9d3e9908c0377f7035efb6608376e0db.ppt
- Количество слайдов: 62

Combination Therapy for Type 2 Diabetes Springfield, IL, Nov 15, 2003 Paul Davidson, MD, FACE Atlanta Diabetes Associates Atlanta, Georgia

ACE / AACE Targets for Glycemic Control Hb. A 1 c < 6. 5 % Fasting/preprandial glucose< 110 mg/d. L Postprandial glucose < 140 mg/d. L ACE / AACE Consensus Conference, Washington DC August 2001

Goals of Intensive Diabetes Management A Normal Hb. A 1 c Is Not Everything. It Is the Only Thing!
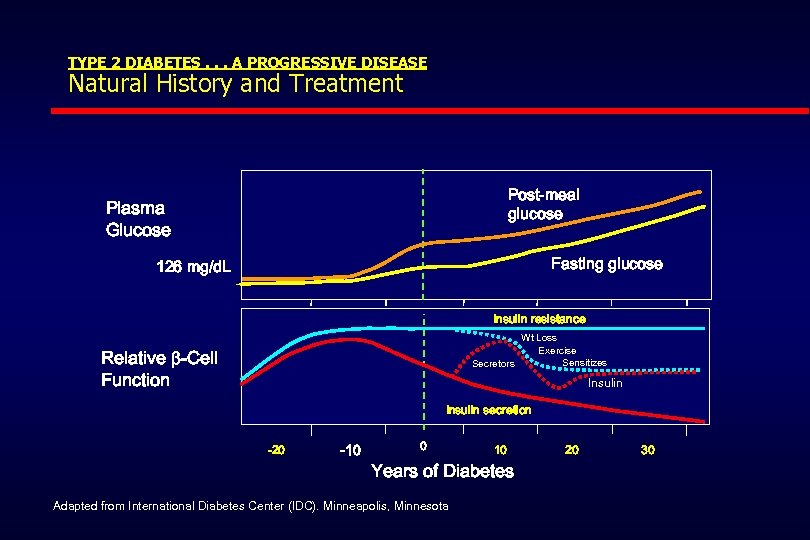
TYPE 2 DIABETES. . . A PROGRESSIVE DISEASE Natural History and Treatment Post-meal glucose Plasma Glucose Fasting glucose 126 mg/d. L Insulin resistance Wt Loss Exercise Sensitizes Secretors Relative -Cell Function Insulin secretion -20 -10 0 10 Years of Diabetes Adapted from International Diabetes Center (IDC). Minneapolis, Minnesota 20 30
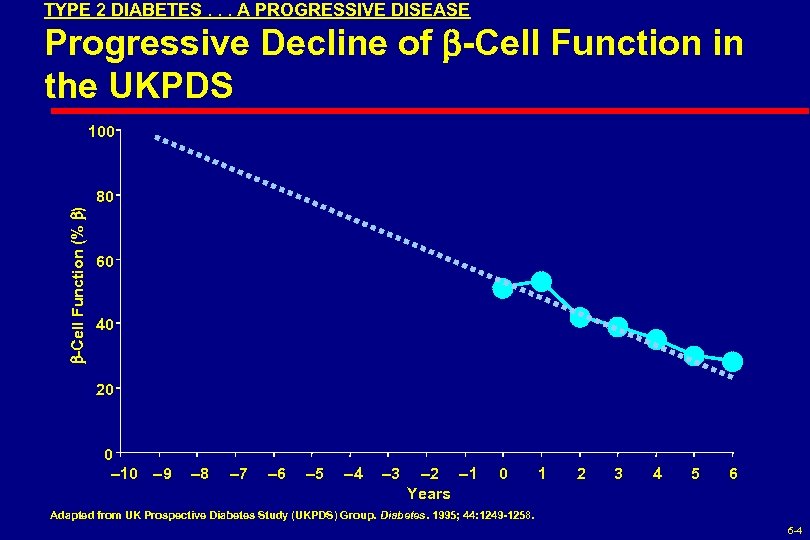
TYPE 2 DIABETES. . . A PROGRESSIVE DISEASE Progressive Decline of -Cell Function in the UKPDS 100 -Cell Function (% ) 80 60 40 20 0 10 9 8 7 6 5 4 3 2 1 Years 0 1 2 3 4 5 6 Adapted from UK Prospective Diabetes Study (UKPDS) Group. Diabetes. 1995; 44: 1249 -1258. 6 -4
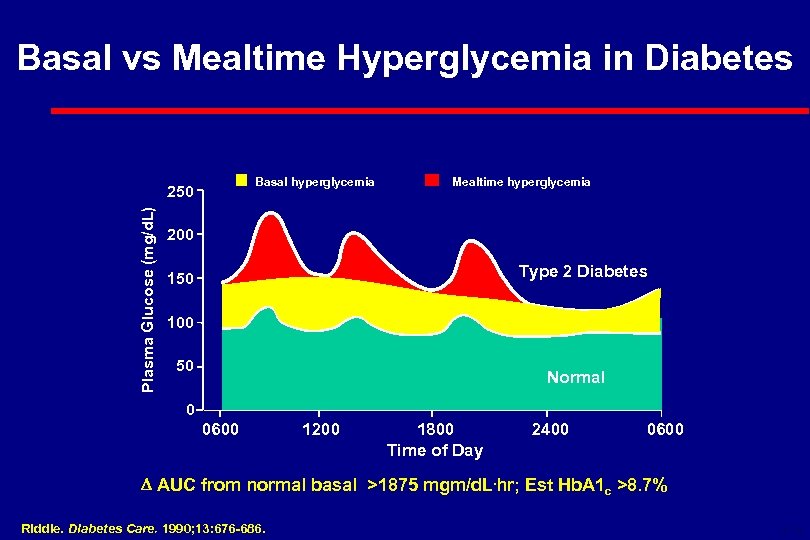
Basal vs Mealtime Hyperglycemia in Diabetes Basal hyperglycemia Plasma Glucose (mg/d. L) 250 Mealtime hyperglycemia 200 Type 2 Diabetes 150 100 50 Normal 0 0600 1200 1800 Time of Day 2400 0600 AUC from normal basal >1875 mgm/d. L. hr; Est Hb. A 1 c >8. 7% Riddle. Diabetes Care. 1990; 13: 676 -686. 6 -18
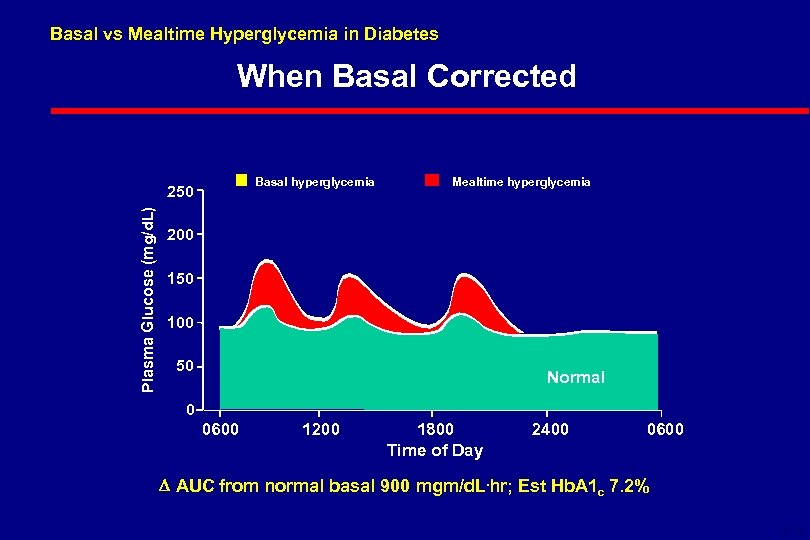
Basal vs Mealtime Hyperglycemia in Diabetes When Basal Corrected Basal hyperglycemia Plasma Glucose (mg/d. L) 250 Mealtime hyperglycemia 200 150 100 50 Normal 0 0600 1200 1800 Time of Day 2400 0600 AUC from normal basal 900 mgm/d. L. hr; Est Hb. A 1 c 7. 2% 6 -18
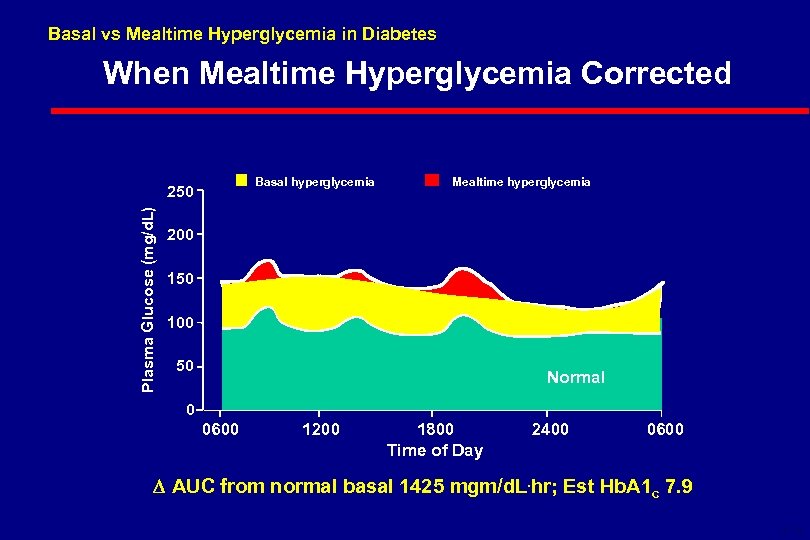
Basal vs Mealtime Hyperglycemia in Diabetes When Mealtime Hyperglycemia Corrected Basal hyperglycemia Plasma Glucose (mg/d. L) 250 Mealtime hyperglycemia 200 150 100 50 Normal 0 0600 1200 1800 Time of Day 2400 0600 AUC from normal basal 1425 mgm/d. L. hr; Est Hb. A 1 c 7. 9 6 -18
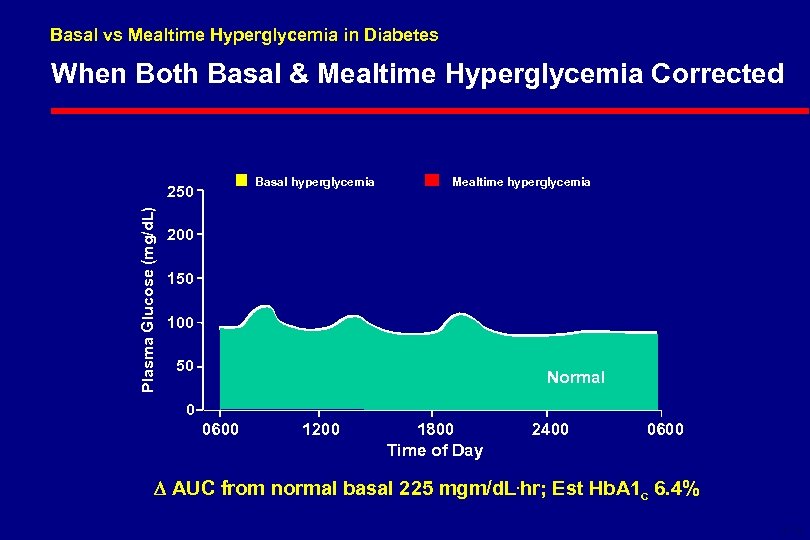
Basal vs Mealtime Hyperglycemia in Diabetes When Both Basal & Mealtime Hyperglycemia Corrected Basal hyperglycemia Plasma Glucose (mg/d. L) 250 Mealtime hyperglycemia 200 150 100 50 Normal 0 0600 1200 1800 Time of Day 2400 0600 AUC from normal basal 225 mgm/d. L. hr; Est Hb. A 1 c 6. 4% 6 -18

Step Therapy l Diet l Exercise l Sulfonylurea or Metformin l Add Alternate Agent l Add hs NPH l Switch to Mixed Insulin bid l Switch to Multiple Dose Insulin Utilitarian, Common Sense, Recommended Prone to Failure from Misscheduling and Mismanagement

Stumble Therapy l YAG Diet l Golf Cart Exercise l Sample of the Week Medication – Interupted, – Not Combined l Poor Understanding of Goals l Poor Monitoring Hb. A 1 c >8% (If Seen) Informed Patient Refers Self Elsewhere

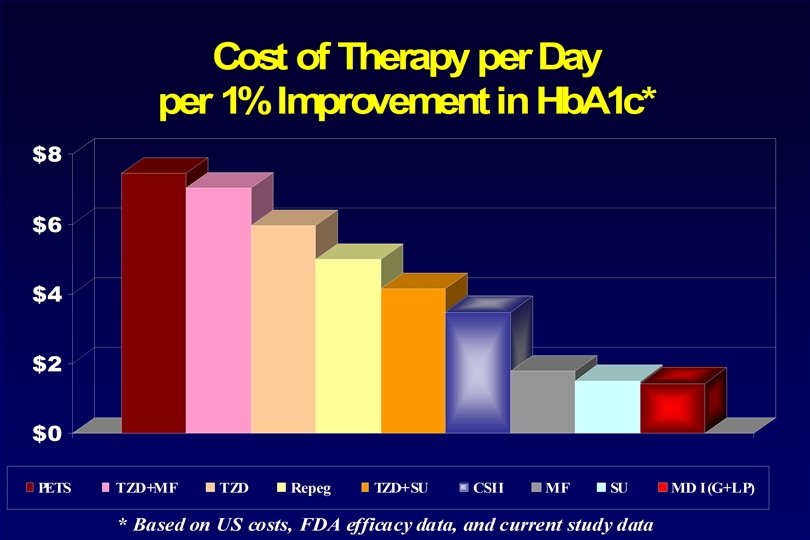
PETS Therapy Step--Spelled Backwards All at once, nothing first, Just like bubbles, when they burst. l Start with Fast to Glucose <126 mg/d. L – IV Insulin l Feed PSMF Diet l Add SU, MF, TZD, Repaglanide + prn Lispro for BG <150 l “Normal” BG from Day 1 l Monitor BG qid l See Patient Monthly, HFP l Hb. A 1 c Bimonthly GI Problems: Cut MF Hypoglycemia: Cut SU Hypoglycemia Again: Cut Repaglinide Allow 2 Month to See TZD Effect
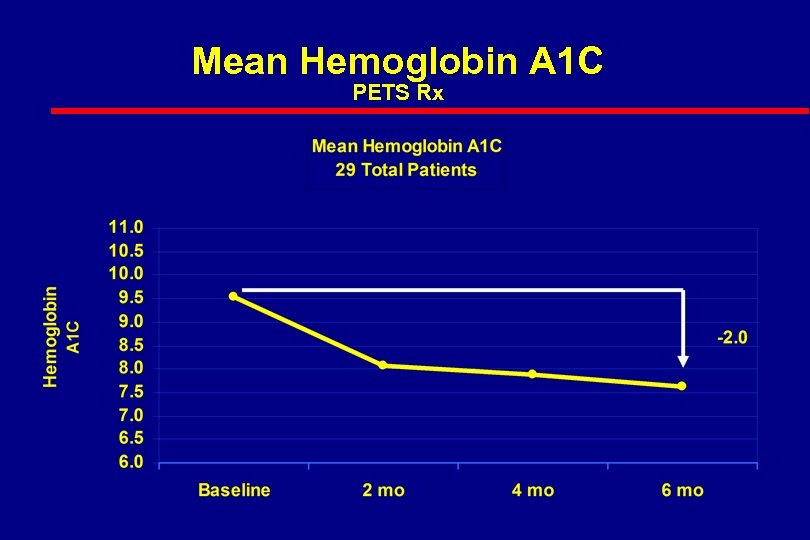
Mean Hemoglobin A 1 C PETS Rx

Insulin only The most powerful agent we have to control glucose
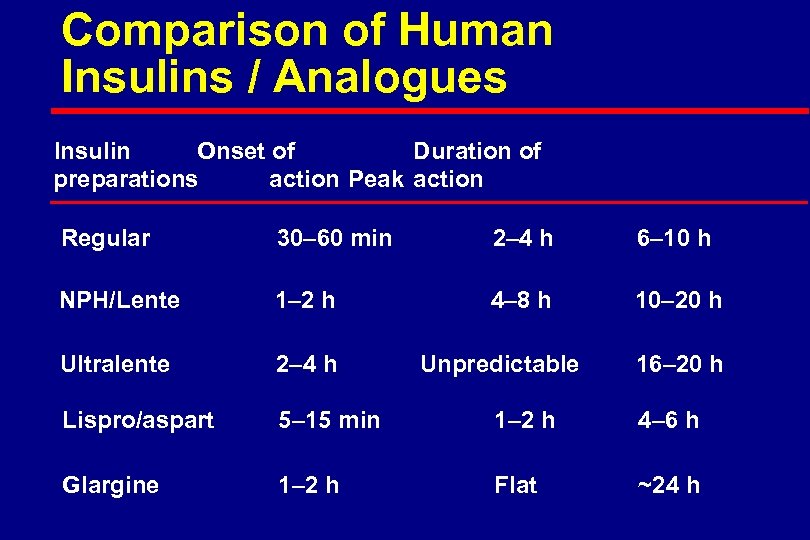
Comparison of Human Insulins / Analogues Insulin Onset of Duration of preparations action Peak action Regular 30– 60 min 2– 4 h 6– 10 h NPH/Lente 1– 2 h 4– 8 h 10– 20 h Ultralente 2– 4 h Lispro/aspart 5– 15 min 1– 2 h 4– 6 h Glargine 1– 2 h Flat ~24 h Unpredictable 16– 20 h
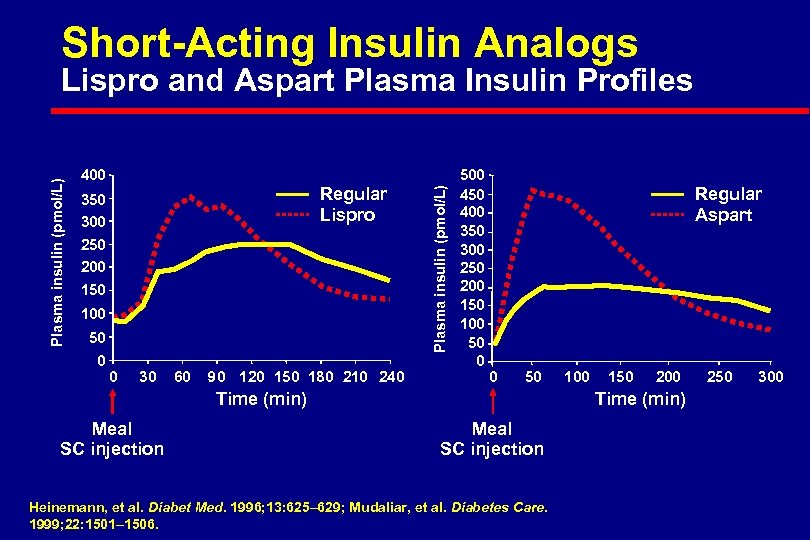
Short-Acting Insulin Analogs 400 Regular Lispro 350 300 250 200 150 100 50 0 0 30 60 90 120 150 180 210 240 Plasma insulin (pmol/L) Lispro and Aspart Plasma Insulin Profiles 500 450 400 350 300 250 200 150 100 50 0 Regular Aspart 0 50 Time (min) Meal SC injection 100 150 200 Time (min) Meal SC injection Heinemann, et al. Diabet Med. 1996; 13: 625– 629; Mudaliar, et al. Diabetes Care. 1999; 22: 1501– 1506. 250 300

Short-Acting Analogs Lispro and Aspart l Convenient administration immediately prior to meals l Faster onset of action l Limit postprandial hyperglycemic peaks l Shorter duration of activity – Reduce late postprandial hypoglycemia – Frequent late postprandial hyperglycemia l Need for basal insulin replacement revealed
Limitations of NPH, Lente, and Ultralente l Do not mimic basal insulin profile – Variable absorption – Pronounced peaks – Less than 24 -hour duration of action l Cause unpredictable hypoglycemia – Major factor limiting insulin adjustments – More weight gain
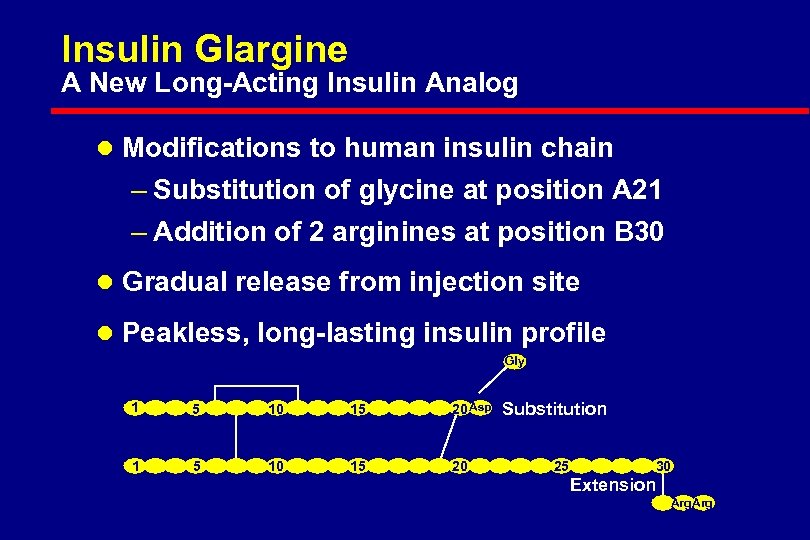
Insulin Glargine A New Long-Acting Insulin Analog l Modifications to human insulin chain – Substitution of glycine at position A 21 – Addition of 2 arginines at position B 30 l Gradual release from injection site l Peakless, long-lasting insulin profile Gly 1 5 10 15 20 Asp 1 5 10 15 20 Substitution 25 30 Extension Arg
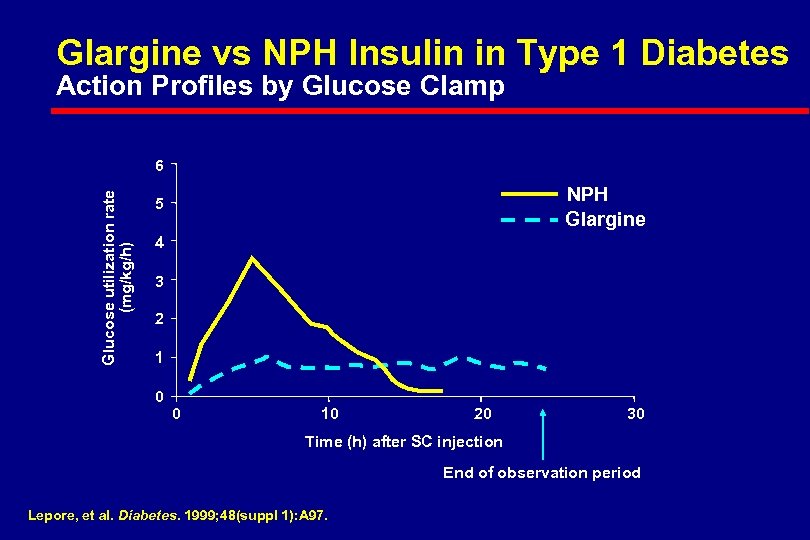
Glargine vs NPH Insulin in Type 1 Diabetes Action Profiles by Glucose Clamp Glucose utilization rate (mg/kg/h) 6 NPH Glargine 5 4 3 2 1 0 0 10 20 30 Time (h) after SC injection End of observation period Lepore, et al. Diabetes. 1999; 48(suppl 1): A 97.
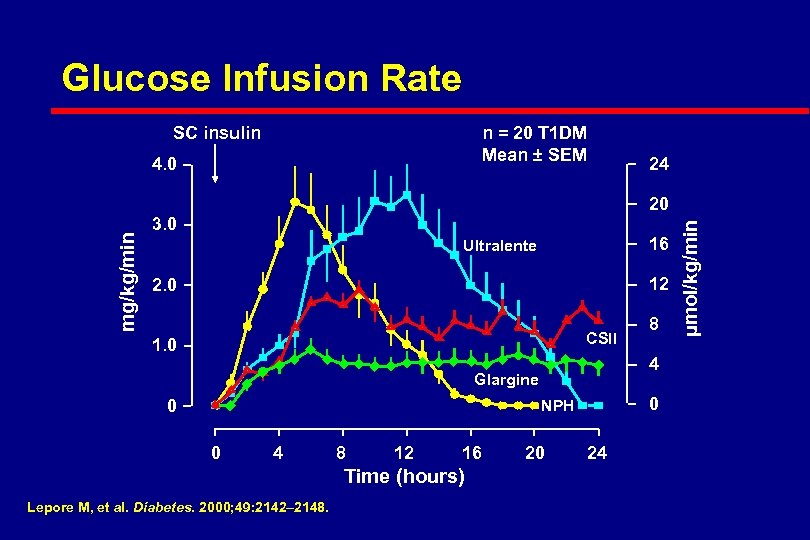
Glucose Infusion Rate SC insulin n = 20 T 1 DM Mean ± SEM 4. 0 24 3. 0 16 Ultralente 12 2. 0 CSII 1. 0 4 Glargine 0 0 NPH 0 4 8 12 16 Time (hours) Lepore M, et al. Diabetes. 2000; 49: 2142– 2148. 8 20 24 µmol/kg/min mg/kg/min 20
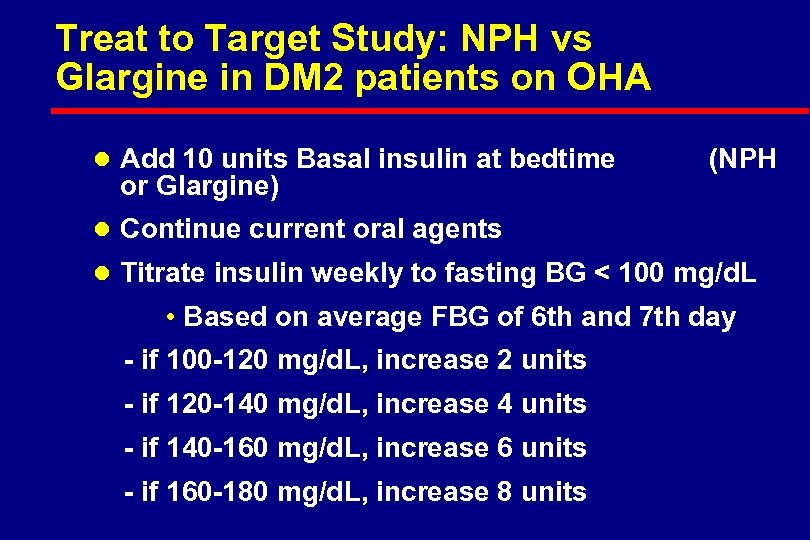
Treat to Target Study: NPH vs Glargine in DM 2 patients on OHA l Add 10 units Basal insulin at bedtime or Glargine) (NPH l Continue current oral agents l Titrate insulin weekly to fasting BG < 100 mg/d. L • Based on average FBG of 6 th and 7 th day - if 100 -120 mg/d. L, increase 2 units - if 120 -140 mg/d. L, increase 4 units - if 140 -160 mg/d. L, increase 6 units - if 160 -180 mg/d. L, increase 8 units
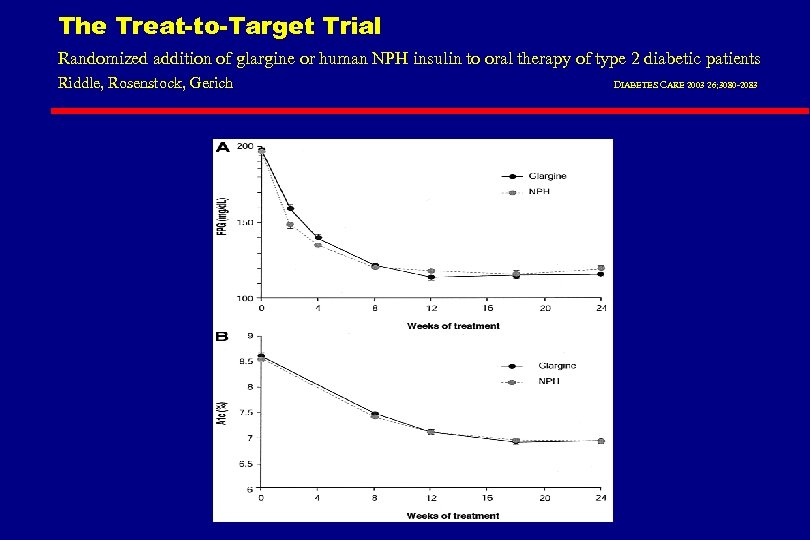
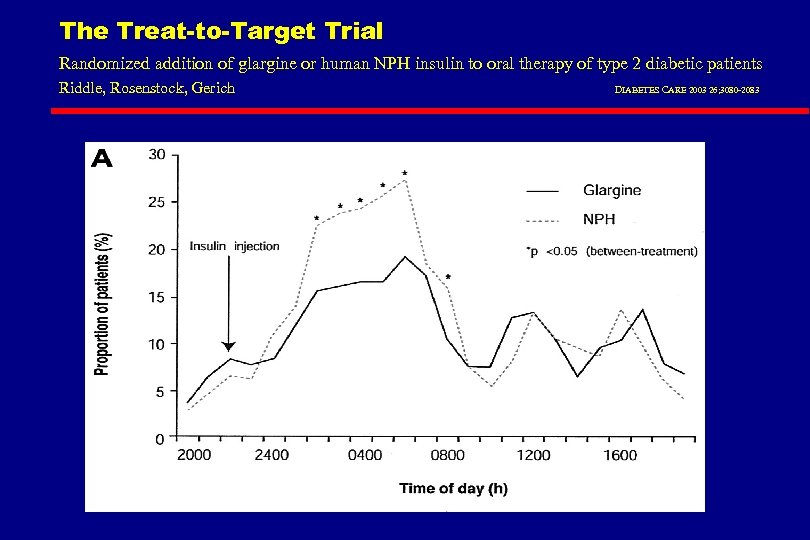
The Treat-to-Target Trial Randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients Riddle, Rosenstock, Gerich DIABETES CARE 2003 26; 3080 -2083
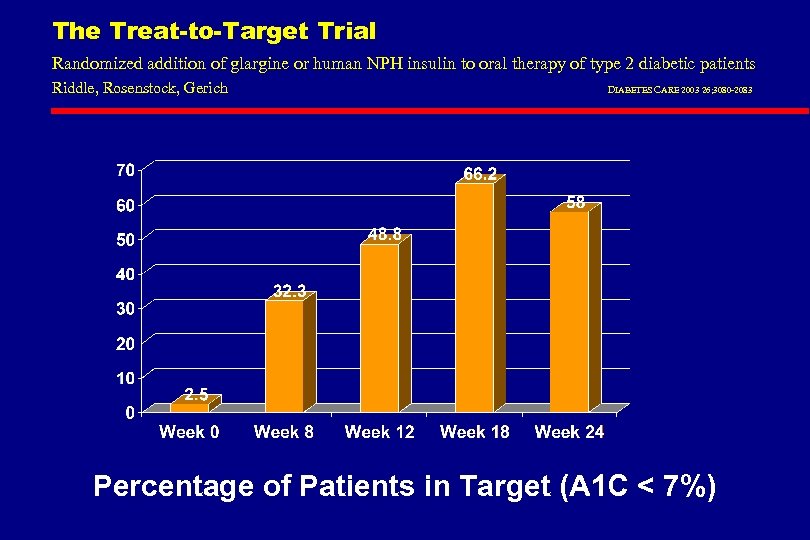
The Treat-to-Target Trial Randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients Riddle, Rosenstock, Gerich DIABETES CARE 2003 26; 3080 -2083 Percentage of Patients in Target (A 1 C < 7%)
The Treat-to-Target Trial Randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients Riddle, Rosenstock, Gerich DIABETES CARE 2003 26; 3080 -2083
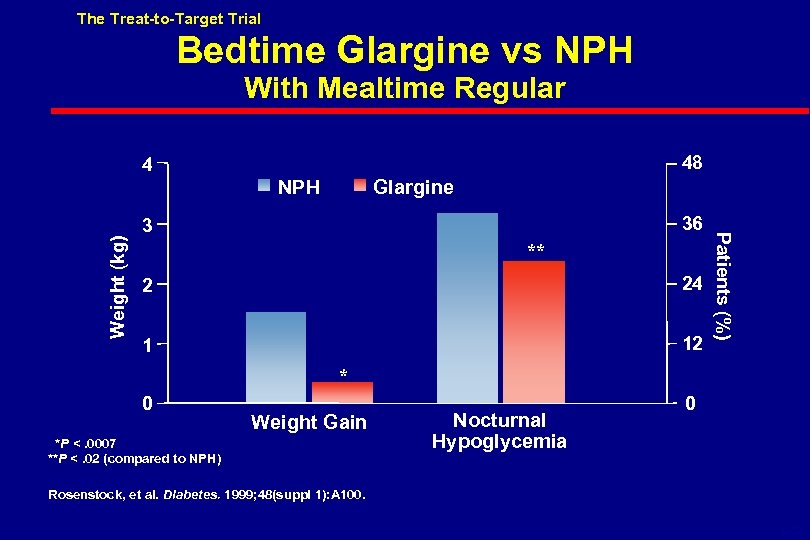
The Treat-to-Target Trial . Bedtime Glargine vs NPH With Mealtime Regular 48 4 Glargine 36 3 ** 2 24 1 12 Patients (%) Weight (kg) NPH * 0 Weight Gain *P <. 0007 **P <. 02 (compared to NPH) Nocturnal Hypoglycemia 0 Rosenstock, et al. Diabetes. 1999; 48(suppl 1): A 100. 6 -52
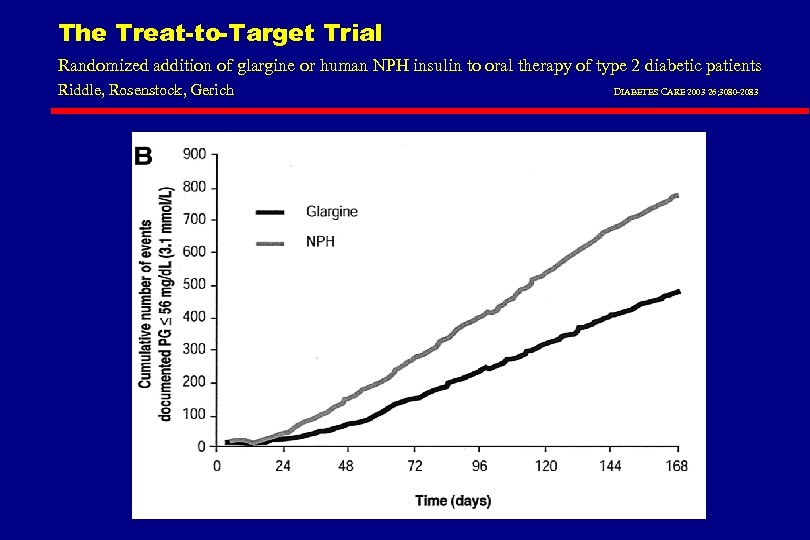
The Treat-to-Target Trial Randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients Riddle, Rosenstock, Gerich DIABETES CARE 2003 26; 3080 -2083
Treatment to Target Study: NPH vs Glargine in DM 2 patients on OHA l 57% had Hb. A 1 c <7% l Nocturnal Hypoglycemia reduced by 42% in the Glargine group l 33% had Hb. A 1 c <7% without any nighttime hypoglycemia in glargine group l Results significantly better than with NPH

Establishing Basal Requirement for Glargine Body Weight in pounds x 0. 1 Give twice in first day? Average am BG x 2 after five days Add to Glargine (BG-100)/10 Repeat weekly Example: 200# 20 units glargine stat and q hs AM BG averages 200 on 6 th and 7 th day Add (BG-100)10 to glargine, i. e. increase from 20 to 30 units q hs 2 nd week--average 130 increase glargine from 30 to 33

Overall Summary: Glargine l Insulin glargine has the following clinical benefits – Once-daily dosing because of its prolonged duration of action and smooth, peakless timeaction profile – Comparable or better glycemic control (FBG) – Lower risk of nocturnal hypoglycemic events – Safety profile similar to that of human insulin

Goals of Intensive Diabetes Management l Near-normal glycemia – Hb. A 1 c less than 6. 5% l Avoid short-term crisis – Hypoglycemia – Hyperglycemia – DKA l Minimize long-term complications l Improve QOL

Type 2 Diabetes … A Progressive Disease Over time, all patients will need insulin to control glucose

Insulin Therapy in Type 2 Diabetes Indications l Significant hyperglycemia at presentation l Hyperglycemia on maximal doses of oral agents l Decompensation – – Acute injury, stress, infection, myocardial ischemia Severe hyperglycemia with ketonemia and/or ketonuria Uncontrolled weight loss Use of diabetogenic medications (e. g. corticosteroids) l Surgery l Pregnancy l Renal or hepatic disease

MIMICKING NATURE WITH INSULIN THERAPY All persons need both basal and mealtime insulin (endogenous or exogenous) to control glucose 6 -19
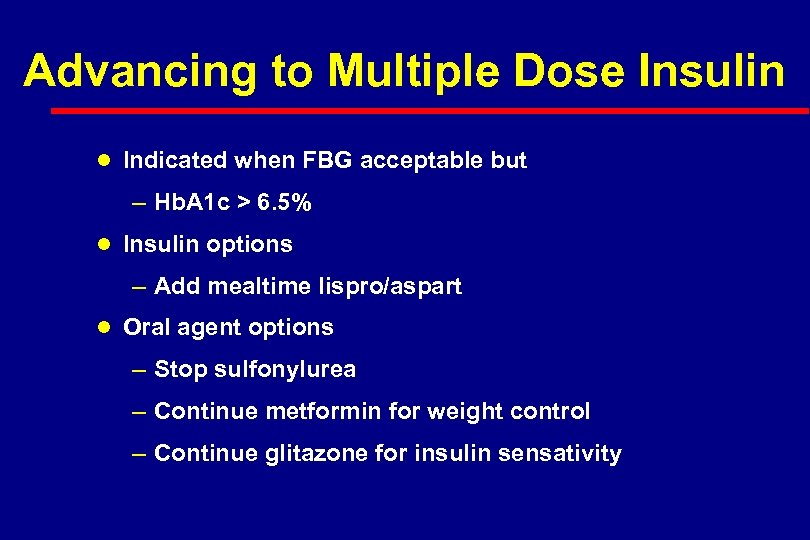
Advancing to Multiple Dose Insulin l Indicated when FBG acceptable but – Hb. A 1 c > 6. 5% l Insulin options – Add mealtime lispro/aspart l Oral agent options – Stop sulfonylurea – Continue metformin for weight control – Continue glitazone for insulin sensativity
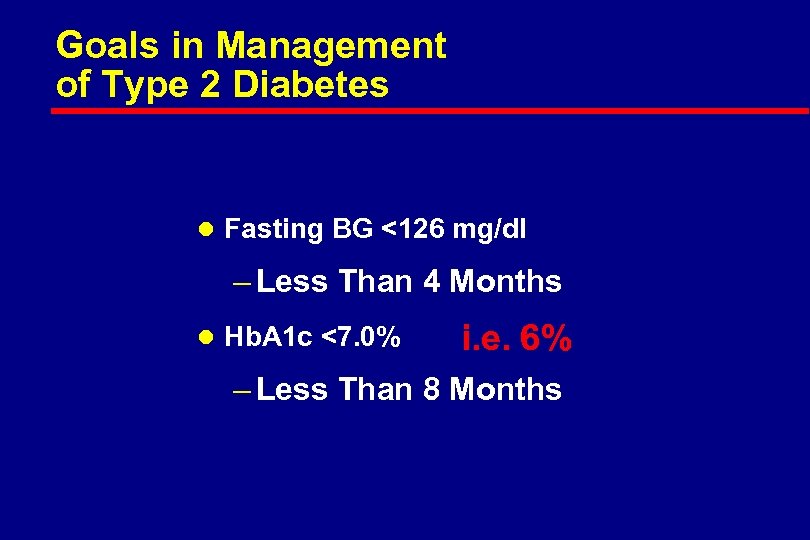
Goals in Management of Type 2 Diabetes l Fasting BG <126 mg/dl – Less Than 4 Months l Hb. A 1 c <7. 0% i. e. 6% – Less Than 8 Months
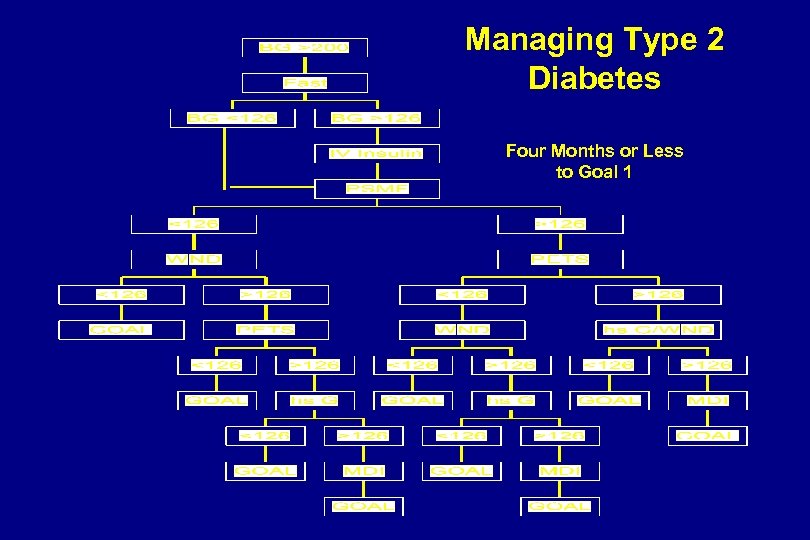
Managing Type 2 Diabetes Four Months or Less to Goal 1
Managing Type 2 Diabetes Goal 2 (Hb. A 1 c <7. 0%)

GEMS--Glargine Evening Mealtime Secretagogue l Basal Dosing – (Weight in #`s x 0. 1) • Glargine hs l Prior to Meals – Short Acting Secretagogue • Rapaglinide 2 mg • Nateglinide 120 mg – Glimepiride 2 mg

Routine Hospital Care for Type 2 Diabetes The Case for GEMS l Usually metformin contra-indicated l Glargine insulin required for normal am glucose – Stress or steroids l Interrupted and/or unreliable food intake l Nursing routine problems – Lispro insulin at time of tray – Reluctance to give lispro with normoglycemia l Supplemental lispro with elevated glucose l Short-acting secretagogue in half hour before tray – Little risk of hypoglycemia if limited intake

Infections in Diabetes l One BG >220 mg/dl results in 5. 8 times increase in nosocomial infection rate l Two hours hyperglycemia results in impaired WBC function for weeks Pomposelli, New England Deaconess, J Parenteral and Enteral Nutrition 22: 77 -81, 1998

Intravenous Insulin with Severe Illness Three major recent studies l. DIGAMI: Prospective Randomised Study of Intensive Insulin Treatment on Long Term Survival After Acute Myocardial Infarction in Patients with Diabetes Mellitus Malmberg, et al. BMJ. 1997; 314: 1512 -1515. l. Portland: Continuous Insulin Infusion Reduces Mortality in Patients with Diabetes Undergoing Coronary Artery Bypass Grafting Furnary et al J Thorac Cardiovasc Surg 2003; 123: 1007 -21 l. Leuven: Intensive Insulin Therapy in Critically Ill Patients Van den Berghe et al N Engl J Med 2001; 345: 1359 -67

DIGAMI Study Diabetes, Insulin Glucose Infusion in Acute Myocardial Infarction(1997) l Acute MI With BG >200 mg/dl l Intensive Insulin Treatment l IV Insulin For >24 Hours l Four Insulin Injections/Day For >3 Months l Reduced Risk of Mortality By 28% Over 3. 4 Years 51% in Those Not Previous Diagnosed Malmberg BMJ 1997; 314: 1512
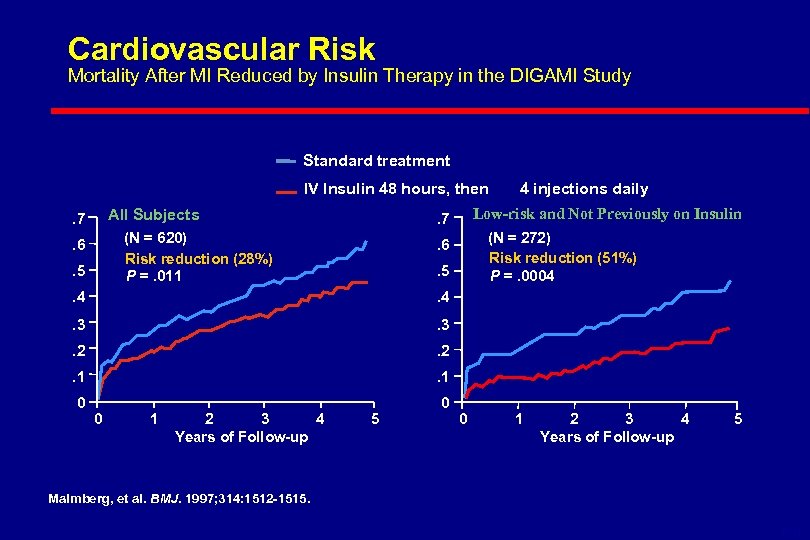
Cardiovascular Risk Mortality After MI Reduced by Insulin Therapy in the DIGAMI Study Standard treatment IV Insulin 48 hours, then All Subjects . 7. 5 Low-risk and Not Previously on Insulin . 7 (N = 620) Risk reduction (28%) P =. 011 . 6 (N = 272) Risk reduction (51%) P =. 0004 . 6. 5 . 4 . 3 . 2 . 1 0 0 4 injections daily 0 1 2 3 4 Years of Follow-up 5 Malmberg, et al. BMJ. 1997; 314: 1512 -1515. 6 -11
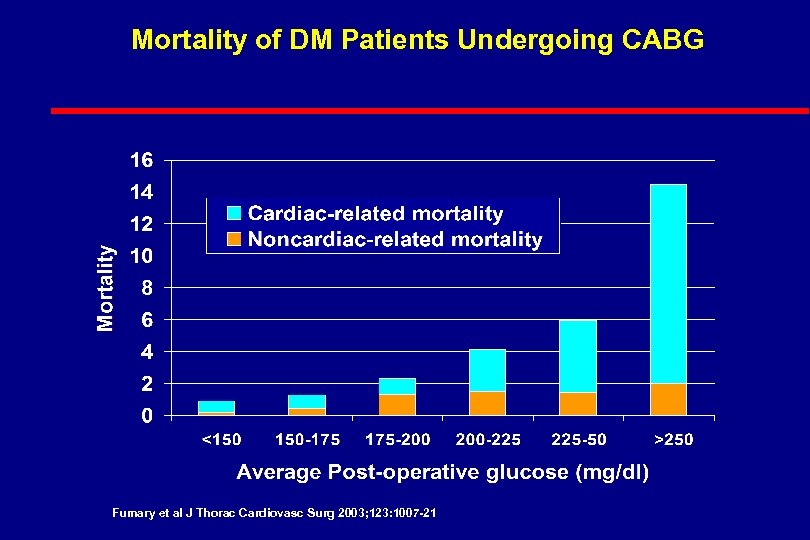
Mortality of DM Patients Undergoing CABG Fumary et al J Thorac Cardiovasc Surg 2003; 123: 1007 -21
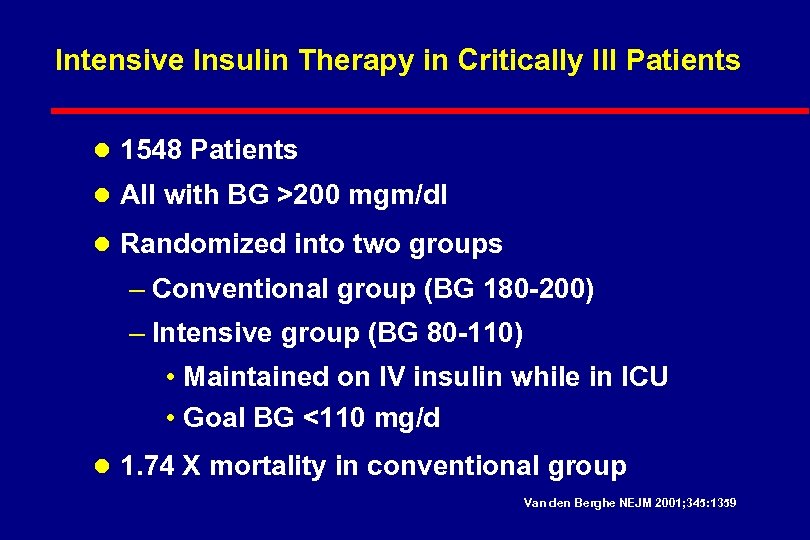
Intensive Insulin Therapy in Critically Ill Patients l 1548 Patients l All with BG >200 mgm/dl l Randomized into two groups – Conventional group (BG 180 -200) – Intensive group (BG 80 -110) • Maintained on IV insulin while in ICU • Goal BG <110 mg/d l 1. 74 X mortality in conventional group Van den Berghe NEJM 2001; 345: 1359
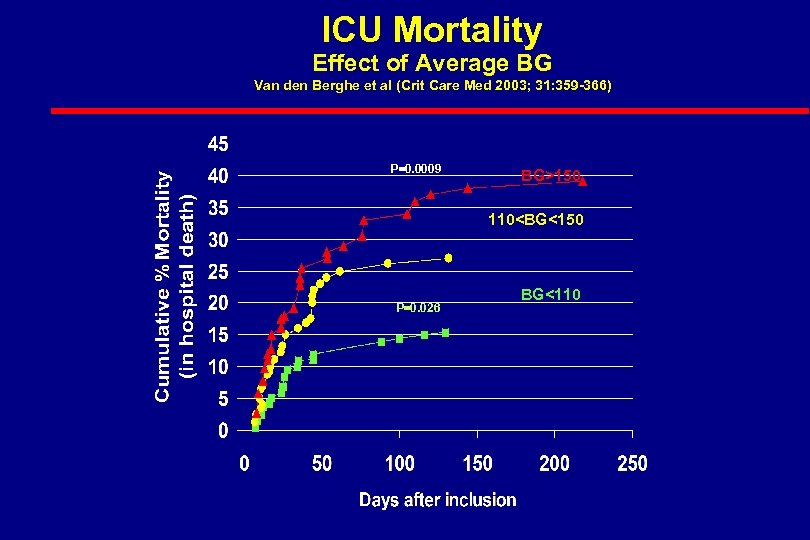
ICU Mortality Effect of Average BG Van den Berghe et al (Crit Care Med 2003; 31: 359 -366) P=0. 0009 BG>150 110<BG<150 P=0. 026 BG<110
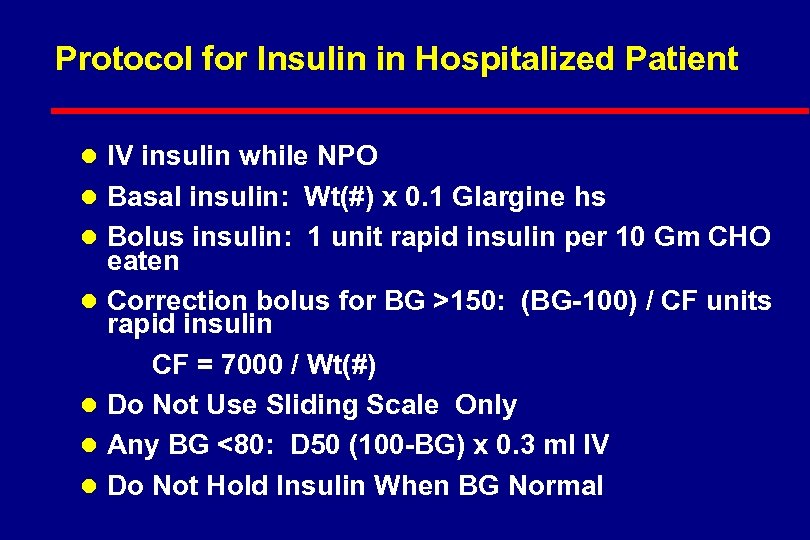
Protocol for Insulin in Hospitalized Patient l IV insulin while NPO l Basal insulin: Wt(#) x 0. 1 Glargine hs l Bolus insulin: 1 unit rapid insulin per 10 Gm CHO l l eaten Correction bolus for BG >150: (BG-100) / CF units rapid insulin CF = 7000 / Wt(#) Do Not Use Sliding Scale Only Any BG <80: D 50 (100 -BG) x 0. 3 ml IV Do Not Hold Insulin When BG Normal

If Hb. A 1 c is Not to Goal i. e. 6. 5% l SMBG – frequency – recording – memory meter l Diet – accurate CHO counting – appropriate CHO/insulin bolusing l Infusion site areas l Overtreatment of low BG l Delayed or undertreatment of high BG
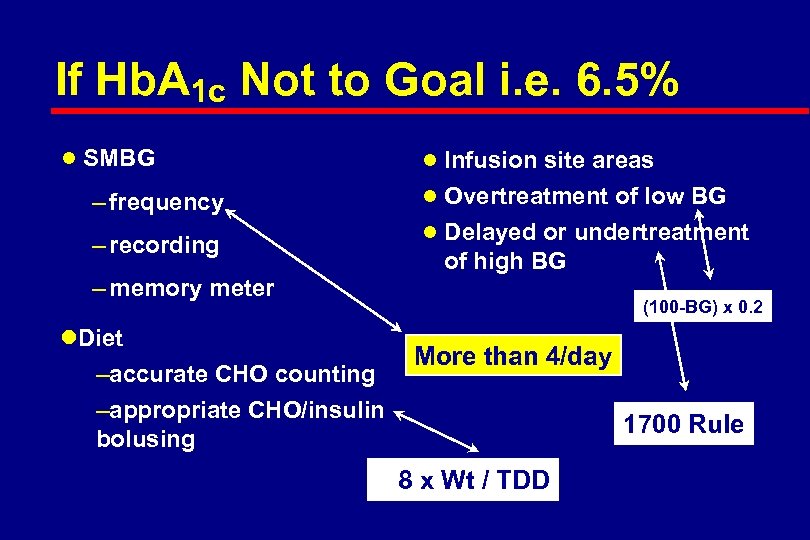
If Hb. A 1 c Not to Goal i. e. 6. 5% l SMBG l Infusion site areas l Overtreatment of low BG – frequency l Delayed or undertreatment – recording of high BG – memory meter (100 -BG) x 0. 2 l. Diet –accurate CHO counting –appropriate CHO/insulin bolusing More than 4/day 2. 8 x Wt / TDD 1700 Rule
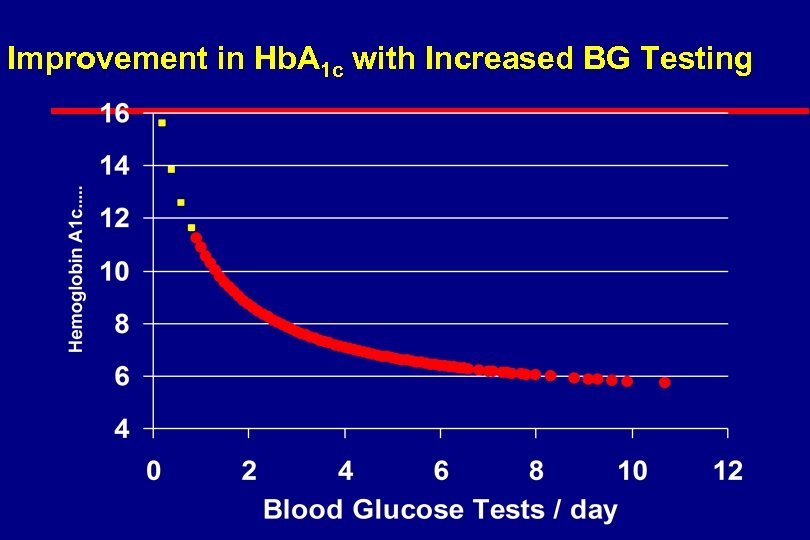
Improvement in Hb. A 1 c with Increased BG Testing
If Hb. A 1 c Not to Goal i. e. 6. 5% l SMBG l Infusion site areas l Overtreatment of low BG – frequency l Delayed or undertreatment – recording of high BG – memory meter (100 -BG) x 0. 2 l. Diet –accurate CHO counting –appropriate CHO/insulin bolusing More than 4/day 2. 8 x Wt / TDD 1700 Rule
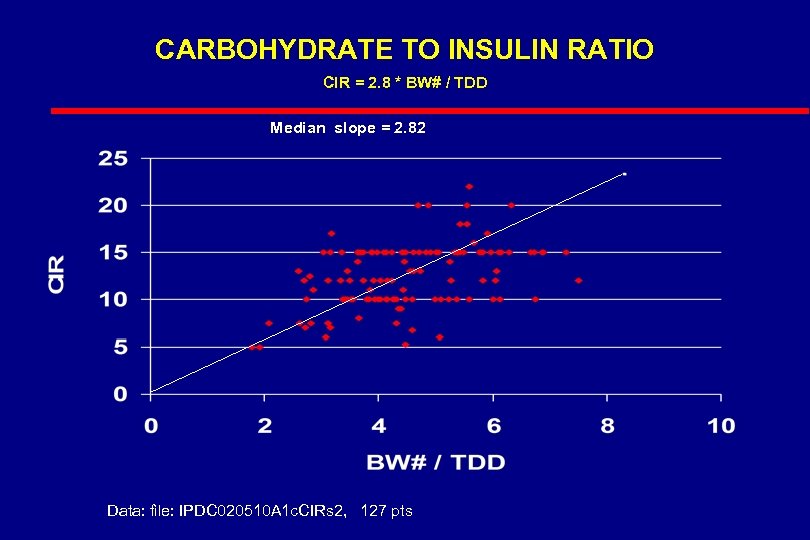
CARBOHYDRATE TO INSULIN RATIO CIR = 2. 8 * BW# / TDD Median slope = 2. 82 Data: file: IPDC 020510 A 1 c. CIRs 2, 127 pts
If Hb. A 1 c Not to Goal i. e. 6. 5% l SMBG l Infusion site areas l Overtreatment of low BG – frequency l Delayed or undertreatment – recording of high BG – memory meter (100 -BG) x 0. 2 l. Diet –accurate CHO counting –appropriate CHO/insulin bolusing More than 4/day 2. 8 x Wt / TDD 1700 Rule
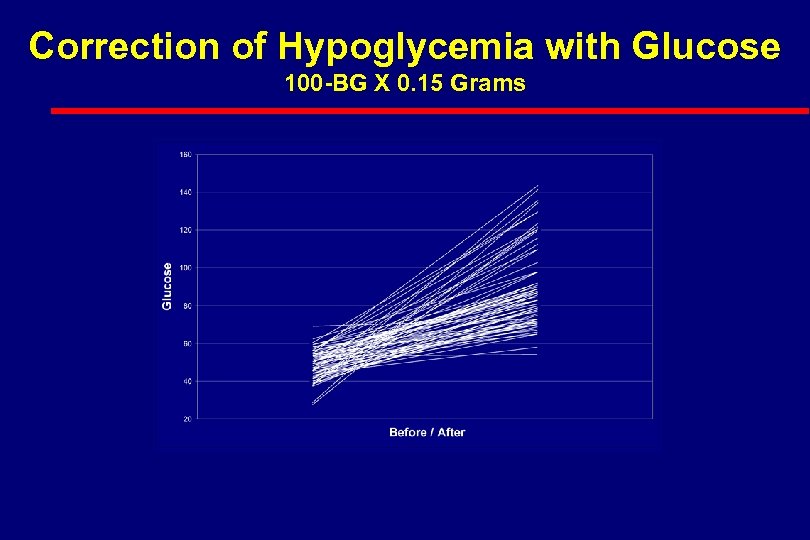
Correction of Hypoglycemia with Glucose 100 -BG X 0. 15 Grams
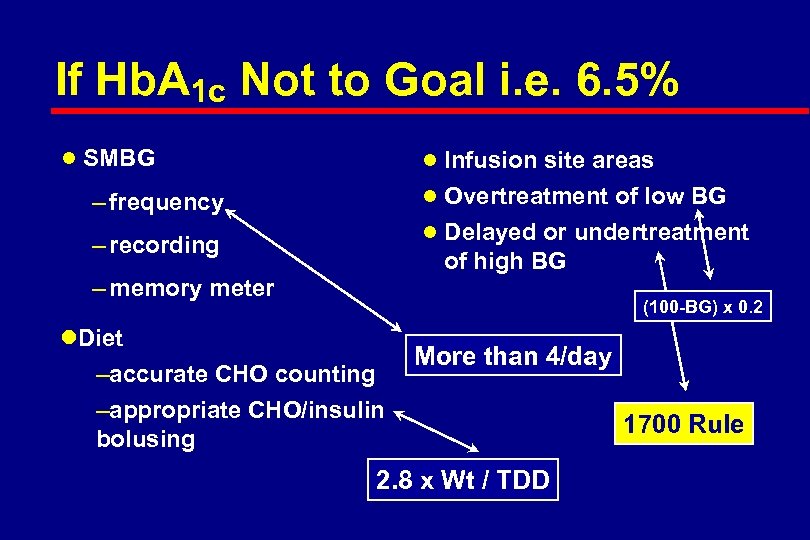
If Hb. A 1 c Not to Goal i. e. 6. 5% l SMBG l Infusion site areas l Overtreatment of low BG – frequency l Delayed or undertreatment – recording of high BG – memory meter (100 -BG) x 0. 2 l. Diet –accurate CHO counting –appropriate CHO/insulin bolusing More than 4/day 2. 8 x Wt / TDD 1700 Rule
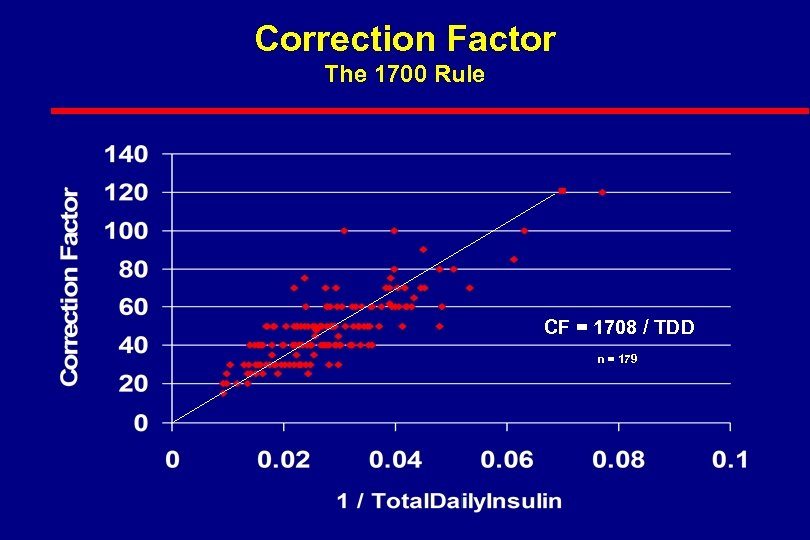
Correction Factor The 1700 Rule CF = 1708 / TDD n = 179
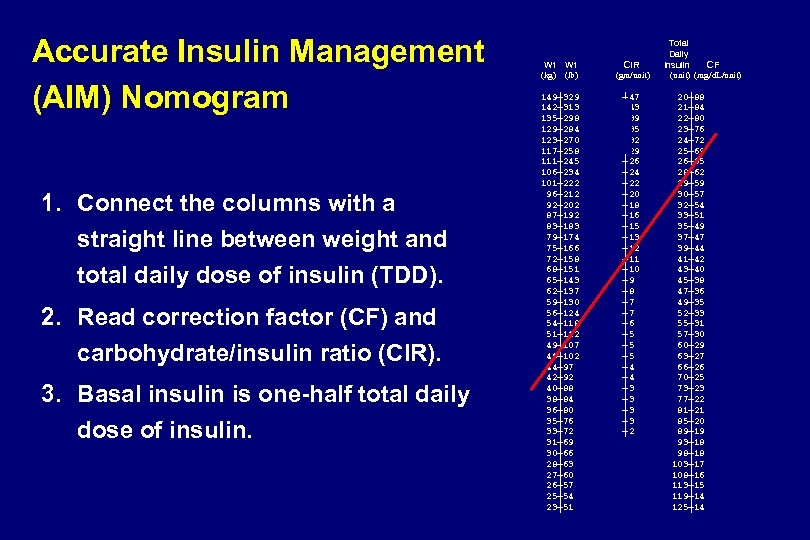
Total Daily Wt CIR Insulin CF (kg) (lb) (gm/unit) (mg/d. L/unit) Accurate Insulin Management (AIM) Nomogram 1. Connect the columns with a straight line between weight and total daily dose of insulin (TDD). 2. Read correction factor (CF) and carbohydrate/insulin ratio (CIR). 3. Basal insulin is one-half total daily dose of insulin. 149┼ 329 142┼ 313 135┼ 298 129┼ 284 123┼ 270 117┼ 258 111┼ 245 106┼ 234 101┼ 222 96┼ 212 92┼ 202 87┼ 192 83┼ 183 79┼ 174 75┼ 166 72┼ 158 68┼ 151 65┼ 143 62┼ 137 59┼ 130 56┼ 124 54┼ 118 51┼ 112 49┼ 107 46┼ 102 44┼ 97 42┼ 92 40┼ 88 38┼ 84 36┼ 80 35┼ 76 33┼ 72 31┼ 69 30┼ 66 28┼ 63 27┼ 60 26┼ 57 25┼ 54 23┼ 51 ┼ 47 ┼ 43 ┼ 39 ┼ 35 ┼ 32 ┼ 29 ┼ 26 ┼ 24 ┼ 22 ┼ 20 ┼ 18 ┼ 16 ┼ 15 ┼ 13 ┼ 12 ┼ 11 ┼ 10 ┼ 9 ┼ 8 ┼ 7 ┼ 6 ┼ 5 ┼ 5 ┼ 4 ┼ 3 ┼ 3 ┼ 2 ┼ 2 ┼ 2 ┼ 1 ┼ 1 20┼ 88 21┼ 84 22┼ 80 23┼ 76 24┼ 72 25┼ 69 26┼ 65 28┼ 62 29┼ 59 30┼ 57 32┼ 54 33┼ 51 35┼ 49 37┼ 47 39┼ 44 41┼ 42 43┼ 40 45┼ 38 47┼ 36 49┼ 35 52┼ 33 55┼ 31 57┼ 30 60┼ 29 63┼ 27 66┼ 26 70┼ 25 73┼ 23 77┼ 22 81┼ 21 85┼ 20 89┼ 19 93┼ 18 98┼ 18 103┼ 17 108┼ 16 113┼ 15 119┼ 14 125┼ 14

Future of Diabetes Management Improvements in Insulin & Delivery l Insulin analogs and inhaled insulin l Smart external pumps l Internal pumps l Real-time sensors l Closed-loop systems l Unconceived-of solutions

Conclusion Intensive therapy to target is the only way to treat patients with diabetes 1. Metformin and/or TZD + Glinide or Sulfonylurea (PETS) 2. Glargine + Glinide or Sulfonylurea (GEMS) 3. Glargine + Lispro/Aspart (MDI) 4. Insulin Pump (CSII)

QUESTIONS? l For a copy or viewing of these slides – Contact • www. adaendo. com l How can I get use of Glucommander? (Computer-directed IV insulin program) – Available for review on internet, • www. glucommander. com – Contact us: • Glucommander@adaendo. com
9d3e9908c0377f7035efb6608376e0db.ppt