a73353c732448b8fe1ba1686b774186a.ppt
- Количество слайдов: 23

Collaborative study for establishment of the first national standard for Parvovirus B 19 DNA NAT assays Yi-Chen Yang, Drug Biology Division Bureau of Food and Drug Analysis Department of Health, Taiwan So. GAT XX June 2007

Background • NAT requirements in Taiwan ( 2002 ) – NAT test for Parvovirus B 19 is suggested on plasma pool or mini-pool the cut-off limit of B 19 DNA should be < 105 IU/m. L • WHO International standard for B 19 virus DNA (99/800) • European Pharmacopoeia Biological Reference Preparation (BRP) for B 19 Virus DNA Testing of Plasma Pools by NAT • To facilitate the implementation of the policy in Taiwan – national standard and working reagent for human parvovirus B 19 DNA NAT assays 2

National Standards for NAT • Reasons for Preparation – Difference genotypes among countries and regions (HBV, HCV) – Limited vials of international standards – One of BFDA’s task: supply of reference standards • Intended use u National standard : as a laboratory standard or reference material u Working reagent : as a run control for routine NAT assays • Blood/cell/tissure donor screening by NAT assays • Plasma pool screening by NAT assays • Testing for Class III IVD marketing approval • Quality control of IVD • Post marketing surveillance of IVD • Research However it is for the user to establish suitability of purpose 3
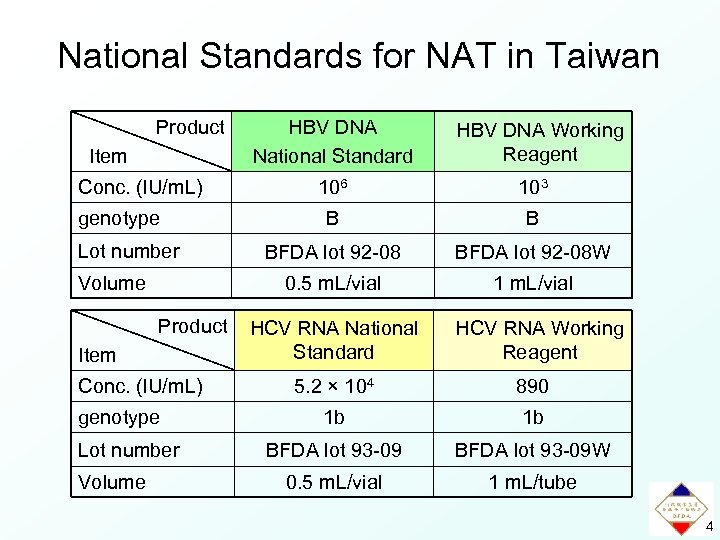
National Standards for NAT in Taiwan Product Item Conc. (IU/m. L) HBV DNA Working Reagent Lot number Volume Product Item Conc. (IU/m. L) genotype Lot number 106 103 B B BFDA lot 92 -08 W 0. 5 m. L/vial genotype Volume HBV DNA National Standard 1 m. L/vial HCV RNA National Standard HCV RNA Working Reagent 5. 2 × 104 890 1 b 1 b BFDA lot 93 -09 W 0. 5 m. L/vial 1 m. L/tube 4

Objective § To establish the B 19 DNA national standard Ø 106 IU/m. L, 0. 5 m. L/vial Ø around 1, 000 vials § To prepare the B 19 DNA working reagent Ø 104 IU/m. L, 1 m. L/vial Ø around 1, 000 vials 5
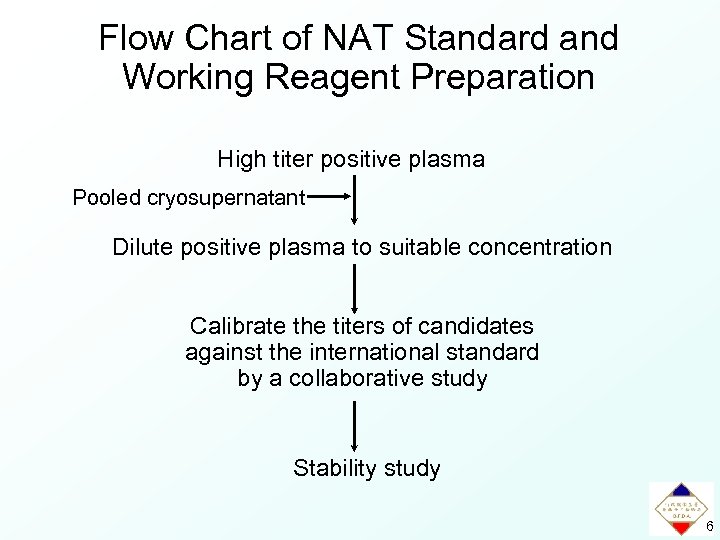
Flow Chart of NAT Standard and Working Reagent Preparation High titer positive plasma Pooled cryosupernatant Dilute positive plasma to suitable concentration Calibrate the titers of candidates against the international standard by a collaborative study Stability study 6

Preparation of B 19 DNA National Standard and Working Reagent • High titer positive plasma – Screening for other viruses ü Anti-HIV 1/2, HBs. Ag, Anti-HCV by EIA: (-) ü HAV RNA, HBV DNA, HCV RNA & HIV-1 RNA by NAT: (-) – Quantitative analysis of B 19 DNA – DNA sequencing/ Nucleotide-nucleotide BLAST (NCBI) • Diluent : Pooled human cryosupernatant ü Anti-HIV 1/2, HBs. Ag, Anti-HCV by EIA: All (-) ü HAV RNA, HBV DNA, HCV RNA, HIV-1 RNA & B 19 DNA by NAT : All (-) ü Anti-B 19 Ig. M, Anti-B 19 Ig. G by EIA: All (-) • Check the titers of preparations in 3 different assay methods – Light. Cycler Parvovirus B 19 Quantification Kit – Real. Art Parvo B 19 LC kit – In-house assay 7

International Collaborative Study for B 19 DNA Standards • Participating Labs including: 10 Labs from 7 countries – – Official Medicine Control Laboratories (OMCL) NAT testing laboratory Manufacturers of plasma products Manufacturers of in vitro diagnostics • Each Lab received 3 vials of each sample, and 1 vial of WHO B 19 DNA IS (code: 99/800) – Perform 3 independent assays for each sample – Calibrate the candidates against the IS 8
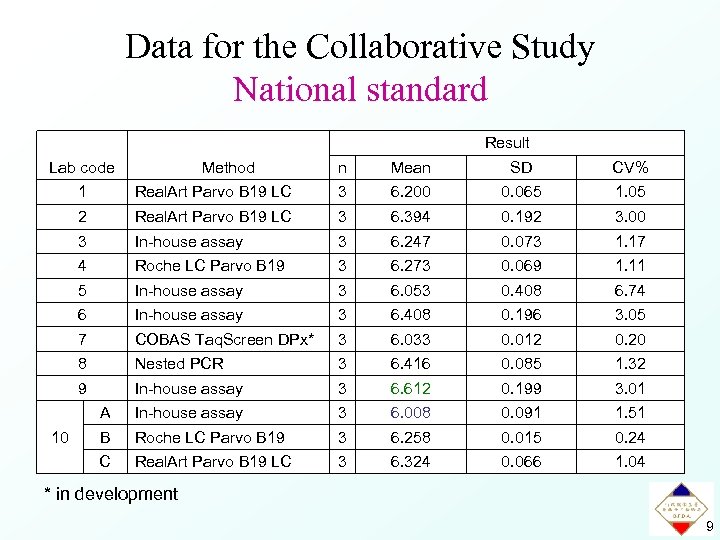
Data for the Collaborative Study National standard Result Lab code Method n Mean SD CV% 1 3 6. 200 0. 065 1. 05 2 Real. Art Parvo B 19 LC 3 6. 394 0. 192 3. 00 3 In-house assay 3 6. 247 0. 073 1. 17 4 Roche LC Parvo B 19 3 6. 273 0. 069 1. 11 5 In-house assay 3 6. 053 0. 408 6. 74 6 In-house assay 3 6. 408 0. 196 3. 05 7 COBAS Taq. Screen DPx* 3 6. 033 0. 012 0. 20 8 Nested PCR 3 6. 416 0. 085 1. 32 9 In-house assay 3 6. 612 0. 199 3. 01 A In-house assay 3 6. 008 0. 091 1. 51 B Roche LC Parvo B 19 3 6. 258 0. 015 0. 24 C 10 Real. Art Parvo B 19 LC 3 6. 324 0. 066 1. 04 * in development 9
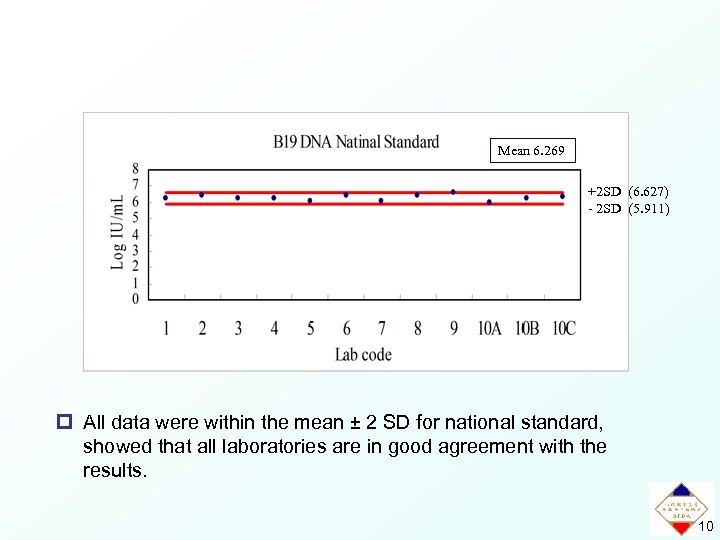
Mean 6. 269 +2 SD (6. 627) - 2 SD (5. 911) p All data were within the mean ± 2 SD for national standard, showed that all laboratories are in good agreement with the results. 10
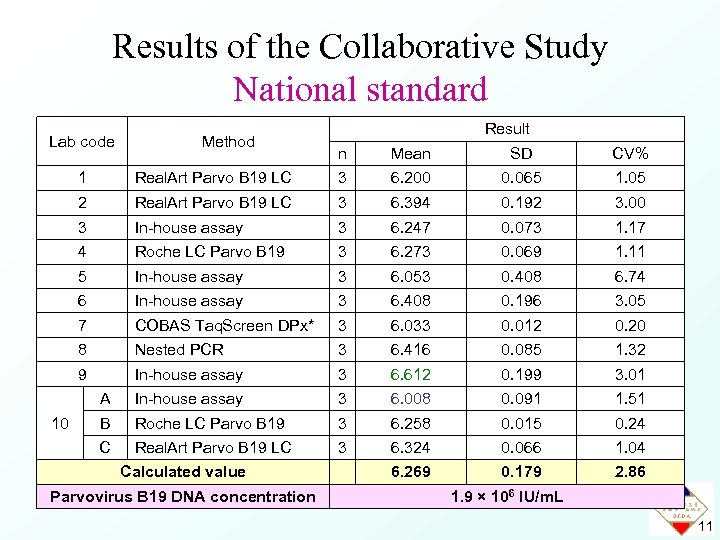
Results of the Collaborative Study National standard Lab code Method Result n Mean SD CV% 1 3 6. 200 0. 065 1. 05 2 Real. Art Parvo B 19 LC 3 6. 394 0. 192 3. 00 3 In-house assay 3 6. 247 0. 073 1. 17 4 Roche LC Parvo B 19 3 6. 273 0. 069 1. 11 5 In-house assay 3 6. 053 0. 408 6. 74 6 In-house assay 3 6. 408 0. 196 3. 05 7 COBAS Taq. Screen DPx* 3 6. 033 0. 012 0. 20 8 Nested PCR 3 6. 416 0. 085 1. 32 9 In-house assay 3 6. 612 0. 199 3. 01 A In-house assay 3 6. 008 0. 091 1. 51 B Roche LC Parvo B 19 3 6. 258 0. 015 0. 24 C 10 Real. Art Parvo B 19 LC 3 6. 324 0. 066 1. 04 6. 269 0. 179 2. 86 Calculated value Parvovirus B 19 DNA concentration 1. 9 × 106 IU/m. L 11
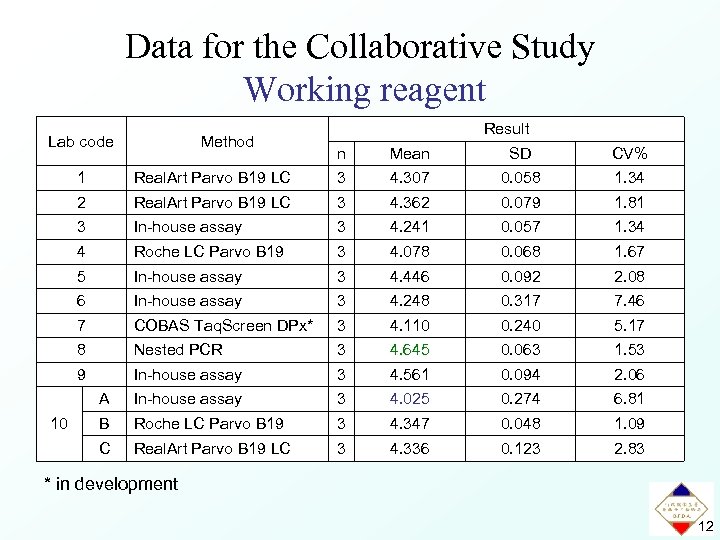
Data for the Collaborative Study Working reagent Lab code Method Result n Mean SD CV% 1 3 4. 307 0. 058 1. 34 2 Real. Art Parvo B 19 LC 3 4. 362 0. 079 1. 81 3 In-house assay 3 4. 241 0. 057 1. 34 4 Roche LC Parvo B 19 3 4. 078 0. 068 1. 67 5 In-house assay 3 4. 446 0. 092 2. 08 6 In-house assay 3 4. 248 0. 317 7. 46 7 COBAS Taq. Screen DPx* 3 4. 110 0. 240 5. 17 8 Nested PCR 3 4. 645 0. 063 1. 53 9 In-house assay 3 4. 561 0. 094 2. 06 A In-house assay 3 4. 025 0. 274 6. 81 B Roche LC Parvo B 19 3 4. 347 0. 048 1. 09 C 10 Real. Art Parvo B 19 LC 3 4. 336 0. 123 2. 83 * in development 12
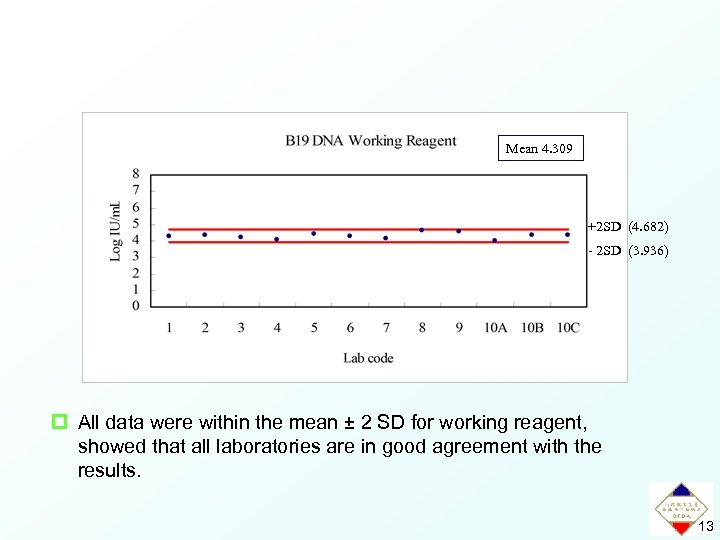
Mean 4. 309 +2 SD (4. 682) - 2 SD (3. 936) p All data were within the mean ± 2 SD for working reagent, showed that all laboratories are in good agreement with the results. 13
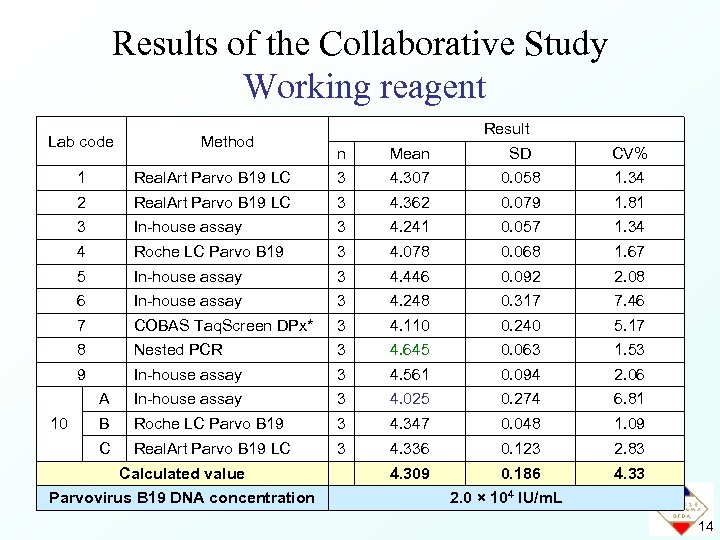
Results of the Collaborative Study Working reagent Lab code Method Result n Mean SD CV% 1 3 4. 307 0. 058 1. 34 2 Real. Art Parvo B 19 LC 3 4. 362 0. 079 1. 81 3 In-house assay 3 4. 241 0. 057 1. 34 4 Roche LC Parvo B 19 3 4. 078 0. 068 1. 67 5 In-house assay 3 4. 446 0. 092 2. 08 6 In-house assay 3 4. 248 0. 317 7. 46 7 COBAS Taq. Screen DPx* 3 4. 110 0. 240 5. 17 8 Nested PCR 3 4. 645 0. 063 1. 53 9 In-house assay 3 4. 561 0. 094 2. 06 A In-house assay 3 4. 025 0. 274 6. 81 B Roche LC Parvo B 19 3 4. 347 0. 048 1. 09 C 10 Real. Art Parvo B 19 LC 3 4. 336 0. 123 2. 83 4. 309 0. 186 4. 33 Calculated value Parvovirus B 19 DNA concentration 2. 0 × 104 IU/m. L 14
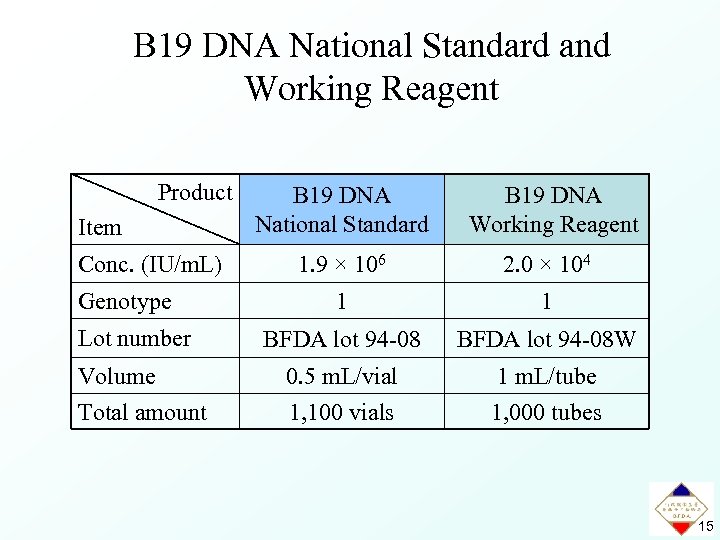
B 19 DNA National Standard and Working Reagent Product Item Conc. (IU/m. L) Genotype Lot number Volume Total amount B 19 DNA National Standard B 19 DNA Working Reagent 1. 9 × 106 2. 0 × 104 1 1 BFDA lot 94 -08 W 0. 5 m. L/vial 1, 100 vials 1 m. L/tube 1, 000 tubes 15
Stability Study for B 19 DNA Standards • Check the titers in 2 different assay methods – Real. Art Parvo B 19 LC kit – In-house assay • Performed 3 independent assays for each method 16
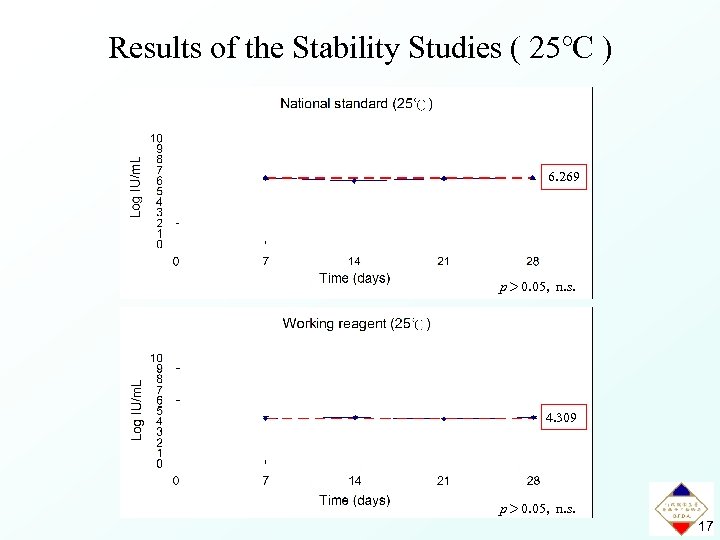
Results of the Stability Studies ( 25℃ ) 6. 269 p> 0. 05, n. s. 4. 309 p> 0. 05, n. s. 17
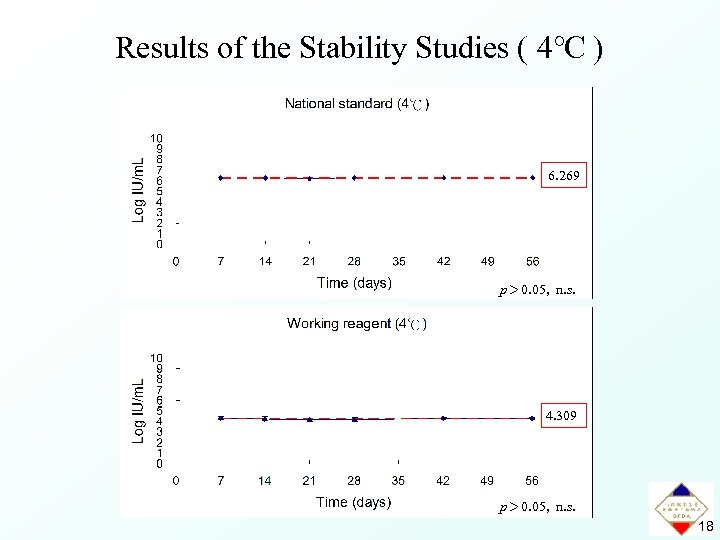
Results of the Stability Studies ( 4℃ ) 6. 269 p> 0. 05, n. s. 4. 309 p> 0. 05, n. s. 18
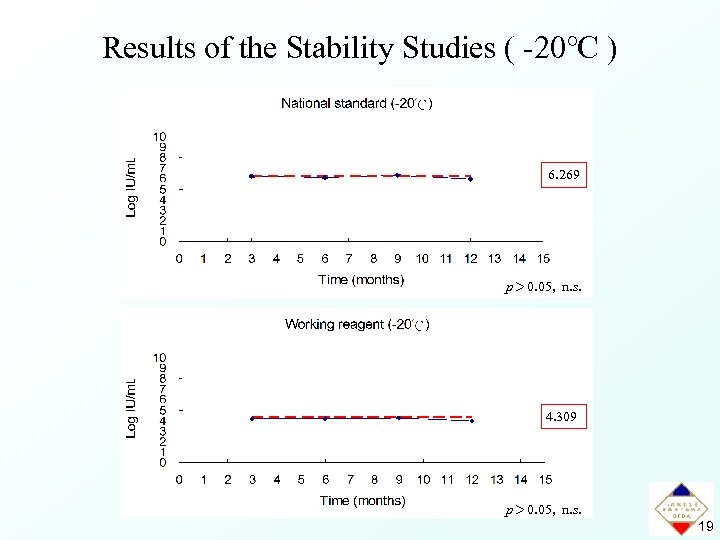
Results of the Stability Studies ( -20℃ ) 6. 269 p> 0. 05, n. s. 4. 309 p> 0. 05, n. s. 19
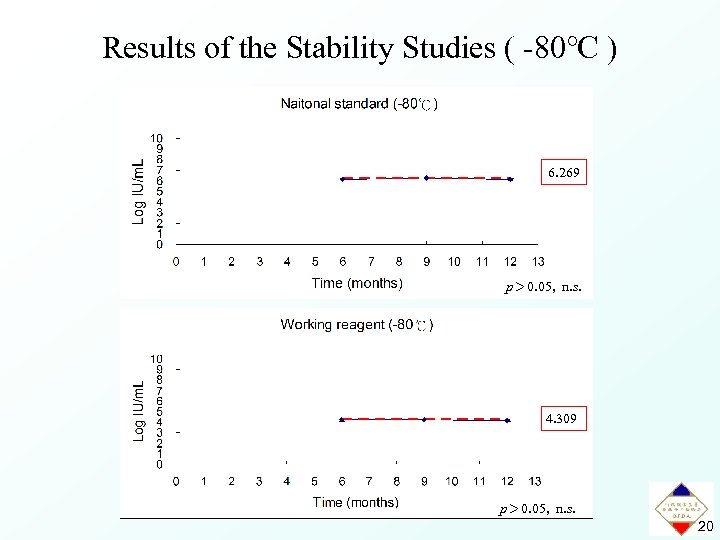
Results of the Stability Studies ( -80℃ ) 6. 269 p> 0. 05, n. s. 4. 309 p> 0. 05, n. s. 20

Summary • In this international collaborative study, a high level of agreement between results was obtained from different laboratories. • The first national standard and working reagent for B 19 DNA NAT assays with an assigned potency of 1. 9 × 106 IU/m. L and 2. 0 × 104 IU/m. L, respectively, were established. • The national standard and working reagent were stable at 25℃ for 4 weeks, 4℃ for 8 weeks, -20℃ and -80℃ for at least 12 months. 21

Acknowledgements • thanks to all participants of the collaborative study group – – – – – Dr. M. Y. Yu Dr. C. M. Nübling, Dr. M. Chudy Dr. S. Baylis Dr. Y. Okada Dr. M. Gessner, Dr. A. Klotz Dr. D. Johnstone Dr. R. Smith Dr. T. Grewing Dr. D. Sizmann, Dr. A. Schubert – Dr. J. Saldanha – Dr. A. Heath CBER, USA PEI, Germany NIBSC, UK NIID, Japan Baxter AG, Austria CSL Bioplasma, Australia NGI, USA QIAGEN, Germany Roche, USA NIBSC, UK your attention Thank you for 22
NAT requirement in Taiwan (Dec. 19 2002 ) to improve the safety of blood products 1. NAT tests on plasma pool are required: negative for HIV, HBV, HCV 2. Virus inactivation/removal steps for enveloped and nonenveloped viruses: two steps or one step ( shown to be reliably effective ) 3. For S/D treated blood products One additional step should be performed e. g. monoclonal purification or nanofiltration ( at least 4 log reduction of HAV ) or The plasma pool should be HAV NAT(-) before the manufacturing process 4. NAT test for Parvovirus B 19 is suggested on plasma pool or mini-pool the cut-off limit of B 19 DNA should be < 105 IU/ml 23
a73353c732448b8fe1ba1686b774186a.ppt