23da6e94a342ed9ac37edc3f5eec03d9.ppt
- Количество слайдов: 28

Colchicine for Patients with Diabetic Nephropathy A Randomized Double blinded Control study Limor Marko, MSc Supervisor: Shaye Kivity, MD

Diabetic nephropathy The leading cause today for ESRF in the western world. Multifactorial intervention may slow the rate of albuminuria and renal injury: Glycemic control HTN control ACE-I/ARBs
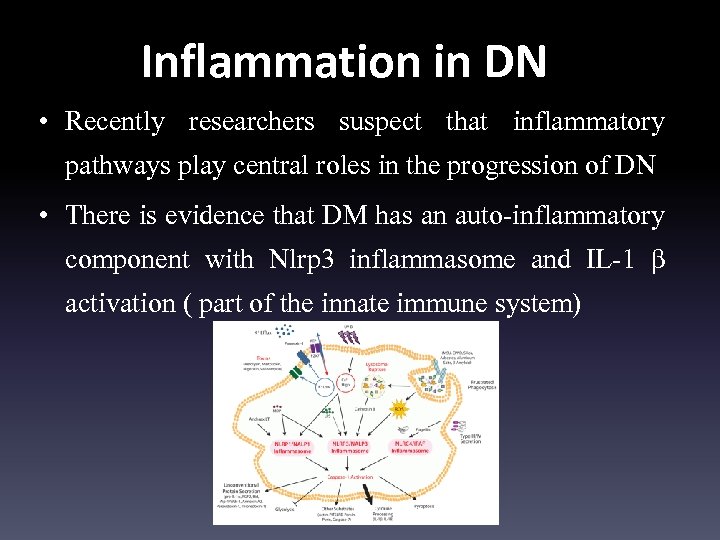
Inflammation in DN • Recently researchers suspect that inflammatory pathways play central roles in the progression of DN • There is evidence that DM has an auto-inflammatory component with Nlrp 3 inflammasome and IL-1 β activation ( part of the innate immune system)

Colchicine • Relatively safe anti-inflammatory drug • Used to treat and reverse albuminuria in FMF nephropathy • The pathogenesis of FMF and autoinflammatory diseases is related to activation of the inflammasome via Nf-Kappa. B, and Interleukin -1 pathways

Colchicine reduces proteinuria in rats with experimental DM This effect was attributed to its anti-inflammatory effect: Attenuated mesangial inflammatory cell infiltration, possibly by inhibiting: Monocyte chemotactic protein-1(MCP-1) Intercellular adhesion molecule-1 (ICAM-1)
Aim and hypothesis Aims of the Study: A randomized double blinded controlled study to test the efficacy and safety of colchicine treatment for DN. Working hypothesis Patients with DN treated with colchicine will have a significant reduction in the progression of proteinuria compared to placebo, with minimal side effects

Methods • Double blinded placebo control clinical trial • 40 patients with DN will be randomized to receive oral colchicine or placebo at a ratio of 1: 1. (supplied by RAFA ) • Colchicine treatment will be initiated at 1 mg per day, and increased to 2 mg if tolerated • Both groups will be treated for 18 months. • 24 hr. Protein will be assessed at: – At baseline – Every 3 mo. during the study period – A year after end of treatment • At Screening and at end of study thorough assessments
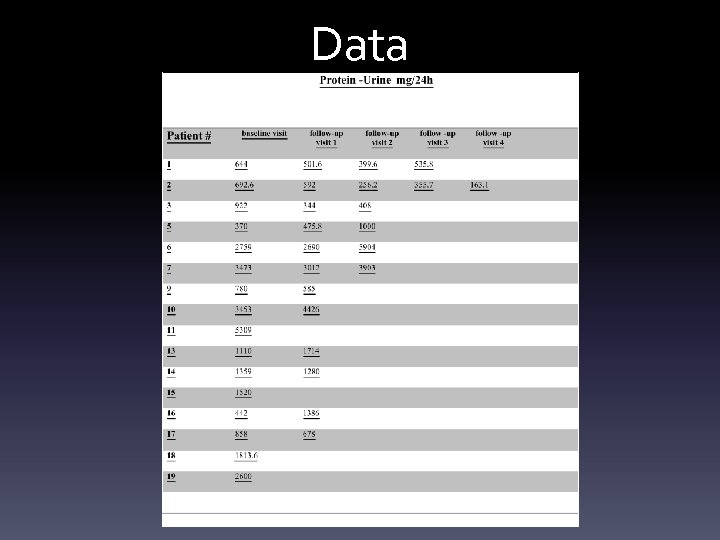
Thorough assessment at screening and end 24 hr urine collection for protein. Urine protein-to-creatinine ratio (UPCR) in a spot first- or second-morning urine sample after avoiding exercise Blood creatinine Complete blood count Creatinine phosphokinase ( CPK) Liver function tests Fasting Glucose Test and Hb. A 1 c DM treatment monitoring and comprehensive clinical follow-up, other treatments Blood pressure Total cholesterol, LDL-C, HDL-C, TG

Inclusion criteria: • Patients with DM, age>18 years old, able to sign an informed consent. • 24 hr. proteinuria >0. 5 gr. • Hemoglobin A 1 c in the range of 6 -9%, stable for last year (0. 5±) • Blood creatinine lower than 2 mg/d. L. • Blood pressure lower than 140/90 mm. Hg on stable antihypertensive treatment for at least 3 months. • Treated with ACE or ARB, unless contraindicated

Exclusion criteria: • Malignancy or significant heart, lung or liver disease. • Any GI disease, IBD, malnutrition ( BMI under 18 ) • Psychiatric disease • Any muscle disease, history of rhabdomyelitis, myopathy or myositis. • Any disease causing renal injury/proteinuria apart from DM • Any inflammatory or autoimmune disease • Any infection during the last month.

Outcome measures: • Stabilization or decrease of proteinuria of the treatment group compared to placebo and to initial tests • Stabilization or decrease of fasting glucose or Hb. A 1 C

Current Status of Research • Double blinded study- The study is in its blinded stage. Efficacy results are yet to be available. • 20 subjects have been recruited – 2 withdrew due to side effects – 1 withdrew since wasn’t interested in continuing the study • Screening visit was withheld for all 20 subjects

Study population • Gender: 3 F/ 17 M • Age: 62. 3± 8. 9 • Starting dose: 1 mg (0. 5 mg 1 X 2)
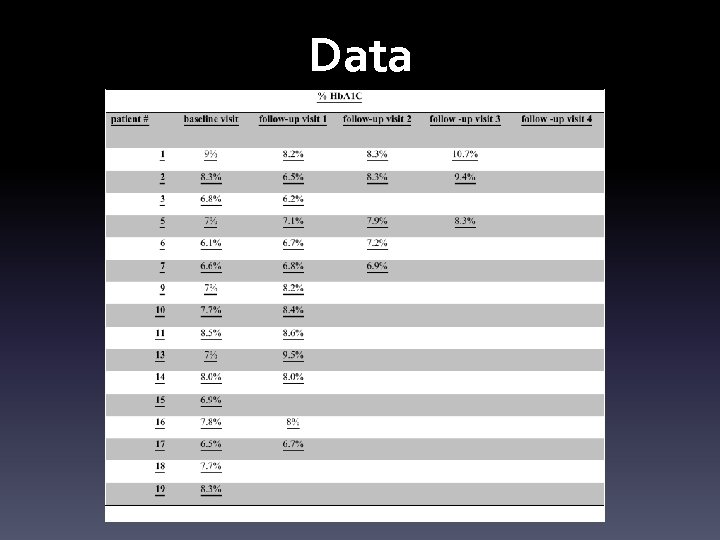
Data
Data
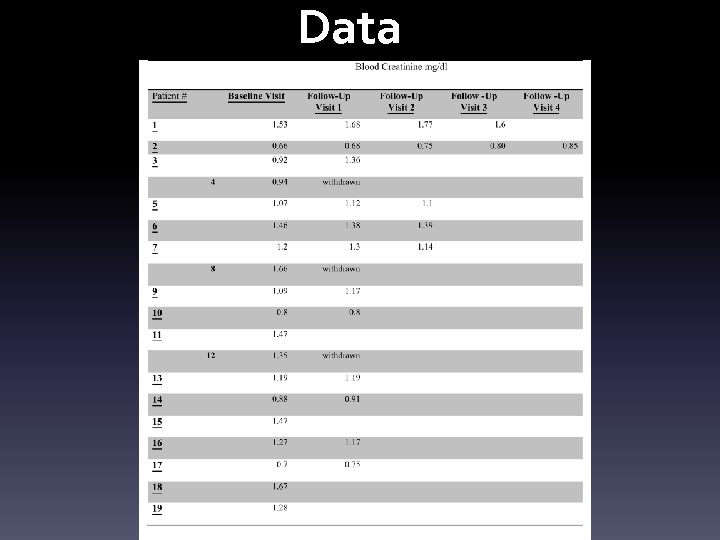
Data

Safety • No SAEs (Serious Adverse Events) were reported up to this point. • Reported adverse events were from 2 patients that suffered from intolerance to the drug, however no major effects were observed.

Future Plan • Complete recruitment of study population. • Estimated time for recruitment completion is 9 months. • Continue patients follow-up according to the study protocol, every 3 months up to 18 months from study entry and 1 year post treatment. • Data analysis upon completion of study

Thank You!

Safety and Efficacy of Interleukin 1 receptor antagonist for patients with FMF resistant to colchicine treatment A retrospective study
Familial Mediterranean Fever • Most common autoinflammatory disease, affecting a patient population estimated at 150, 000 worldwide • Manifests with a typical clinical picture, mainly composed of painful, febrile serositis attacks, including peritonitis, pleuritis and arthritis • Overproduction inflammasome of IL-1β in the FMF-associated

Treatment • Only medication approved for FMF is colchicine. • Eventually leads to the prevention of FMF flares and amyloid complications • Resistance to colchicine prophylaxis in 5 -10% of patients.

Anakinra • Recombinant human interleukin 1 (IL-1) receptor antagonist (IL-1 Ra) • Recent double-blinded randomized control study showed Anakinra to appear as an effective and safe treatment for Colchicine resistant FMF patients

Aim • The primary aim of our study is to investigate the safety and efficacy of Ankanira • The secondary aim of our study is to detect factors predicting response to IL-1 Treatment and examining the clinical and genetical characteristics of FMF colchicine resistant patients

Methods • In this study we plan to identify subjects that were treated with Anakinra in Israel due to colchicine refractory FMF, in the last decade. We will gather demographic and clinical data • We estimate a total of 60 patients

• Inclusion Criteria – Diagnosed according to Tel Hashomer criteria for FMF – Treated with Anakinra for any period • Exclusion criteria – Not treated with Anakinra – Treated with Anakinra for another indication

• First study of this sample size to investigate safety and efficacy of Anakinra treatment. Up to date largest study was on 12 patients • Results may help tailor treatment for patients resistant to colchicine treatment

Thank You!
23da6e94a342ed9ac37edc3f5eec03d9.ppt