eac5ded601b4f024c83833de196291ce.ppt
- Количество слайдов: 77
 Coagulation Testing What is it? Why do we need it POC? Marcia L. Zucker, Ph. D. Director of Clinical Research Educational Services, Edison, NJ
Coagulation Testing What is it? Why do we need it POC? Marcia L. Zucker, Ph. D. Director of Clinical Research Educational Services, Edison, NJ
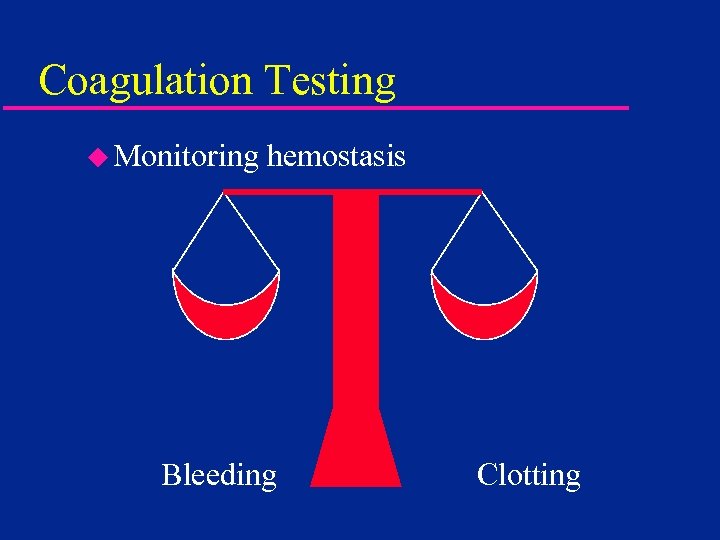 Coagulation Testing u Monitoring hemostasis Bleeding Clotting
Coagulation Testing u Monitoring hemostasis Bleeding Clotting
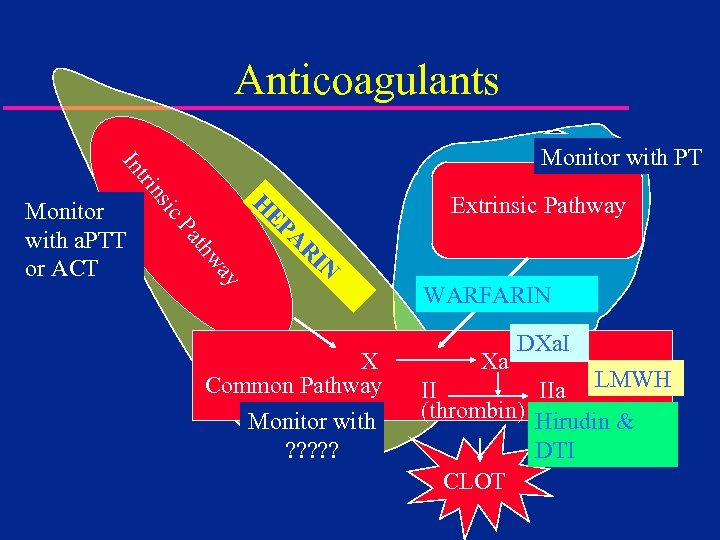 Anticoagulants Extrinsic Pathway H IN R A ay thw Pa EP ic Monitor with a. PTT or ACT ns tri In Monitor with PT X Common Pathway Monitor with ? ? ? WARFARIN Xa DXa. I II IIa LMWH (thrombin) Hirudin & DTI CLOT
Anticoagulants Extrinsic Pathway H IN R A ay thw Pa EP ic Monitor with a. PTT or ACT ns tri In Monitor with PT X Common Pathway Monitor with ? ? ? WARFARIN Xa DXa. I II IIa LMWH (thrombin) Hirudin & DTI CLOT
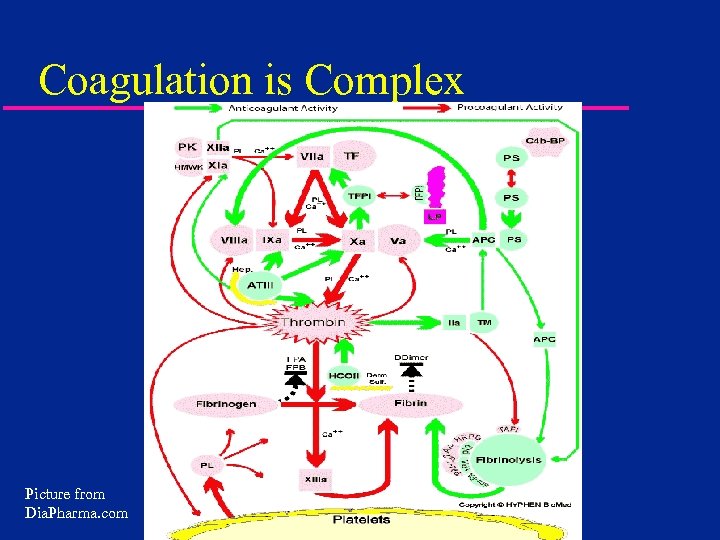 Coagulation is Complex Picture from Dia. Pharma. com
Coagulation is Complex Picture from Dia. Pharma. com
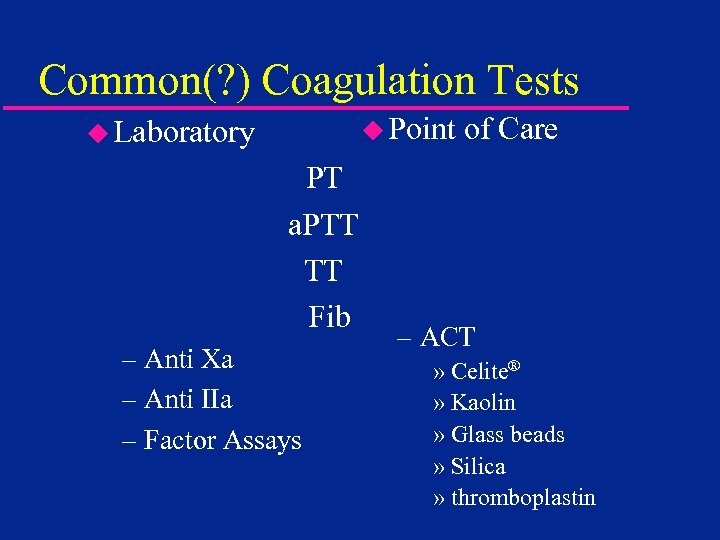 Common(? ) Coagulation Tests u Point u Laboratory PT. . a. PTT TT. . Fib. – Anti Xa – Anti IIa – Factor Assays of Care – ACT » Celite® » Kaolin » Glass beads » Silica » thromboplastin
Common(? ) Coagulation Tests u Point u Laboratory PT. . a. PTT TT. . Fib. – Anti Xa – Anti IIa – Factor Assays of Care – ACT » Celite® » Kaolin » Glass beads » Silica » thromboplastin
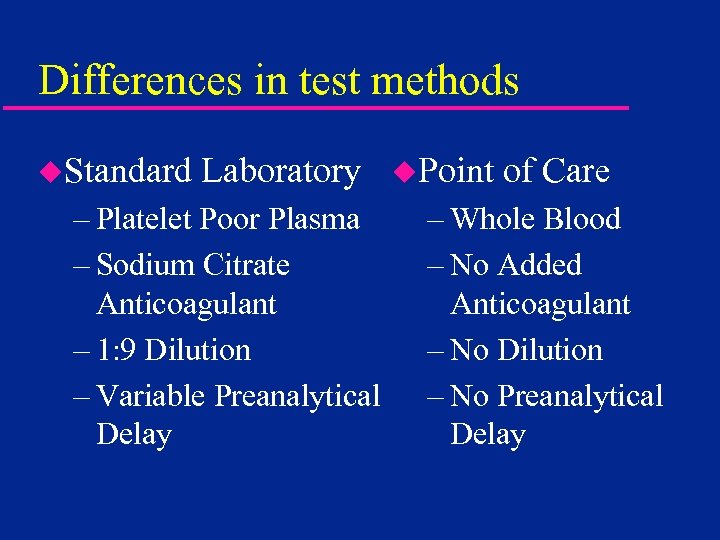 Differences in test methods u. Standard Laboratory – Platelet Poor Plasma – Sodium Citrate Anticoagulant – 1: 9 Dilution – Variable Preanalytical Delay u. Point of Care – Whole Blood – No Added Anticoagulant – No Dilution – No Preanalytical Delay
Differences in test methods u. Standard Laboratory – Platelet Poor Plasma – Sodium Citrate Anticoagulant – 1: 9 Dilution – Variable Preanalytical Delay u. Point of Care – Whole Blood – No Added Anticoagulant – No Dilution – No Preanalytical Delay
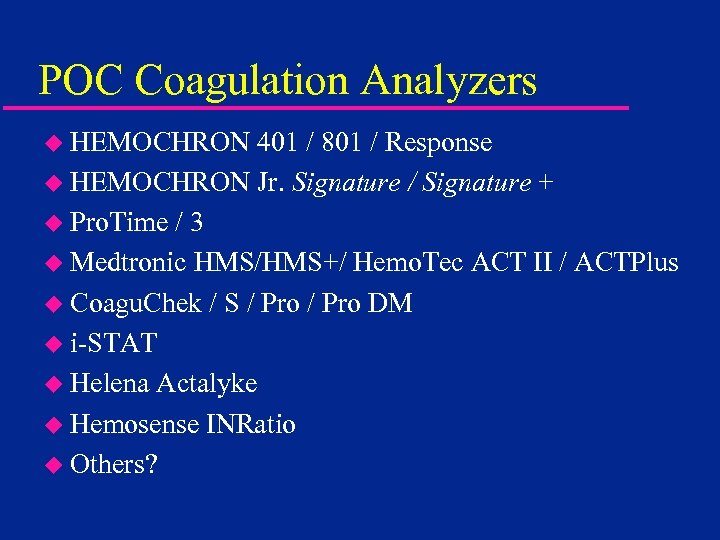 POC Coagulation Analyzers u HEMOCHRON 401 / 801 / Response u HEMOCHRON Jr. Signature / Signature + u Pro. Time / 3 u Medtronic HMS/HMS+/ Hemo. Tec ACT II / ACTPlus u Coagu. Chek / S / Pro DM u i-STAT u Helena Actalyke u Hemosense INRatio u Others?
POC Coagulation Analyzers u HEMOCHRON 401 / 801 / Response u HEMOCHRON Jr. Signature / Signature + u Pro. Time / 3 u Medtronic HMS/HMS+/ Hemo. Tec ACT II / ACTPlus u Coagu. Chek / S / Pro DM u i-STAT u Helena Actalyke u Hemosense INRatio u Others?
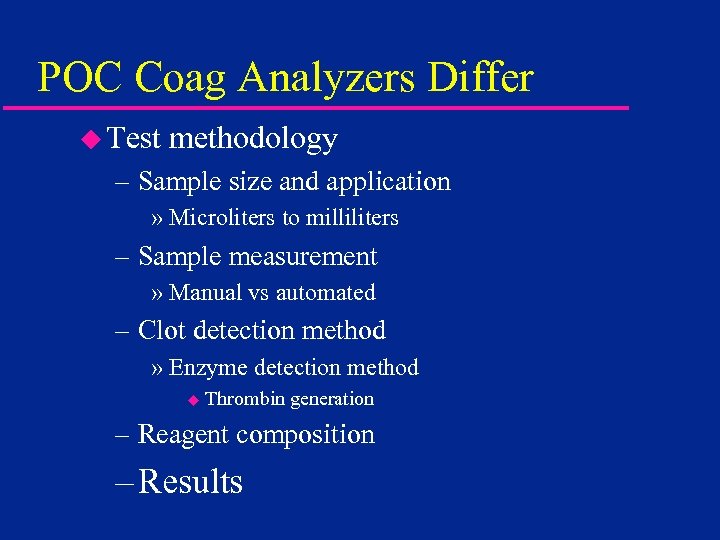 POC Coag Analyzers Differ u Test methodology – Sample size and application » Microliters to milliliters – Sample measurement » Manual vs automated – Clot detection method » Enzyme detection method u Thrombin generation – Reagent composition – Results
POC Coag Analyzers Differ u Test methodology – Sample size and application » Microliters to milliliters – Sample measurement » Manual vs automated – Clot detection method » Enzyme detection method u Thrombin generation – Reagent composition – Results
 Clinical Applications u. Operating Room – Cardiac Surgery – Interventional Cardiology and Radiology u. Critical Care u. Satellite Sites – Dialysis – ECMO – Emergency Room – Anticoagulation Clinic
Clinical Applications u. Operating Room – Cardiac Surgery – Interventional Cardiology and Radiology u. Critical Care u. Satellite Sites – Dialysis – ECMO – Emergency Room – Anticoagulation Clinic
 History of the ACT u. Lee-White clotting time – Manual – No activator – Very slow u 1966 –Hattersley- Activated Clotting Time – Diatomaceous earth activator – Operator defined mixing and clot detection – Global assay - Contact activation of cascade
History of the ACT u. Lee-White clotting time – Manual – No activator – Very slow u 1966 –Hattersley- Activated Clotting Time – Diatomaceous earth activator – Operator defined mixing and clot detection – Global assay - Contact activation of cascade
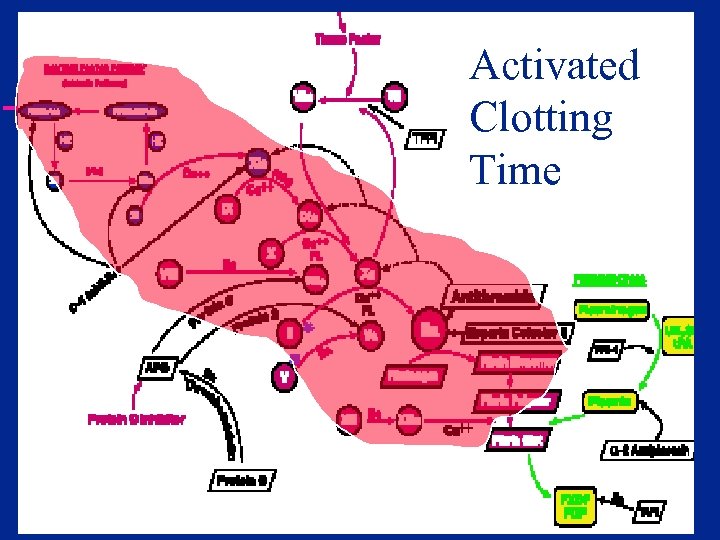 Activated Clotting Time
Activated Clotting Time
 Particulate Contact Activation u Initiation of intrinsic coagulation cascade – Factor XII (Hageman factor) – Prekallikrein (Fletcher factor) u Dramatically shortens contact activation period over Lee-White time u Proposed as both screening assay for coagulation defects and for heparin monitoring
Particulate Contact Activation u Initiation of intrinsic coagulation cascade – Factor XII (Hageman factor) – Prekallikrein (Fletcher factor) u Dramatically shortens contact activation period over Lee-White time u Proposed as both screening assay for coagulation defects and for heparin monitoring
 ACT Automation - 1969 u. HEMOCHRON introduced – semi-automated – less operator dependence – two assays » CA 510 (later FTCA 510) udiatomaceous earth activated » P 214 glass bead activated
ACT Automation - 1969 u. HEMOCHRON introduced – semi-automated – less operator dependence – two assays » CA 510 (later FTCA 510) udiatomaceous earth activated » P 214 glass bead activated
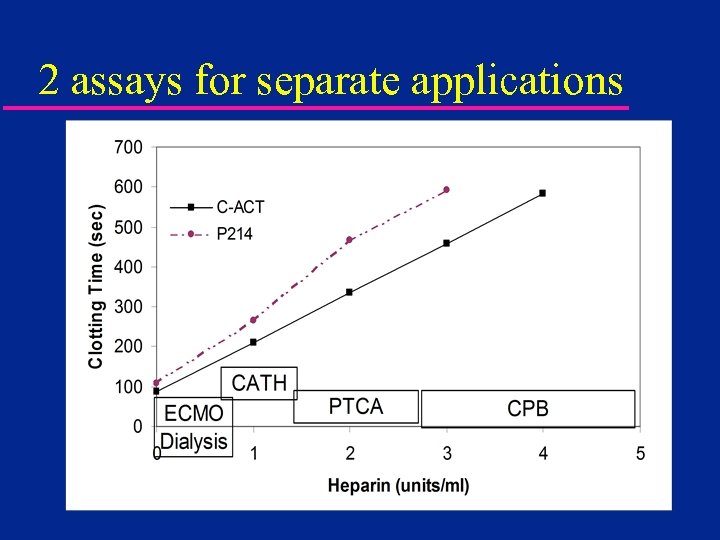 2 assays for separate applications
2 assays for separate applications
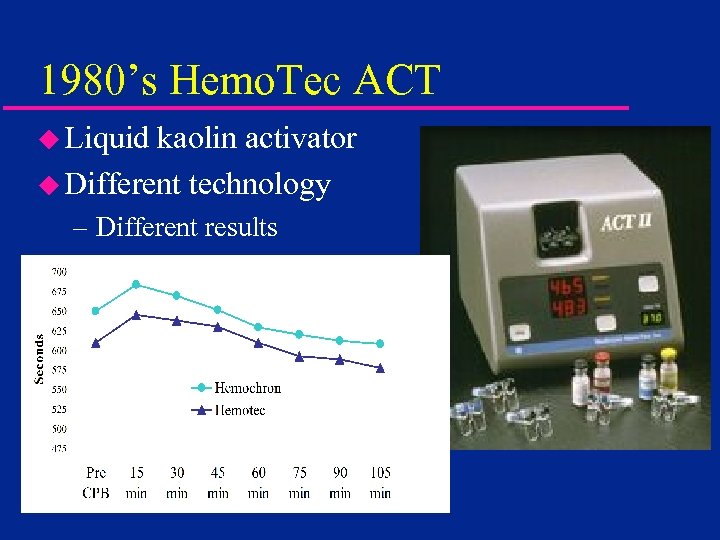 1980’s Hemo. Tec ACT u Liquid kaolin activator u Different technology – Different results
1980’s Hemo. Tec ACT u Liquid kaolin activator u Different technology – Different results
 ACT Differences u. Recognized in literature >20 years – Clinical evaluations of Hemochron appeared in journals mid 1970’s – By 1981, papers appeared showing little correlation between ACT and heparin level – By 1988, papers clearly showed clinically different results between Hemochron and Hemo. Tec u. Differences ignored by clinicians
ACT Differences u. Recognized in literature >20 years – Clinical evaluations of Hemochron appeared in journals mid 1970’s – By 1981, papers appeared showing little correlation between ACT and heparin level – By 1988, papers clearly showed clinically different results between Hemochron and Hemo. Tec u. Differences ignored by clinicians
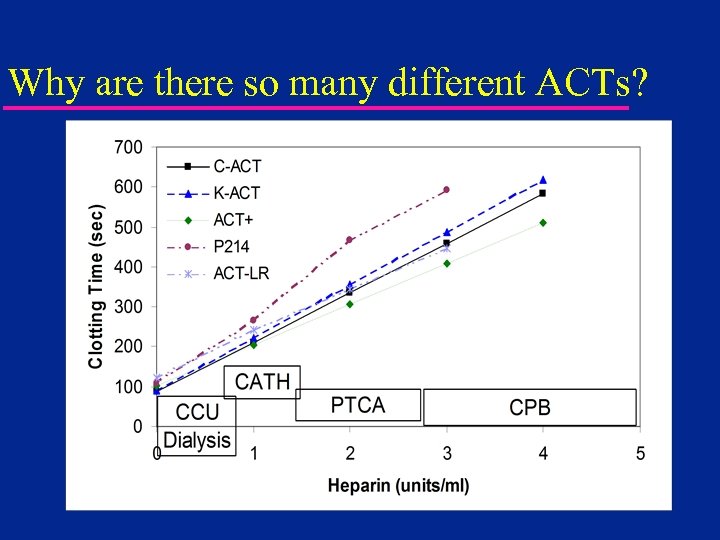 Why are there so many different ACTs?
Why are there so many different ACTs?
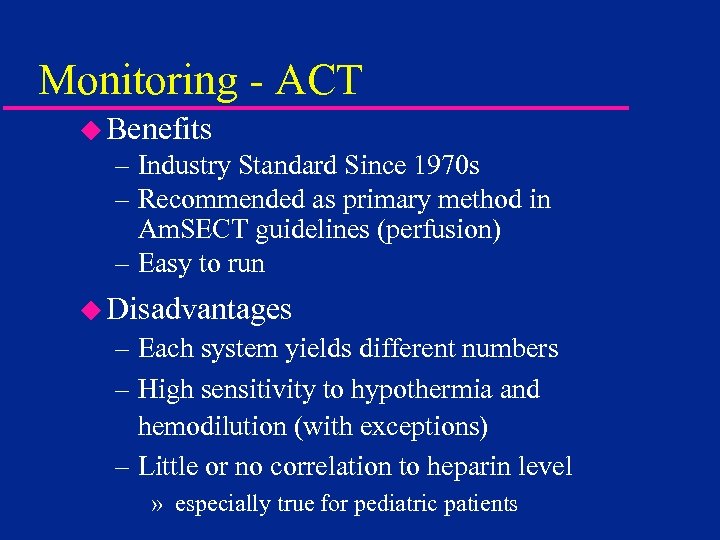 Monitoring - ACT u Benefits – Industry Standard Since 1970 s – Recommended as primary method in Am. SECT guidelines (perfusion) – Easy to run u Disadvantages – Each system yields different numbers – High sensitivity to hypothermia and hemodilution (with exceptions) – Little or no correlation to heparin level » especially true for pediatric patients
Monitoring - ACT u Benefits – Industry Standard Since 1970 s – Recommended as primary method in Am. SECT guidelines (perfusion) – Easy to run u Disadvantages – Each system yields different numbers – High sensitivity to hypothermia and hemodilution (with exceptions) – Little or no correlation to heparin level » especially true for pediatric patients
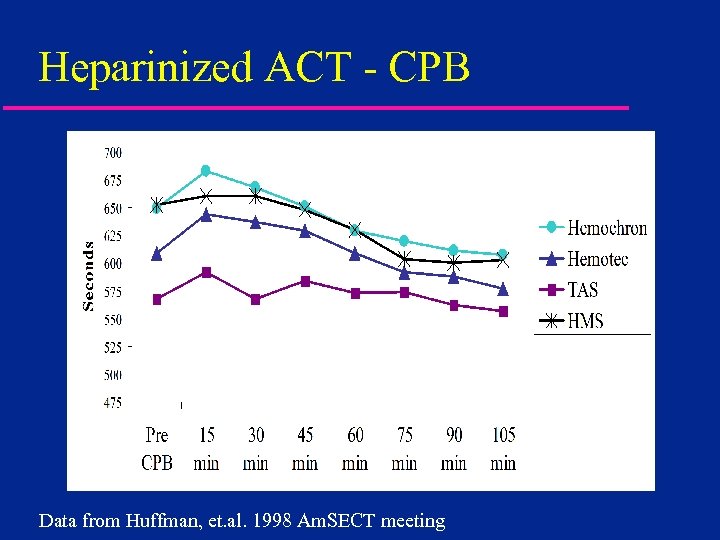 Heparinized ACT - CPB Data from Huffman, et. al. 1998 Am. SECT meeting
Heparinized ACT - CPB Data from Huffman, et. al. 1998 Am. SECT meeting
 Pharmaceutical Intervention u. Amicar or Tranexamic Acid – No effect on standard celite ACT u. Aprotinin – Significant elevation of celite ACT – Two dosing regimens » Full or Half Hammersmith » Both independent of patient size
Pharmaceutical Intervention u. Amicar or Tranexamic Acid – No effect on standard celite ACT u. Aprotinin – Significant elevation of celite ACT – Two dosing regimens » Full or Half Hammersmith » Both independent of patient size
 ACT Monitoring-Aprotinin Treatment u Celite ACT – Not recommended – Still used with target times of >750 seconds u Kaolin ACT – Unaffected by moderate doses of aprotinin – Used with target times of > 480 seconds u ACT+ – Unaffected by ALL doses of aprotinin – Used with target times of > 400 seconds
ACT Monitoring-Aprotinin Treatment u Celite ACT – Not recommended – Still used with target times of >750 seconds u Kaolin ACT – Unaffected by moderate doses of aprotinin – Used with target times of > 480 seconds u ACT+ – Unaffected by ALL doses of aprotinin – Used with target times of > 400 seconds
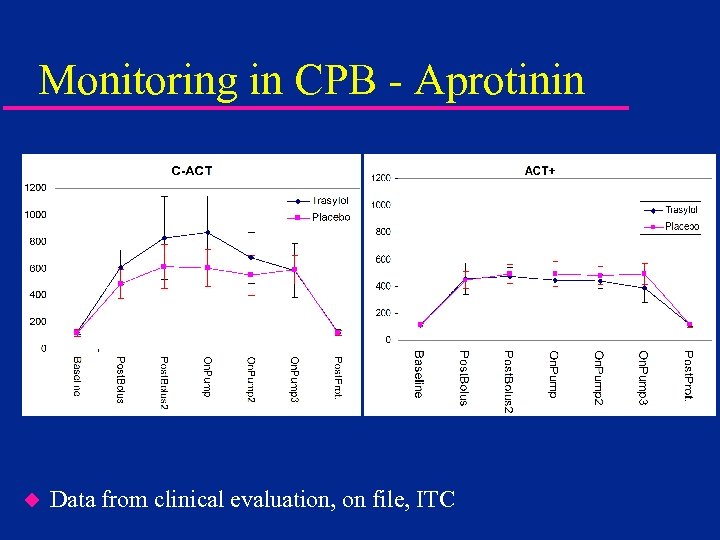 Monitoring in CPB - Aprotinin u Data from clinical evaluation, on file, ITC
Monitoring in CPB - Aprotinin u Data from clinical evaluation, on file, ITC
 Other POC Coag in the OR u a. PTT / PT – Pre- and post-procedural screening u Fibrinogen – Pre- and post-procedural screening u Dosing Assays – Customize heparin and protamine for each patient » HEMOCHRON HRT / PRT » Hepcon HMS – Measure heparin level » Relationship to coagulation status unclear
Other POC Coag in the OR u a. PTT / PT – Pre- and post-procedural screening u Fibrinogen – Pre- and post-procedural screening u Dosing Assays – Customize heparin and protamine for each patient » HEMOCHRON HRT / PRT » Hepcon HMS – Measure heparin level » Relationship to coagulation status unclear
 Other POC Coag in the OR u. Heparin neutralization verification – Ensure complete removal of circulating heparin » a. PTT » PDA-O - ACT based » TT / HNTT - Thrombin Time based » heparinase ACT
Other POC Coag in the OR u. Heparin neutralization verification – Ensure complete removal of circulating heparin » a. PTT » PDA-O - ACT based » TT / HNTT - Thrombin Time based » heparinase ACT
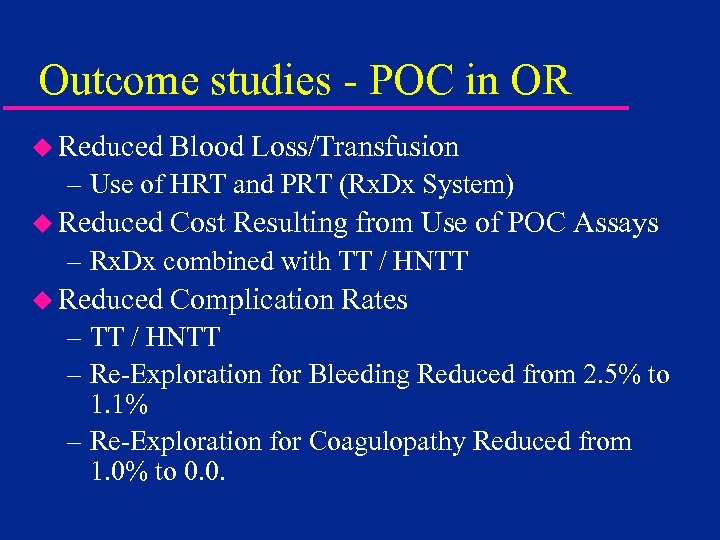 Outcome studies - POC in OR u Reduced Blood Loss/Transfusion – Use of HRT and PRT (Rx. Dx System) u Reduced Cost Resulting from Use of POC Assays – Rx. Dx combined with TT / HNTT u Reduced Complication Rates – TT / HNTT – Re-Exploration for Bleeding Reduced from 2. 5% to 1. 1% – Re-Exploration for Coagulopathy Reduced from 1. 0% to 0. 0.
Outcome studies - POC in OR u Reduced Blood Loss/Transfusion – Use of HRT and PRT (Rx. Dx System) u Reduced Cost Resulting from Use of POC Assays – Rx. Dx combined with TT / HNTT u Reduced Complication Rates – TT / HNTT – Re-Exploration for Bleeding Reduced from 2. 5% to 1. 1% – Re-Exploration for Coagulopathy Reduced from 1. 0% to 0. 0.
 Clinical Applications u. Operating Room – Cardiac Surgery – Interventional Cardiology and Radiology u. Critical Care u. Satellite Sites – Dialysis – ECMO – Emergency Room – Anticoagulation Clinic
Clinical Applications u. Operating Room – Cardiac Surgery – Interventional Cardiology and Radiology u. Critical Care u. Satellite Sites – Dialysis – ECMO – Emergency Room – Anticoagulation Clinic
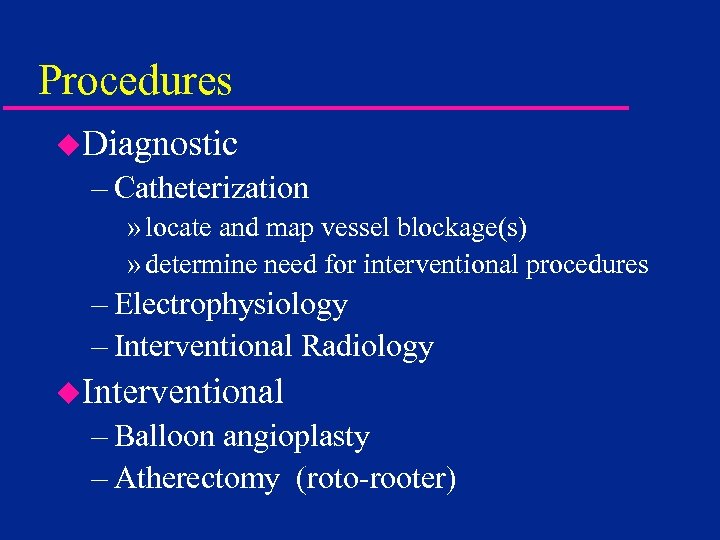 Procedures u. Diagnostic – Catheterization » locate and map vessel blockage(s) » determine need for interventional procedures – Electrophysiology – Interventional Radiology u. Interventional – Balloon angioplasty – Atherectomy (roto-rooter)
Procedures u. Diagnostic – Catheterization » locate and map vessel blockage(s) » determine need for interventional procedures – Electrophysiology – Interventional Radiology u. Interventional – Balloon angioplasty – Atherectomy (roto-rooter)
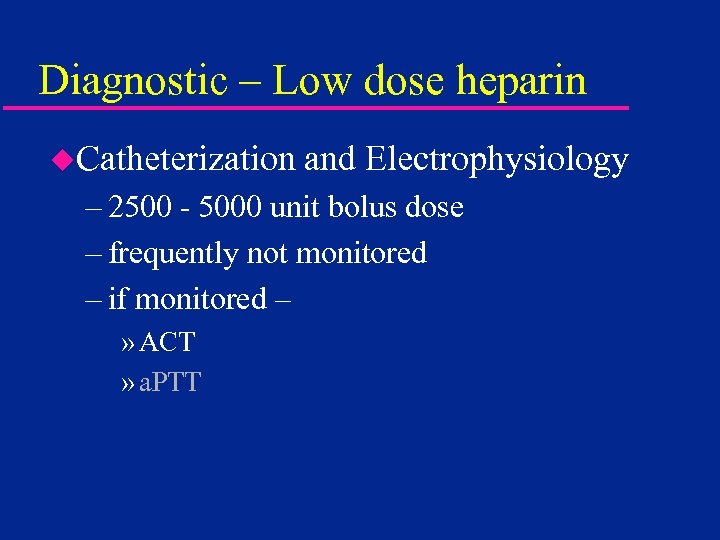 Diagnostic – Low dose heparin u. Catheterization and Electrophysiology – 2500 - 5000 unit bolus dose – frequently not monitored – if monitored – » ACT » a. PTT
Diagnostic – Low dose heparin u. Catheterization and Electrophysiology – 2500 - 5000 unit bolus dose – frequently not monitored – if monitored – » ACT » a. PTT
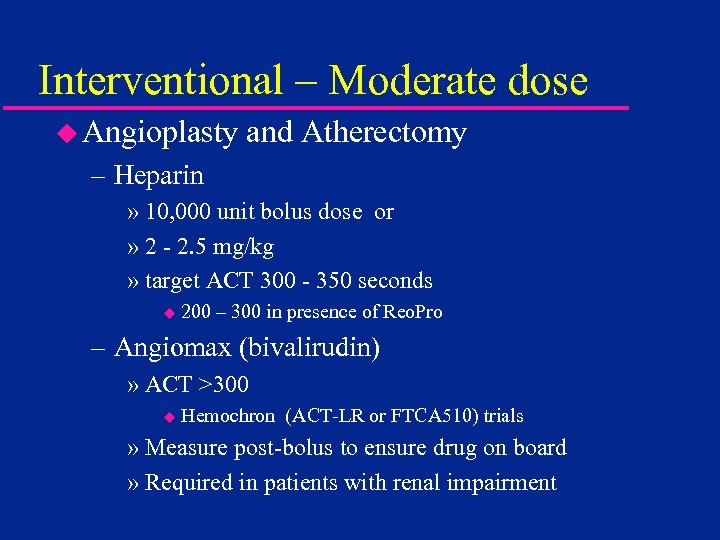 Interventional – Moderate dose u Angioplasty and Atherectomy – Heparin » 10, 000 unit bolus dose or » 2 - 2. 5 mg/kg » target ACT 300 - 350 seconds u 200 – 300 in presence of Reo. Pro – Angiomax (bivalirudin) » ACT >300 u Hemochron (ACT-LR or FTCA 510) trials » Measure post-bolus to ensure drug on board » Required in patients with renal impairment
Interventional – Moderate dose u Angioplasty and Atherectomy – Heparin » 10, 000 unit bolus dose or » 2 - 2. 5 mg/kg » target ACT 300 - 350 seconds u 200 – 300 in presence of Reo. Pro – Angiomax (bivalirudin) » ACT >300 u Hemochron (ACT-LR or FTCA 510) trials » Measure post-bolus to ensure drug on board » Required in patients with renal impairment
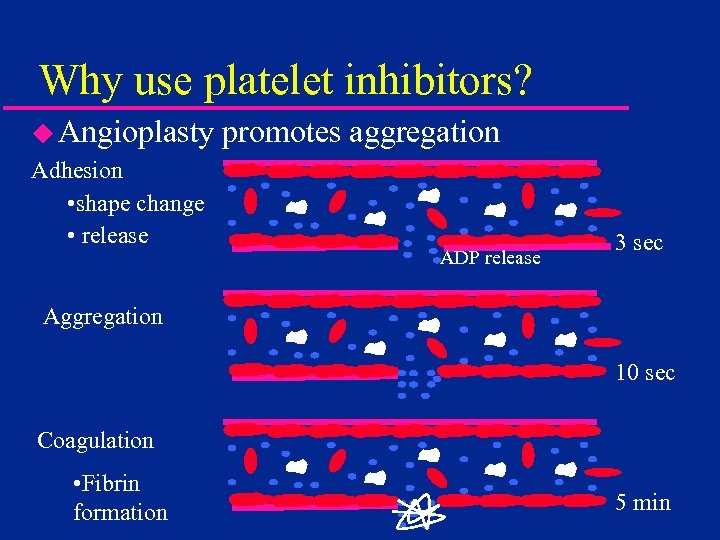 Why use platelet inhibitors? u Angioplasty Adhesion • shape change • release promotes aggregation ADP release 3 sec Aggregation 10 sec Coagulation • Fibrin formation 5 min
Why use platelet inhibitors? u Angioplasty Adhesion • shape change • release promotes aggregation ADP release 3 sec Aggregation 10 sec Coagulation • Fibrin formation 5 min
 Need to inhibit restenosis / reocclusion
Need to inhibit restenosis / reocclusion
 Platelet Inhibitors u. Reo. Pro – elevates ACTs – target time = 250 sec with Reo. Pro » determined using FTCA 510 tube u. Integrelin – No reported clinically significant effects on ACT u. Aggrastat – No reported effects on ACT
Platelet Inhibitors u. Reo. Pro – elevates ACTs – target time = 250 sec with Reo. Pro » determined using FTCA 510 tube u. Integrelin – No reported clinically significant effects on ACT u. Aggrastat – No reported effects on ACT
 Clinical Applications u. Operating Room – Cardiac Surgery – Interventional Cardiology and Radiology u. Critical Care u. Satellite Sites – Dialysis – ECMO – Emergency Room – Anticoagulation Clinic
Clinical Applications u. Operating Room – Cardiac Surgery – Interventional Cardiology and Radiology u. Critical Care u. Satellite Sites – Dialysis – ECMO – Emergency Room – Anticoagulation Clinic
 ACT or a. PTT u. Determine when to pull the femoral sheath – Premature sheath pull can lead to bleeding. – Delayed removal can increase time in CCU. – Target set at each site. » ACT targets range from 150 – 220 seconds » a. PTT targets range from 40 – 70 seconds u Must be linked to heparin sensitivity of reagent used
ACT or a. PTT u. Determine when to pull the femoral sheath – Premature sheath pull can lead to bleeding. – Delayed removal can increase time in CCU. – Target set at each site. » ACT targets range from 150 – 220 seconds » a. PTT targets range from 40 – 70 seconds u Must be linked to heparin sensitivity of reagent used
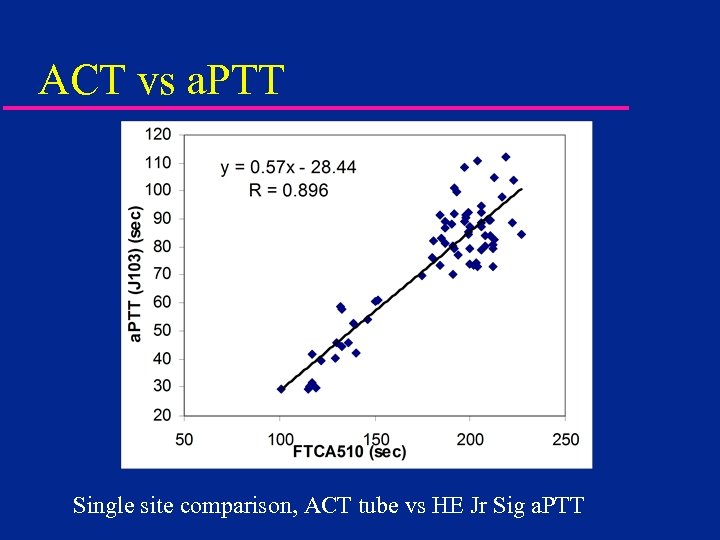 ACT vs a. PTT Single site comparison, ACT tube vs HE Jr Sig a. PTT
ACT vs a. PTT Single site comparison, ACT tube vs HE Jr Sig a. PTT
 ACT or a. PTT u. Monitor heparin therapy – Target times determined by each facility – APTT outcome study » Reduce time to result (112 vs <5 minute) » Reduce time to stabilization » Reduce dose adjustments » Reduce length of stay » By using POC a. PTT instead of lab u Poster at AACC 2000 – Staikos, et. al.
ACT or a. PTT u. Monitor heparin therapy – Target times determined by each facility – APTT outcome study » Reduce time to result (112 vs <5 minute) » Reduce time to stabilization » Reduce dose adjustments » Reduce length of stay » By using POC a. PTT instead of lab u Poster at AACC 2000 – Staikos, et. al.
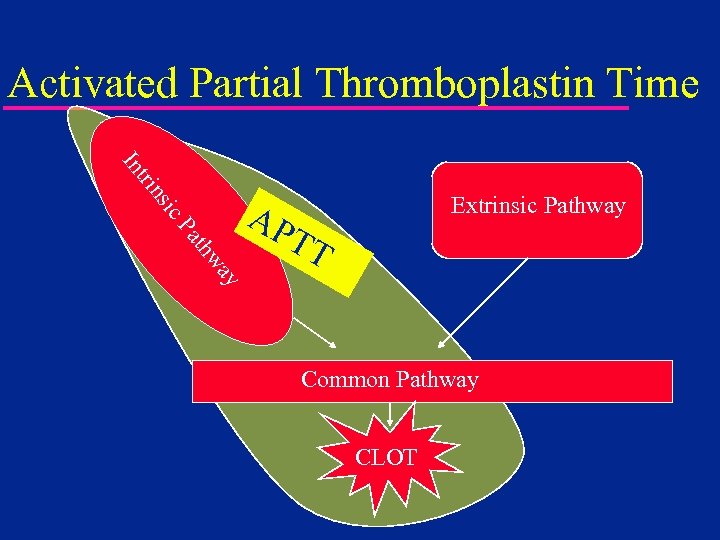 Activated Partial Thromboplastin Time ic ns tri In Extrinsic Pathway ay thw Pa AP TT Common Pathway CLOT
Activated Partial Thromboplastin Time ic ns tri In Extrinsic Pathway ay thw Pa AP TT Common Pathway CLOT
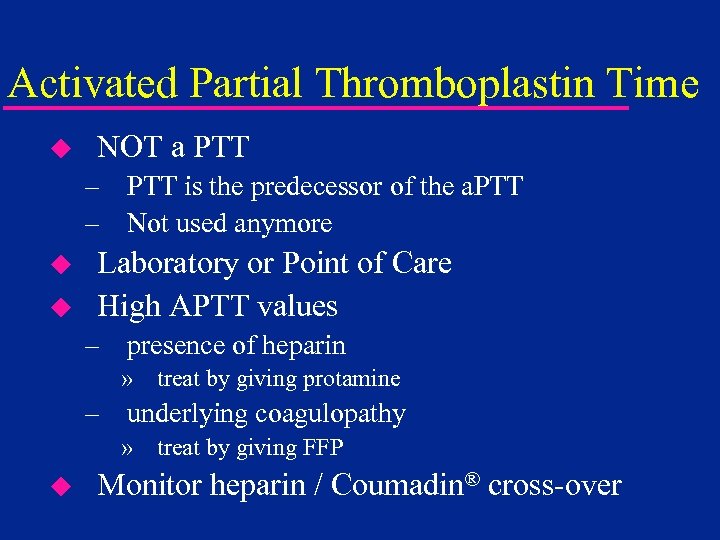 Activated Partial Thromboplastin Time u NOT a PTT – PTT is the predecessor of the a. PTT – Not used anymore u u Laboratory or Point of Care High APTT values – presence of heparin » treat by giving protamine – underlying coagulopathy » treat by giving FFP u Monitor heparin / Coumadin® cross-over
Activated Partial Thromboplastin Time u NOT a PTT – PTT is the predecessor of the a. PTT – Not used anymore u u Laboratory or Point of Care High APTT values – presence of heparin » treat by giving protamine – underlying coagulopathy » treat by giving FFP u Monitor heparin / Coumadin® cross-over
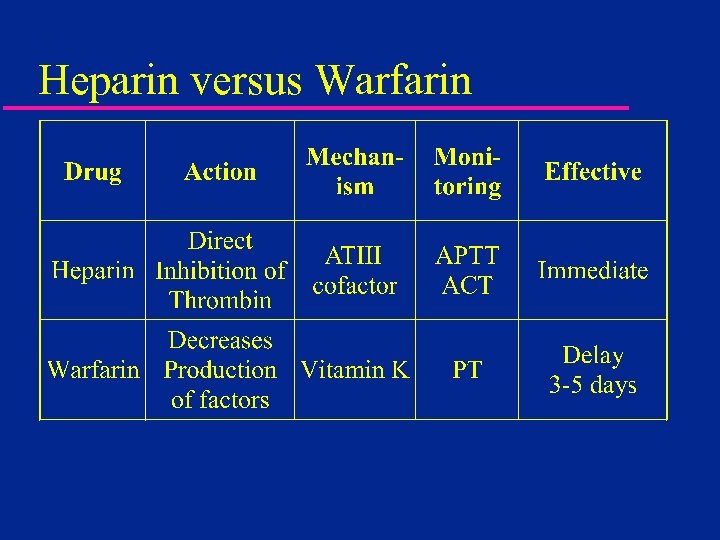 Heparin versus Warfarin
Heparin versus Warfarin
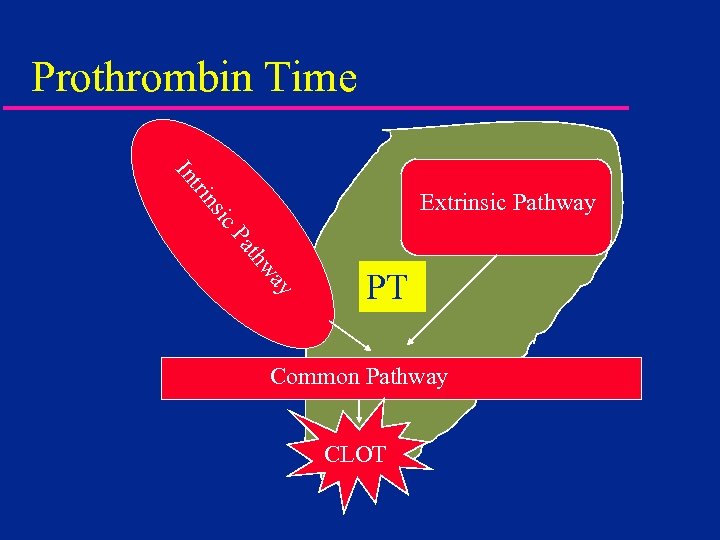 Prothrombin Time tri In ic ns Extrinsic Pathway ay thw Pa PT Common Pathway CLOT
Prothrombin Time tri In ic ns Extrinsic Pathway ay thw Pa PT Common Pathway CLOT
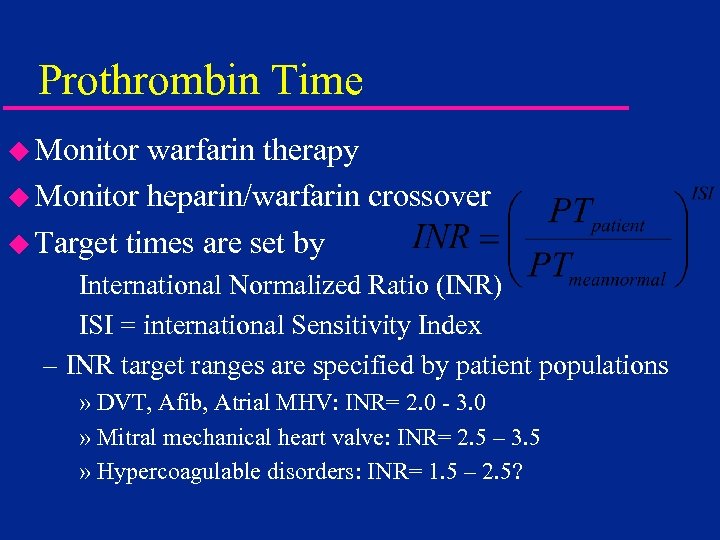 Prothrombin Time u Monitor warfarin therapy u Monitor heparin/warfarin crossover u Target times are set by International Normalized Ratio (INR) ISI = international Sensitivity Index – INR target ranges are specified by patient populations » DVT, Afib, Atrial MHV: INR= 2. 0 - 3. 0 » Mitral mechanical heart valve: INR= 2. 5 – 3. 5 » Hypercoagulable disorders: INR= 1. 5 – 2. 5?
Prothrombin Time u Monitor warfarin therapy u Monitor heparin/warfarin crossover u Target times are set by International Normalized Ratio (INR) ISI = international Sensitivity Index – INR target ranges are specified by patient populations » DVT, Afib, Atrial MHV: INR= 2. 0 - 3. 0 » Mitral mechanical heart valve: INR= 2. 5 – 3. 5 » Hypercoagulable disorders: INR= 1. 5 – 2. 5?
 Will POC Results Match the Lab? (Probably Not) but it WILL Correlate
Will POC Results Match the Lab? (Probably Not) but it WILL Correlate
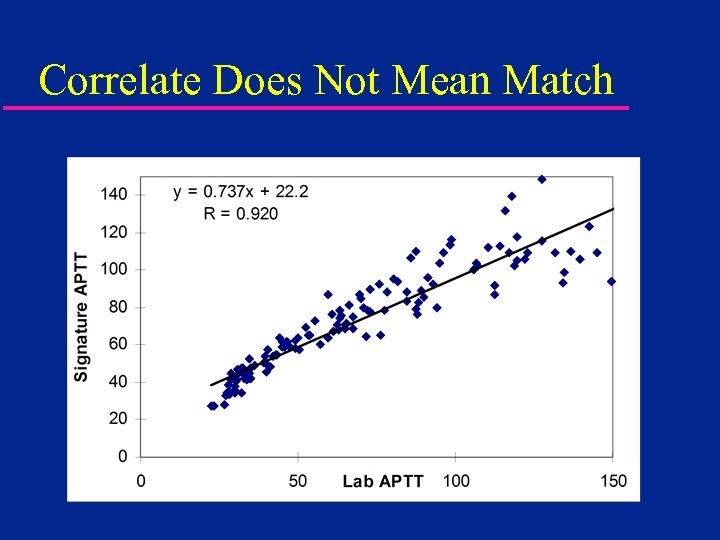 Correlate Does Not Mean Match
Correlate Does Not Mean Match
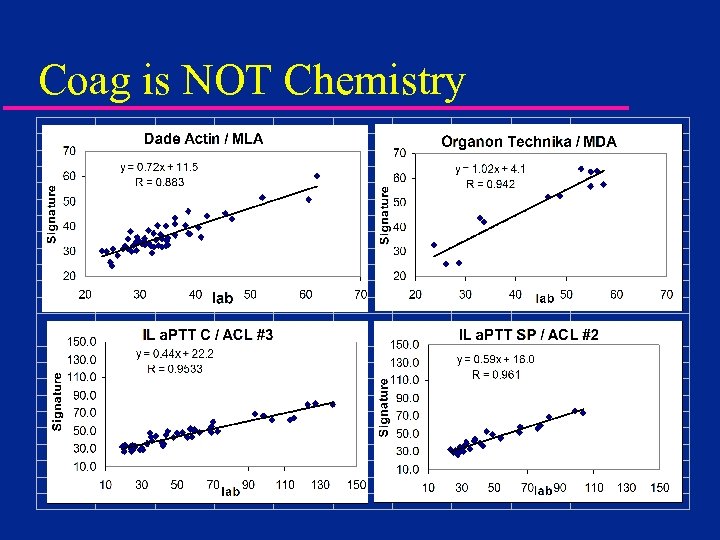 Coag is NOT Chemistry
Coag is NOT Chemistry
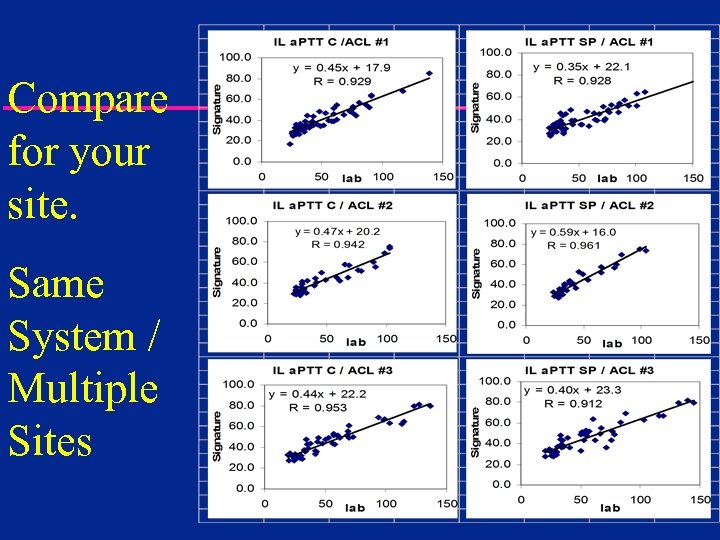 Compare for your site. Same System / Multiple Sites
Compare for your site. Same System / Multiple Sites
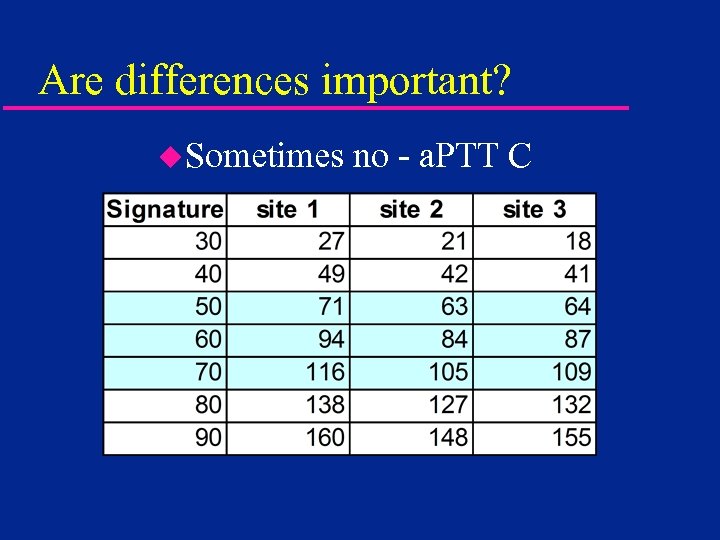 Are differences important? u. Sometimes no - a. PTT C
Are differences important? u. Sometimes no - a. PTT C
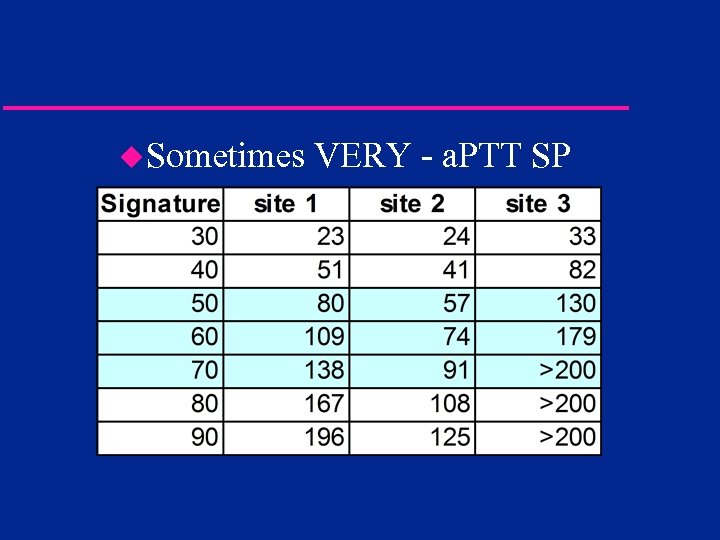 u. Sometimes VERY - a. PTT SP
u. Sometimes VERY - a. PTT SP
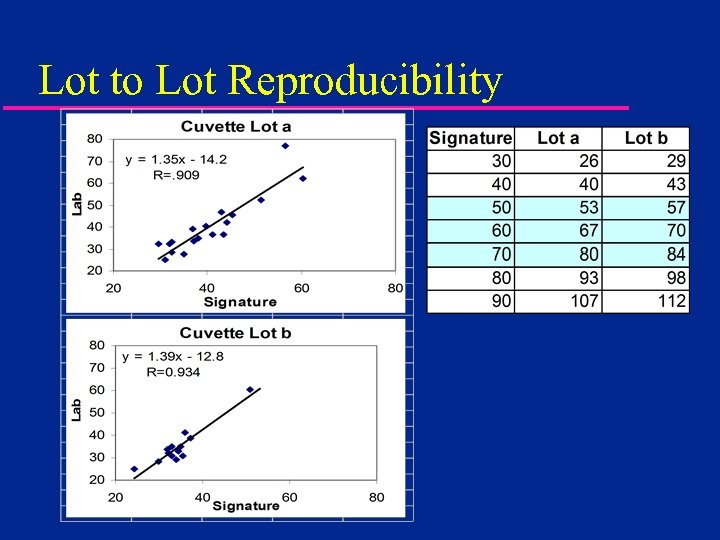 Lot to Lot Reproducibility
Lot to Lot Reproducibility
 Clinical Applications u. Operating Room – Cardiac Surgery – Interventional Cardiology and Radiology u. Critical Care u. Satellite Sites – Dialysis – ECMO – Emergency Room – Anticoagulation Clinic
Clinical Applications u. Operating Room – Cardiac Surgery – Interventional Cardiology and Radiology u. Critical Care u. Satellite Sites – Dialysis – ECMO – Emergency Room – Anticoagulation Clinic
 Dialysis / ECMO u. ACT (or nothing in dialysis) – Majority use P 214 glass activated ACT – Some use ACT-LR; Hemo. Tec LR ACT u. Better Control of Anticoagulation Leads to Increased Dialyzer Reuse – Potential for Long Term Cost Savings – No Compromise in Dialysis Efficacy (Kt/V) » Ouseph, R. et. al. Am J Kidney Dis 35: 89 -94; 2000
Dialysis / ECMO u. ACT (or nothing in dialysis) – Majority use P 214 glass activated ACT – Some use ACT-LR; Hemo. Tec LR ACT u. Better Control of Anticoagulation Leads to Increased Dialyzer Reuse – Potential for Long Term Cost Savings – No Compromise in Dialysis Efficacy (Kt/V) » Ouseph, R. et. al. Am J Kidney Dis 35: 89 -94; 2000
 Emergency Room u ACT; a. PTT; PT; Fibrinogen u Immediate Identification of Coagulopathies – Optimization of Critical Decision Pathways u ACT Allows Early Detection of Traumatic Coagulopathy – Allows Early Treatment Decisions – Aids Damage Control Decisions » Aucar, J. et. al. 1998 SW Surgeons Congress u Optimize Staffing During Off Hours
Emergency Room u ACT; a. PTT; PT; Fibrinogen u Immediate Identification of Coagulopathies – Optimization of Critical Decision Pathways u ACT Allows Early Detection of Traumatic Coagulopathy – Allows Early Treatment Decisions – Aids Damage Control Decisions » Aucar, J. et. al. 1998 SW Surgeons Congress u Optimize Staffing During Off Hours
 Anticoagulation Clinics u Results Available While Patient is Present – Improved Anticoagulation Management – Improved Standard of Care – Staff Efficiency u Immediate Retesting (if needed) – Fingerstick Sampling u Same System for Clinic and Home Bound Patients – Standardized ISI / PT normal » Test System Specific
Anticoagulation Clinics u Results Available While Patient is Present – Improved Anticoagulation Management – Improved Standard of Care – Staff Efficiency u Immediate Retesting (if needed) – Fingerstick Sampling u Same System for Clinic and Home Bound Patients – Standardized ISI / PT normal » Test System Specific
 Anticoagulation Clinics u. Potential for Self-Testing – High Risk Patients – Patients Who Travel Frequently – Home-Bound – Patients in Rural Areas Far from Clinic u. Improved Outcomes Through More Frequent Testing
Anticoagulation Clinics u. Potential for Self-Testing – High Risk Patients – Patients Who Travel Frequently – Home-Bound – Patients in Rural Areas Far from Clinic u. Improved Outcomes Through More Frequent Testing
 Will POC Results Match the Lab? (It will be a lot closer than for a. PTT) but it WILL Correlate
Will POC Results Match the Lab? (It will be a lot closer than for a. PTT) but it WILL Correlate
 How to Compare INR Results u Lower dose? u Keep same dose? u Raise Dose? u Test Again? u Test more often?
How to Compare INR Results u Lower dose? u Keep same dose? u Raise Dose? u Test Again? u Test more often?
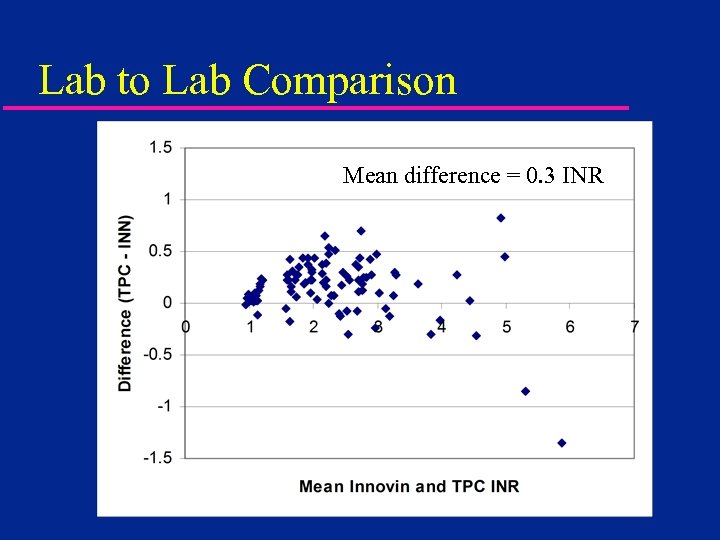 Lab to Lab Comparison Mean difference = 0. 3 INR
Lab to Lab Comparison Mean difference = 0. 3 INR
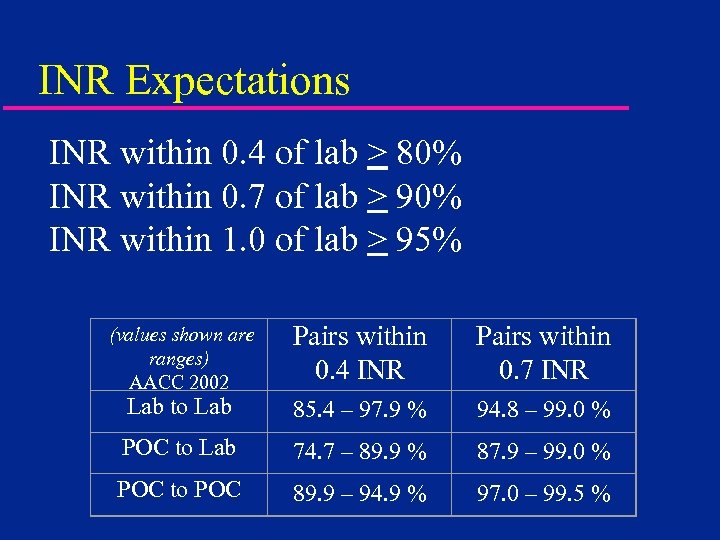 INR Expectations INR within 0. 4 of lab > 80% INR within 0. 7 of lab > 90% INR within 1. 0 of lab > 95% Pairs within 0. 4 INR Pairs within 0. 7 INR Lab to Lab 85. 4 – 97. 9 % 94. 8 – 99. 0 % POC to Lab 74. 7 – 89. 9 % 87. 9 – 99. 0 % POC to POC 89. 9 – 94. 9 % 97. 0 – 99. 5 % (values shown are ranges) AACC 2002
INR Expectations INR within 0. 4 of lab > 80% INR within 0. 7 of lab > 90% INR within 1. 0 of lab > 95% Pairs within 0. 4 INR Pairs within 0. 7 INR Lab to Lab 85. 4 – 97. 9 % 94. 8 – 99. 0 % POC to Lab 74. 7 – 89. 9 % 87. 9 – 99. 0 % POC to POC 89. 9 – 94. 9 % 97. 0 – 99. 5 % (values shown are ranges) AACC 2002
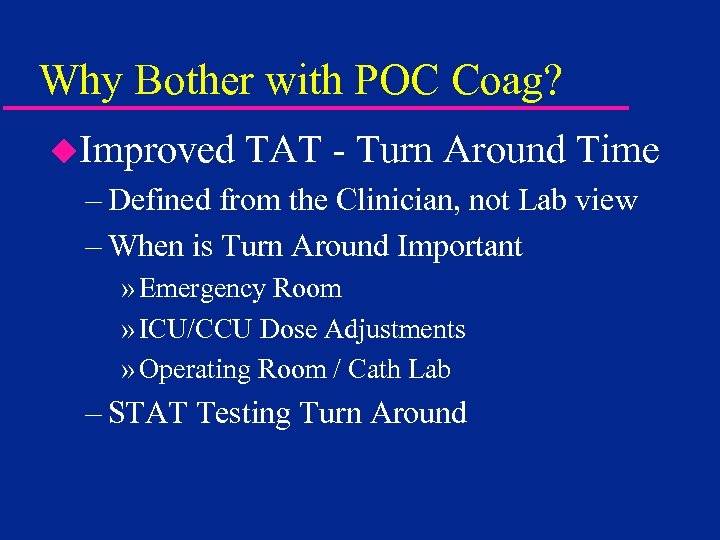 Why Bother with POC Coag? u. Improved TAT - Turn Around Time – Defined from the Clinician, not Lab view – When is Turn Around Important » Emergency Room » ICU/CCU Dose Adjustments » Operating Room / Cath Lab – STAT Testing Turn Around
Why Bother with POC Coag? u. Improved TAT - Turn Around Time – Defined from the Clinician, not Lab view – When is Turn Around Important » Emergency Room » ICU/CCU Dose Adjustments » Operating Room / Cath Lab – STAT Testing Turn Around
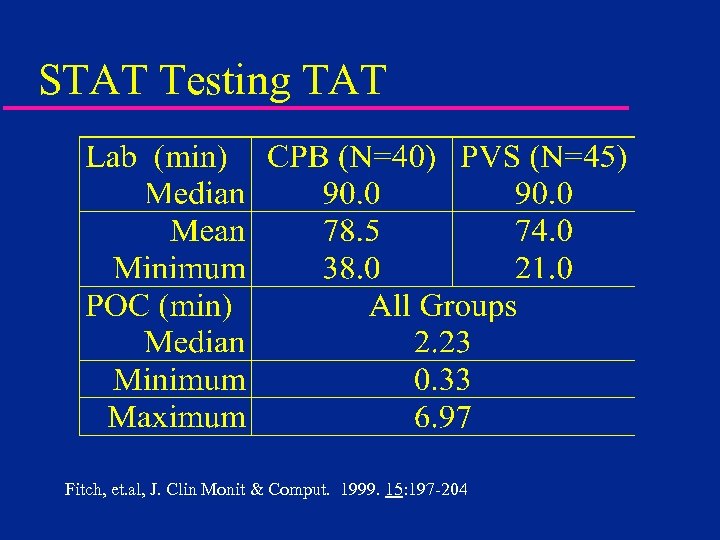 STAT Testing TAT Fitch, et. al, J. Clin Monit & Comput. 1999. 15: 197 -204
STAT Testing TAT Fitch, et. al, J. Clin Monit & Comput. 1999. 15: 197 -204
 Standardized Clinical Interpretation u Defined Assay Sensitivity – Requires Lot to Lot Reproducibility u Defined Reagent Variability – Identical Instrumentation and Reagents at All Testing Sites u Defined Critical Clinical Decision Points – No Change of Normal Ranges or Target Times Between Lots of Test Reagents or Testing Locations
Standardized Clinical Interpretation u Defined Assay Sensitivity – Requires Lot to Lot Reproducibility u Defined Reagent Variability – Identical Instrumentation and Reagents at All Testing Sites u Defined Critical Clinical Decision Points – No Change of Normal Ranges or Target Times Between Lots of Test Reagents or Testing Locations
 What’s the catch? 1. 2. Regulatory compliance Connectivity
What’s the catch? 1. 2. Regulatory compliance Connectivity
 Regulatory compliance - Who sets the rules? – JCAHO » Joint Commission on Accreditation of Health Care Orgs – CAP » College of American Pathologists – FDA » Food and Drug Administration » CDRH u Center for Devices and Radiological Health – CMS » Centers for Medicare and Medicaid Services – CDC » Centers for Disease Control
Regulatory compliance - Who sets the rules? – JCAHO » Joint Commission on Accreditation of Health Care Orgs – CAP » College of American Pathologists – FDA » Food and Drug Administration » CDRH u Center for Devices and Radiological Health – CMS » Centers for Medicare and Medicaid Services – CDC » Centers for Disease Control
 CLIA Applies to ALL Testing Areas u. Central Laboratory u. Satellite Labs – Critical Care – Surgical Suite u. Clinics u. Bedside testing u. Doctor’s office
CLIA Applies to ALL Testing Areas u. Central Laboratory u. Satellite Labs – Critical Care – Surgical Suite u. Clinics u. Bedside testing u. Doctor’s office
 CLIA Regulations for Coagulation u Central Laboratory can hold the CLIA license – Satellites can have independent licensure u Moderately Complex tests – Except – Pro. Time, Coaguchek, INRatio are waived u Requires – Certified Laboratory Director – Record Keeping – Training – Quality Policy
CLIA Regulations for Coagulation u Central Laboratory can hold the CLIA license – Satellites can have independent licensure u Moderately Complex tests – Except – Pro. Time, Coaguchek, INRatio are waived u Requires – Certified Laboratory Director – Record Keeping – Training – Quality Policy
 Implementing POC coag requires: u Method Validation - accuracy – Comparison to current standard » NCCLS Guideline EP-09 recommends 40 samples – Linearity may be used if no current standard – Is assay performance appropriate to clinical needs? u Precision – Controls may be used to establish within and between run variability u Training – Document training of all personnel » high school equivalence or higher education level – competency evaluations at predetermined intervals
Implementing POC coag requires: u Method Validation - accuracy – Comparison to current standard » NCCLS Guideline EP-09 recommends 40 samples – Linearity may be used if no current standard – Is assay performance appropriate to clinical needs? u Precision – Controls may be used to establish within and between run variability u Training – Document training of all personnel » high school equivalence or higher education level – competency evaluations at predetermined intervals
 Implementing POC coag requires: u Linearity NOT required for coag u Calibration “does not apply to unit test systems that cannot be adjusted” u Calibration verification • Current assumption: – Equivalent to CAP POC. 05450 » If the laboratory has more than one method-system for performing tests for a given analyte, are they checked against each other at least twice a year for correlation of patient results? – CLIA requires at least 3 point check
Implementing POC coag requires: u Linearity NOT required for coag u Calibration “does not apply to unit test systems that cannot be adjusted” u Calibration verification • Current assumption: – Equivalent to CAP POC. 05450 » If the laboratory has more than one method-system for performing tests for a given analyte, are they checked against each other at least twice a year for correlation of patient results? – CLIA requires at least 3 point check
 New CLIA Regulations u Work – – in progress New rules published January 2003 Rules in effect March 23, 2003 Interpretive guidelines published Jan 2004 Inspections using new regulations now » 2 year grace period to adapt new rules » Ends Jan 2005 u Quality Assessment Program - Lab Responsibilities – Establish & follow policies/procedures addressing ongoing QA activity. – Take corrective actions as necessary. » Review their effectiveness. » Revise policies/procedures as necessary to prevent recurrence. – Communicate to staff. – Document all assessment activities.
New CLIA Regulations u Work – – in progress New rules published January 2003 Rules in effect March 23, 2003 Interpretive guidelines published Jan 2004 Inspections using new regulations now » 2 year grace period to adapt new rules » Ends Jan 2005 u Quality Assessment Program - Lab Responsibilities – Establish & follow policies/procedures addressing ongoing QA activity. – Take corrective actions as necessary. » Review their effectiveness. » Revise policies/procedures as necessary to prevent recurrence. – Communicate to staff. – Document all assessment activities.
 New CLIA Regulations u Proficiency testing – Changed consensus for PT program grading from 90% to 80%. u Quality Assessment replaces Quality Assurance. – Quality Assessment is interspersed throughout the regulation. – Creates one set of nonwaived QC requirements. u Subpart K - Quality System for Nonwaived Testing – Laboratory is ultimately responsible for ensuring that all components of the analytic process are monitored. – Each laboratory that performs nonwaived testing must meet the applicable analytic systems requirements; unless HHS approves a procedure, specified in the Interpretive Guidelines, that provides equivalent quality testing
New CLIA Regulations u Proficiency testing – Changed consensus for PT program grading from 90% to 80%. u Quality Assessment replaces Quality Assurance. – Quality Assessment is interspersed throughout the regulation. – Creates one set of nonwaived QC requirements. u Subpart K - Quality System for Nonwaived Testing – Laboratory is ultimately responsible for ensuring that all components of the analytic process are monitored. – Each laboratory that performs nonwaived testing must meet the applicable analytic systems requirements; unless HHS approves a procedure, specified in the Interpretive Guidelines, that provides equivalent quality testing
 Equivalent Quality Testing u Traditional: – Testing two levels of external control materials each day of testing – Except coag and blood gases » every 8 hours of use u Equivalent QC Options – #2 -Test systems with internal/procedural controls that monitor a portion of the analytic components, and if the lab successfully completes a thirty day evaluation process, the lab may reduce the frequency of external quality control materials to once per calendar week.
Equivalent Quality Testing u Traditional: – Testing two levels of external control materials each day of testing – Except coag and blood gases » every 8 hours of use u Equivalent QC Options – #2 -Test systems with internal/procedural controls that monitor a portion of the analytic components, and if the lab successfully completes a thirty day evaluation process, the lab may reduce the frequency of external quality control materials to once per calendar week.
 Equivalent Quality Testing u Option #2 – Perform the test system’s internal control procedure(s) in accordance with the manufacturer’s instructions (but not less frequently than once each day of testing) and test two levels of external control material daily for 30 consecutive days of testing. u EQC AND LQC daily (NOT every 8 hours) for 30 days – Then OK to use EQC daily, LQC weekly » Unless manufacturer requires more – Send comments to: Judith Yost » Director, Division of Laboratory Services, CMS » JYost@cms. hhs. gov » (410) 786 -3407
Equivalent Quality Testing u Option #2 – Perform the test system’s internal control procedure(s) in accordance with the manufacturer’s instructions (but not less frequently than once each day of testing) and test two levels of external control material daily for 30 consecutive days of testing. u EQC AND LQC daily (NOT every 8 hours) for 30 days – Then OK to use EQC daily, LQC weekly » Unless manufacturer requires more – Send comments to: Judith Yost » Director, Division of Laboratory Services, CMS » JYost@cms. hhs. gov » (410) 786 -3407
 Routine Quality Control u Instrument Performance Verification – Electronic Quality Control with Numeric Output – Two levels per 8 hour shift (CLIA reg) u Assay Performance Verification – Wet QC as per Manufacturer’s Recommendation » Varies by system u No external QC required for Pro. Time / INRatio in most States » Within system may vary by waived or moderate complexity licensure
Routine Quality Control u Instrument Performance Verification – Electronic Quality Control with Numeric Output – Two levels per 8 hour shift (CLIA reg) u Assay Performance Verification – Wet QC as per Manufacturer’s Recommendation » Varies by system u No external QC required for Pro. Time / INRatio in most States » Within system may vary by waived or moderate complexity licensure
 Ensuring Compliance u Required identification – Mandatory operator ID » Password control » Reuse IDs for some applications – Mandatory patient ID » Reuse IDs for some applications u Lockout – Force QC at specific times » QC must pass to run patient samples – Lockout non-compliant or untrained operators – Disallow specific assays
Ensuring Compliance u Required identification – Mandatory operator ID » Password control » Reuse IDs for some applications – Mandatory patient ID » Reuse IDs for some applications u Lockout – Force QC at specific times » QC must pass to run patient samples – Lockout non-compliant or untrained operators – Disallow specific assays
 Connectivity u. Multiple definitions – Download to computer » To LIS or to HIS or to both or to data management software » Real time and / or batch » QC data, patient data, or both
Connectivity u. Multiple definitions – Download to computer » To LIS or to HIS or to both or to data management software » Real time and / or batch » QC data, patient data, or both
 Connectivity u. Bidirectional communication – Send data to instrument » Reset lockouts » Load configurations u Operator tables u QC frequency u QC ranges u Reuse availability » Vary configuration by clinical setting
Connectivity u. Bidirectional communication – Send data to instrument » Reset lockouts » Load configurations u Operator tables u QC frequency u QC ranges u Reuse availability » Vary configuration by clinical setting
 Solutions u System specific configuration – e. g. HCM for Signature+ HRDM for Response u System specific data management – e. g. Report. Maker for Signature / + HRDM for Response Rapid. Link for Bayer Rapid. Point Data. Care for Roche Coagu. Chek / S / DM / Pro u Link to systems designed for glucose – Abbott and Roche state they will connect with any POC instrumentation
Solutions u System specific configuration – e. g. HCM for Signature+ HRDM for Response u System specific data management – e. g. Report. Maker for Signature / + HRDM for Response Rapid. Link for Bayer Rapid. Point Data. Care for Roche Coagu. Chek / S / DM / Pro u Link to systems designed for glucose – Abbott and Roche state they will connect with any POC instrumentation
 Solutions u Manufacturer neutral interface – MAS RALS-plus – Telcor Quick Serv – Manufacturer works with interface supplier to ensure compatibility – Interface supplier works with LIS / HIS supplier to ensure compatibility – Likely more options as CIC guidelines implemented (NCCLS POCT 1 -A)
Solutions u Manufacturer neutral interface – MAS RALS-plus – Telcor Quick Serv – Manufacturer works with interface supplier to ensure compatibility – Interface supplier works with LIS / HIS supplier to ensure compatibility – Likely more options as CIC guidelines implemented (NCCLS POCT 1 -A)
 Why Bother with POC Coag? u. Once compliance issues addressed – – Improved Clinical Outcome – Reduced LOS – Length of Stay – Improved, timely patient care
Why Bother with POC Coag? u. Once compliance issues addressed – – Improved Clinical Outcome – Reduced LOS – Length of Stay – Improved, timely patient care


