substantially similar to prior art monohydrate
 cefadroxil hemihydrate - Gema, Zenith is hemihydrate "substantially" similar to : the (non-patented) prior art monohydrate? the (patented) Bouzard monohydrate? How much does it cost to resolve this question?
cefadroxil hemihydrate - Gema, Zenith is hemihydrate "substantially" similar to : the (non-patented) prior art monohydrate? the (patented) Bouzard monohydrate? How much does it cost to resolve this question?
 • • • Federal Court litigation : 2003 = $4, 995, 000. 00 Patent Office litigation : 2003 = $375, 000. 00 Patent prosecution : 2003 = $80, 000. 00 – a generic manufacturer obtaining its own patent may be an inexpensive "insurance policy"
• • • Federal Court litigation : 2003 = $4, 995, 000. 00 Patent Office litigation : 2003 = $375, 000. 00 Patent prosecution : 2003 = $80, 000. 00 – a generic manufacturer obtaining its own patent may be an inexpensive "insurance policy"
 Cefadroxil patents (continued) BMS sues. BMS says the hemihydrate transitions in vivo into the Bouzard monohydrate form, before conversion into bioactive monohydrate. Judge concludes, "there is no known way to actually sample the contents of patients' stomachs at the precise moment and conduct x-ray diffraction analysis"
Cefadroxil patents (continued) BMS sues. BMS says the hemihydrate transitions in vivo into the Bouzard monohydrate form, before conversion into bioactive monohydrate. Judge concludes, "there is no known way to actually sample the contents of patients' stomachs at the precise moment and conduct x-ray diffraction analysis"
 Cefadroxil patents (continued) no evidence of infringement * cautionary note on patent drafting * if evidence exists, intimates that Bz monohydrate was made in stomachs of past patients taking prior-art bioactive form of cefadroxil if Bz monohydrate is not new, then the Bz monohydrate patent must be revoked
Cefadroxil patents (continued) no evidence of infringement * cautionary note on patent drafting * if evidence exists, intimates that Bz monohydrate was made in stomachs of past patients taking prior-art bioactive form of cefadroxil if Bz monohydrate is not new, then the Bz monohydrate patent must be revoked
 Next Steps 1 Identify third-party patents –BEFORE investing in R&D –e. g. , landscaping study 2 Identify bars to patentability –BEFORE investing in R&D –e. g. , in-house “paper” research 3 Identify your competitor's strengths. . .
Next Steps 1 Identify third-party patents –BEFORE investing in R&D –e. g. , landscaping study 2 Identify bars to patentability –BEFORE investing in R&D –e. g. , in-house “paper” research 3 Identify your competitor's strengths. . .
 Mark Pohl, Understanding Patent Issues for Formulation Development, Proceedings of Emerging Trends and Technologies in Formulation and Dosage Form Selection for Drug Delivery (18 -20 April 2005) (International Quality & Productivity Center, publ. ) ABSTRACT: New technology – even in such ostensibly mundane areas such as formulation technology - may determine clinical and commercial success. Developing new technology can, however, require significant funding. To protect researchers' access to adequate funding in the future, it may be necessary to commercially protect the innovations made today. We will discuss how to commercially protect new biopharmaceuticals technology using patents. We will review the various requirements to obtain a patent, and focus more closely on the more difficult requirements. We will also discuss several actual examples of pharmaceutical companies' problems and successes in seeking patent protection.
Mark Pohl, Understanding Patent Issues for Formulation Development, Proceedings of Emerging Trends and Technologies in Formulation and Dosage Form Selection for Drug Delivery (18 -20 April 2005) (International Quality & Productivity Center, publ. ) ABSTRACT: New technology – even in such ostensibly mundane areas such as formulation technology - may determine clinical and commercial success. Developing new technology can, however, require significant funding. To protect researchers' access to adequate funding in the future, it may be necessary to commercially protect the innovations made today. We will discuss how to commercially protect new biopharmaceuticals technology using patents. We will review the various requirements to obtain a patent, and focus more closely on the more difficult requirements. We will also discuss several actual examples of pharmaceutical companies' problems and successes in seeking patent protection.
 An email newsletter on pharmaceutical patent law is available by providing your email address: Mark POHL, Esq. , Patent Attorney Mark. Pohl@Licensing. Law. Net (C) 2010 Pharmaceutical Patent Attorneys, LLC 55 Madison Avenue, 4 th floor Morristown, New Jersey 07960 -7397 USA www. Licensing. Law. Net Certain information has been redacted from this presentation to make it suitable for distribution to the public. Clients may obtain an unredacted copy on request.
An email newsletter on pharmaceutical patent law is available by providing your email address: Mark POHL, Esq. , Patent Attorney Mark. Pohl@Licensing. Law. Net (C) 2010 Pharmaceutical Patent Attorneys, LLC 55 Madison Avenue, 4 th floor Morristown, New Jersey 07960 -7397 USA www. Licensing. Law. Net Certain information has been redacted from this presentation to make it suitable for distribution to the public. Clients may obtain an unredacted copy on request.


