1779945354c9d77cb6a0b001e34d41fa.ppt
- Количество слайдов: 9

Comparing the New EIAs with Old Standbys: Florida Bureau of Laboratories Verification Data HIV Diagnostics: New Developments and Challenges Feb. 28, 2005 Berry Bennett, MPH Retrovirology Section Chief FL. Bureau of Laboratories Jacksonville, FL.

Did you know? • New CLIA standard for laboratories who introduce a new test system (MC or HC) on or after April 24, 2003; “Verification of performance specifications. Each laboratory that introduces an unmodified, FDA-cleared or approved test system must do the following before reporting patient test results. ” - 42 CFR 493. 1253(b)(1) revised 10/1/2004 (2 -year phase-in period? ) Interpretive Guidelines “A laboratory may use the manufacturer’s performance specifications as a guideline, but is responsible for verifying the manufacturer’s analytical claims before initiating patient testing. ” Analytical claims include but not limited to sensitivity, specificity, accuracy, precision, detection range or interfering substances, if applicable. “For some qualitative tests, the laboratory may verify the manufacturers’ specifications by testing known positive and negative samples to assure that the expected results are obtained. ” Check with local AHCA for acceptance of in-house samples, performance panels, or manufacturer’s assistance/panels. Laboratories must determine panel size and construct for their population to verify performance.

Florida’s HIV Screening Change, Effective January 2004 Reasons: 1) Indications from the manufacturer that a product substitution most likely would take place. 2) Encouragement from the FDA to manufacturers to include HIV-1 Group O sensitivity as well as group M subtype performance. From: Bio. Rad HIV-1/2 synthetic peptide EIA To: Bio. Rad HIV-1/HIV-2 Plus O EIA Sample size: >1, 000 routine fresh serum samples (~1. 9% seropositivity) (If construction allowed) Expectations & Concerns: 1) Based on Bio. Rad’s seroconversion data, we expected increased sensitivity. 2) As with all new assays we were concerned about compromising specificity as sensitivity increases. 2) Data suggests the new assay could potentially detect more HIV acute infections. 3) We were concerned about an increase number of indeterminate and negative Western Blots.

Florida’s HIV-1/HIV-2 Plus O EIA Initial Verification n = 883* ( 23 r/r, 860 nr ) Bio. Rad HIV-1/2 synthetic peptide EIA: P. Insert claim = 100% sensitivity, 99. 87% specificity FBOL = 99. 0% specificity (9 false pos) Bio. Rad HIV-1/HIV-2 Plus O EIA: P. Insert claim = 100% sensitivity, 99. 89% specificity FBOL = 100% sensitivity, 99. 3% specificity (6 false pos) * evaluation cut short due to laboratory construction.
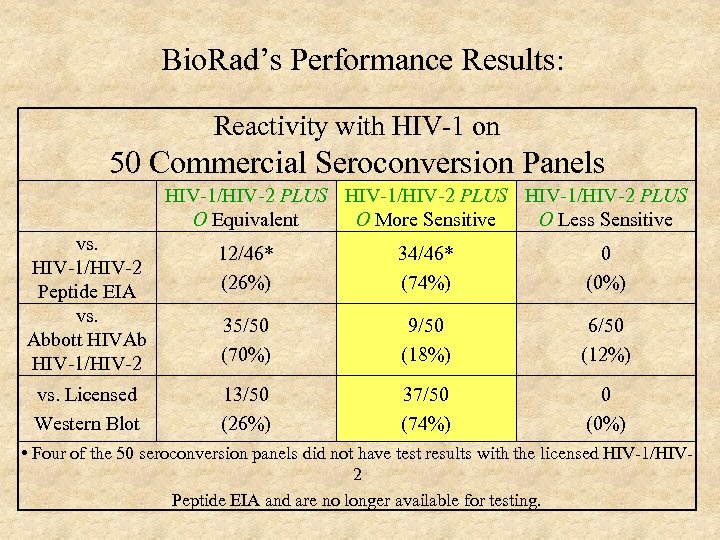
Bio. Rad’s Performance Results: Reactivity with HIV-1 on 50 Commercial Seroconversion Panels HIV-1/HIV-2 PLUS O Equivalent O More Sensitive O Less Sensitive vs. HIV-1/HIV-2 Peptide EIA vs. Abbott HIVAb HIV-1/HIV-2 12/46* (26%) 34/46* (74%) 0 (0%) 35/50 (70%) 9/50 (18%) 6/50 (12%) vs. Licensed Western Blot 13/50 (26%) 37/50 (74%) 0 (0%) • Four of the 50 seroconversion panels did not have test results with the licensed HIV-1/HIV 2 Peptide EIA and are no longer available for testing.
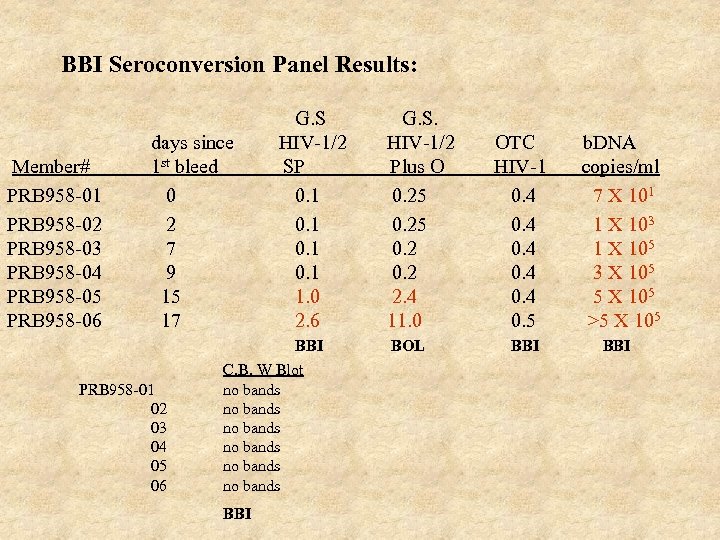
BBI Seroconversion Panel Results: Member# PRB 958 -01 PRB 958 -02 PRB 958 -03 PRB 958 -04 PRB 958 -05 PRB 958 -06 days since 1 st bleed 0 2 7 9 15 17 PRB 958 -01 02 03 04 05 06 G. S HIV-1/2 SP 0. 1 1. 0 2. 6 BBI C. B. W Blot no bands no bands BBI G. S. HIV-1/2 Plus O 0. 25 0. 2 2. 4 11. 0 BOL OTC HIV-1 0. 4 0. 5 b. DNA copies/ml 7 X 101 1 X 103 1 X 105 3 X 105 5 X 105 >5 X 105 BBI
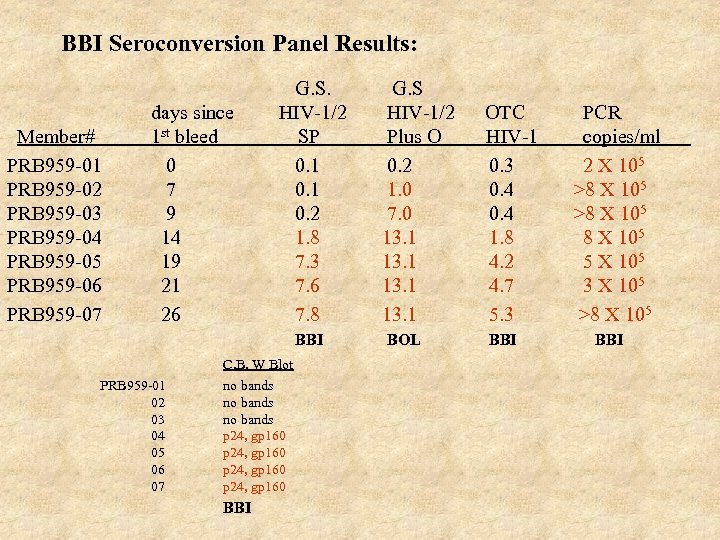
BBI Seroconversion Panel Results: Member# PRB 959 -01 PRB 959 -02 PRB 959 -03 PRB 959 -04 PRB 959 -05 PRB 959 -06 PRB 959 -07 days since 1 st bleed 0 7 9 14 19 21 26 G. S. HIV-1/2 SP 0. 1 0. 2 1. 8 7. 3 7. 6 7. 8 BBI C. B. W Blot PRB 959 -01 02 03 04 05 06 07 no bands p 24, gp 160 BBI G. S HIV-1/2 Plus O 0. 2 1. 0 7. 0 13. 1 BOL OTC HIV-1 0. 3 0. 4 1. 8 4. 2 4. 7 5. 3 BBI PCR copies/ml 2 X 105 >8 X 105 5 X 105 3 X 105 >8 X 105 BBI
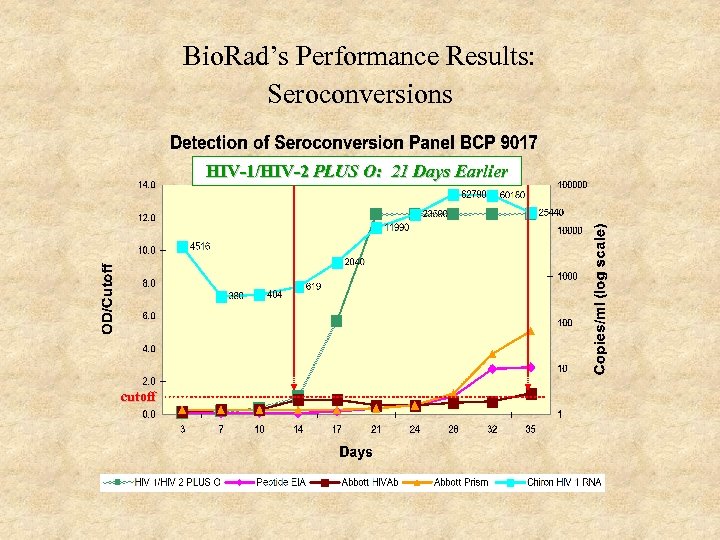
Bio. Rad’s Performance Results: Seroconversions HIV-1/HIV-2 PLUS O: 21 Days Earlier cutoff

Florida’s Lessons Learned: • Most likely one will see increased number of Western Blot indeterminates and/or negatives; a) Poor client redraw/follow up makes it difficult to confirm acute infection sensitivity. b) Original sample submission and/or sample handling may not be satisfactory for NAAT. c) A need for alternative more sensitive confirmation assays. d) A need to monitor automated EIA instrument performance, especially washer calibration. e) Examine unconfirmed repeatedly reactive EIAs for trends such as pregnancy, rheumatoid factor, etc. if such information is provided. • Recommend monitoring technical staff’s competency assessment in sample processing to avoid cross contamination in very sensitive assays. Bio. Rad’s 2004 Technical Bulletin addressed sample cross contamination. “ Serological markers such as HBs. Ag and anti-HIV may be present in serum at very high concentrations, with titers exceeding 1 in 106 in some specimens. Data demonstrates that 3 rd generation HIV tests are often more sensitive than Western Blot and/or 2 nd generation HIV EIAs. ”
1779945354c9d77cb6a0b001e34d41fa.ppt