5f01b0d423ef10fdc0bcc75e5813eda7.ppt
- Количество слайдов: 38
Coagulation Screening Test Mr. Mohammed A. Jabar
PREANALYTICAL VARIABLES INCLUDING SAMPLE COLLECTION Many misleading results in blood coagulation arise not from errors in testing but from carelessness in the preanalytical phase. Ideally the results of blood tests should accurately reflect the values in vivo. When blood is withdrawn from a vessel, changes begin to take place in the components of blood coagulation. Some occur almost immediately, such as platelet activation and the initiation of the clotting mechanism dependent on surface contact.

Sample Collection Anticoagulant of choice 3. 8% or 3. 2% Sodium Citrate 3. 2 % Preferred as the standard measure due to stability and closeness to the plasma osmolality Anticoagulant/blood ratio is critical (1: 9) Exact amount of blood must be drawn. No short draws are acceptable, this will falsely increase results due to presence of too much anticoagulant CLSI guideline is +/- 10 % of fill line Purpose of the anticoagulant is to bind or chelate calcium to prevent clotting of specimen
Sample Collection Other anticoagulants, including oxalate, heparin, and EDTA, are unacceptable. The labile factors (factors V and VIII) are unstable in oxalate, whereas heparin and EDTA directly inhibit the coagulation process and interfere with end-point determinations. Additional benefits of trisodium citrate are that the calcium ion is neutralised more rapidly in citrate, and APTT tests are more sensitive to the presence of heparin.

Sample Collection Sidenote: Samples with High hematocrits NCCLS recommends adjusting anticoagulant ratio for patients with hematocrits exceeding 55% High hematocrits may cause falsely prolonged test results due to an over- anticoagulated sample Formula correction achieves a 40% hematocrit
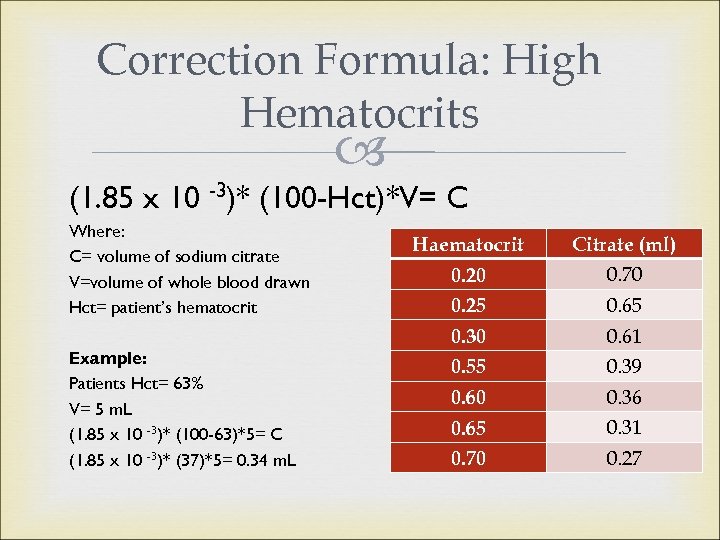
Correction Formula: High Hematocrits (1. 85 x 10 -3)* (100 -Hct)*V= C Where: C= volume of sodium citrate V=volume of whole blood drawn Hct= patient’s hematocrit Example: Patients Hct= 63% V= 5 m. L (1. 85 x 10 -3)* (100 -63)*5= C (1. 85 x 10 -3)* (37)*5= 0. 34 m. L Haematocrit Citrate (ml) 0. 20 0. 70 0. 25 0. 65 0. 30 0. 61 0. 55 0. 39 0. 60 0. 36 0. 65 0. 31 0. 70 0. 27

Site Selection Untraumatic venipuncture is required Traumatic venipunctures release tissue factor and initiate coagulation Fingersticks/Heelsticks are not allowed Indwelling IV line draws are discouraged Contain heparin Falsely increased results Order of Draw Evacuated tube system Blue top is 1 st or 2 nd tube. If 2 nd tube drawn, 1 st top must be anticoagulant free (i. e. red top)

Storage Requirements Prothrombin Time: PT ◦ Uncentrifuged or centrifuged with plasma remaining on top of cells in unopened tube kept at 2 -4 o. C or 18 -24 o C must be tested within 24 hours of collection Activated Partial Thrombin Time: APTT ◦ Uncentrifuged or centrifuged with plasma remaining on top of cells in unopened tube kept at 2 -4 o. C or 18 -24 o C must be tested within 4 hours of collection

Storage Requirements Other Assays Fibrinogen, Thrombin Time, Factor Assays Centrifuged with plasma remaining on top of cells in unopened tube kept at 2 -4 o. C or 18 -24 o. C must be tested within 4 hours of collection

Storage Requirements Other general notes Perform coagulation tests ASAP Specimen may deteriorate rapidly (especially factors V and VIII) If the testing is not completed within specified times, plasma should be removed from the cells and placed in a frost freezer - 20 o. C for two weeks -70 o. C for six months

Transportation of Specimen Send specimen on ice OR deliver to lab ASAP Separate cells from plasma immediately via centrifugation
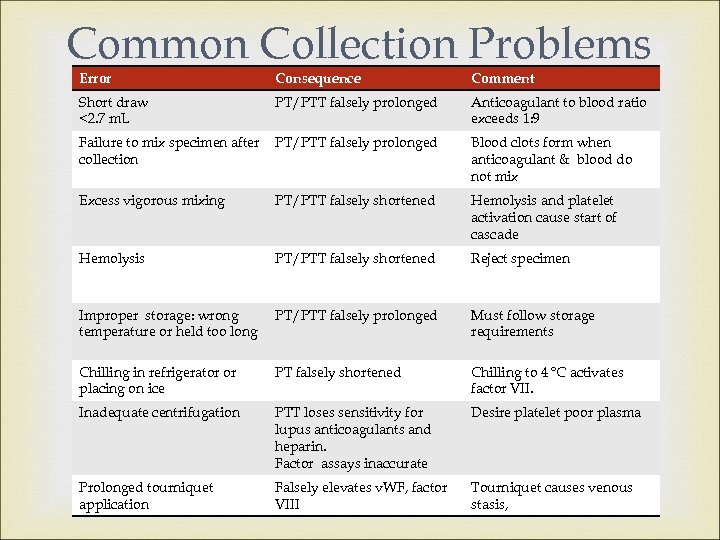
Common Collection Problems Error Consequence Comment Short draw <2. 7 m. L PT/PTT falsely prolonged Anticoagulant to blood ratio exceeds 1: 9 Failure to mix specimen after collection PT/PTT falsely prolonged Blood clots form when anticoagulant & blood do not mix Excess vigorous mixing PT/PTT falsely shortened Hemolysis and platelet activation cause start of cascade Hemolysis PT/PTT falsely shortened Reject specimen Improper storage: wrong temperature or held too long PT/PTT falsely prolonged Must follow storage requirements Chilling in refrigerator or placing on ice PT falsely shortened Chilling to 4 o. C activates factor VII. Inadequate centrifugation PTT loses sensitivity for lupus anticoagulants and heparin. Factor assays inaccurate Desire platelet poor plasma Prolonged tourniquet application Falsely elevates v. WF, factor VIII Tourniquet causes venous stasis,
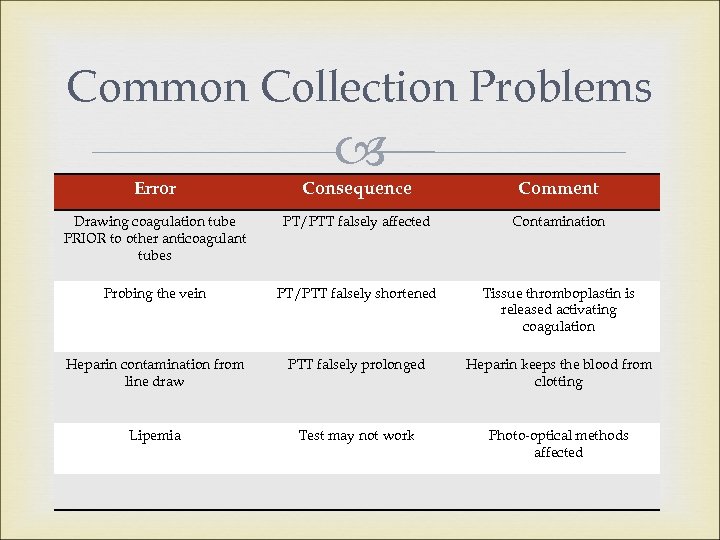
Common Collection Problems Error Consequence Comment Drawing coagulation tube PRIOR to other anticoagulant tubes PT/PTT falsely affected Contamination Probing the vein PT/PTT falsely shortened Tissue thromboplastin is released activating coagulation Heparin contamination from line draw PTT falsely prolonged Heparin keeps the blood from clotting Lipemia Test may not work Photo-optical methods affected
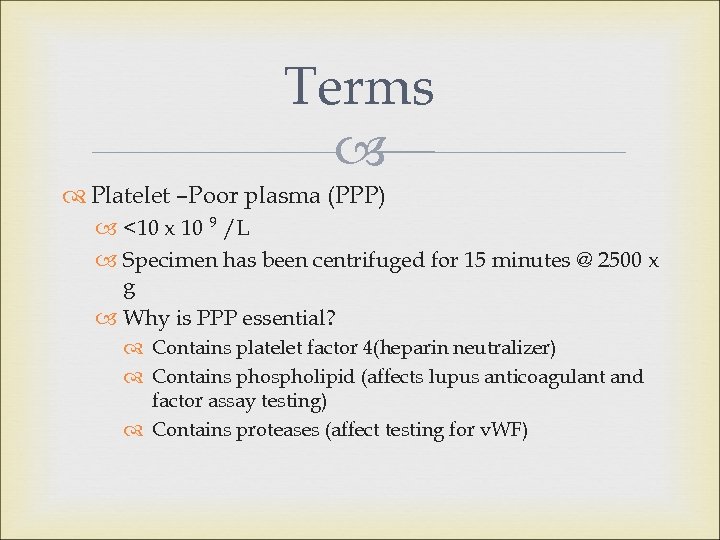
Terms Platelet –Poor plasma (PPP) <10 x 10 9 /L Specimen has been centrifuged for 15 minutes @ 2500 x g Why is PPP essential? Contains platelet factor 4(heparin neutralizer) Contains phospholipid (affects lupus anticoagulant and factor assay testing) Contains proteases (affect testing for v. WF)

Terms Platelet-Rich plasma(PRP) Used in platelet function studies 200 -300 x 10 9 /L Specimen has been centrifuged for 10 minutes @ 200 x g
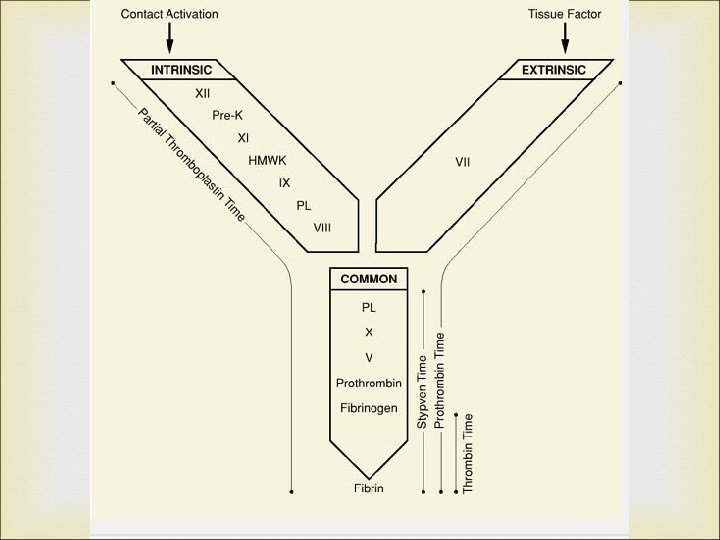
Protime /Prothrombin(PT) The prothrombin time is therefore the time required for the plasma to clot after an excess of thromboplastin and an optimal concentration of calcium have been added. The PT measures functional activity of the extrinsic and common pathways. The PT evaluates patients suspected of having an inherited or acquired deficiency in these pathways.
PT Reagent Calcium ions and Thromboplastin from brain tissue (Rabbit). Thromboplastin (Tissue Factor) protein-lipid complex found in tissues outside blood vessels. Thromboplastins were originally tissue extracts obtained from different species and different organs containing tissue factor and phospholipid. Because of the potential hazard of viral and other infections from handling human brain, it should no longer be used as a source of thromboplastin. The majority of animal thromboplastins now in use are extracts of rabbit brain or lung.
PT Reagent The introduction of recombinant thromboplastins has resulted in a move away from rabbit brain thromboplastin. They are manufactured using recombinant human tissue factor produced in Escherichia coli and synthetic phospholipids, which do not contain any other clotting factors such as prothrombin, factor VII, and factor X. Therefore they are highly sensitive to factor deficiencies and oral anticoagulant–treated patient plasma samples and have an International Sensitivity Index (ISI) close to 1.
PT Reagent Calibration standard PT reagent Reagents are calibrated against established by the WHO. ISI = International Sensitivity Index. ISI is assigned by the manufacturer for each lot of reagent using reference material from WHO. The lower the ISI the more sensitive the Reagent 1. ISI of 1. 8 to 2. 4 = Low sensitivity 2. ISI of 1. 4 to 1. 8 = Average sensitivity 3. ISI 1. 0 to 1. 4 = High Sensitivity

Principle: The procedure uses a tissue thromboplastin reagent with Ca. Cl (Calcium Chloride) to provide a one step procedure for evaluating plasma clotting. This test was devised on the assumption that when an optimal amount of calcium and an excess of thromboplastin are added to decalcified plasma, the rate of coagulation depends on the concentration of prothrombin in the plasma.

Principle: The normal values for the prothrombin time range from 10. 0 to 13. 0 seconds. An elevated prothrombin time may indicate the presence of 1. Vitamin K deficiency, 2. DIC, 3. liver disease, 4. Presence of FSP’s

Principle: 5. a deficiency in one or more of the following factors: 1) 2) 3) 4) 5) 6) Factor I (Fibrinogen) Factor II (Prothrombin) Factor V (Proaccelerin, Labile Factor) Factor VII (Proconvertin, Stable Factor) Factor X (Stuart-Prower Factor) Factor XIII (Fibrin Stabilizing Factor) 6. In addition, inhibitors can cause prolonged PT’s.

When is it ordered? Used to monitor oral anticoagulant therapy (Warfarin / Coumadin). When a patient who is not taking anti-coagulant drugs has signs or symptoms of a bleeding disorder When a patient is to undergo an invasive medical procedure, such as surgery, to ensure normal clotting ability.
Clot formation can be detected by 1. Manual or Using, semiautomated or automated devices. 2. Electromechanical methods. 3. Optical Method. 4. Electrochemical Method.
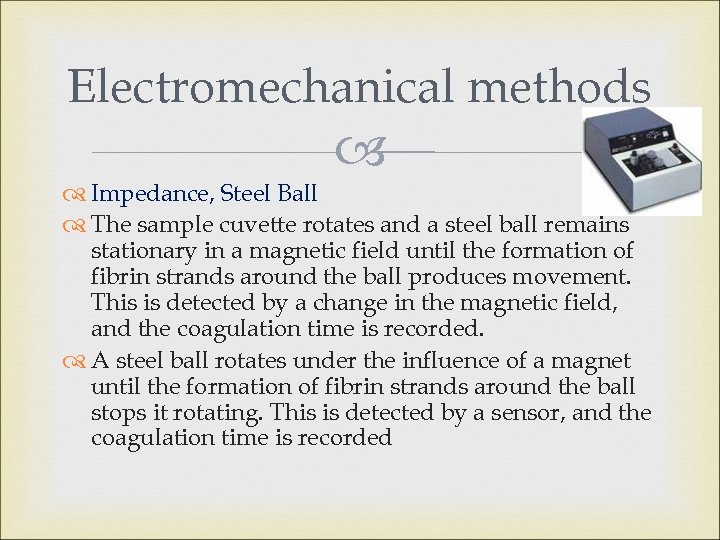
Electromechanical methods Impedance, Steel Ball The sample cuvette rotates and a steel ball remains stationary in a magnetic field until the formation of fibrin strands around the ball produces movement. This is detected by a change in the magnetic field, and the coagulation time is recorded. A steel ball rotates under the influence of a magnet until the formation of fibrin strands around the ball stops it rotating. This is detected by a sensor, and the coagulation time is recorded

Photo Optical A. Scattered Light Detection for Clotting Assays (660 nm) The turbidity during the formation of a fibrin clot is measured as an increase in scattered light intensity when exposed to light at a wavelength of 660 nm. B. Transmitted Light Detection for Chromogenic Assays (405 nm, 575 nm, 800 nm) Colour production leads to a change in light absorbance, which is detected as a change in transmitted light. Over time the change in absorbance per minute is calculated (Δ OD/min). Various wavelengths can be used such as 405 nm, 575 nm, and 800 nm. C. Transmitted Light Detection for Immunoassays (405 nm, 575 nm, 800 nm)
Electrochemical A. INRatio Meter (Hemosense) Near Patient Testing Devices The INRatio single-use test strip is made of laminated layers of transparent plastic. Each test strip has a sample well where blood is applied, three channels through which the blood sample flows to reach the testing areas, reagents to start the coagulation process, and electrodes that interface with the INRatio meter. The device detects a change in electrical resistance when blood clots.
Procedure Reconstitute tissue thromboplastin according to instructions. Label the thromboplastin with the time, date and initials. The thromboplastin reagent is stabile for 7 days after reconstitution. Allow to sit 10 -15 minutes, then invert gently several times. Mix well prior to pipetting any of this reagent at any step in this procedure. Pipet 1 -2 mls, using a plastic pipet, of the tissue thromboplastin-calcium chloride reagent ( PT reagent) into a glass test tube and place in a 37 water bath incubator.
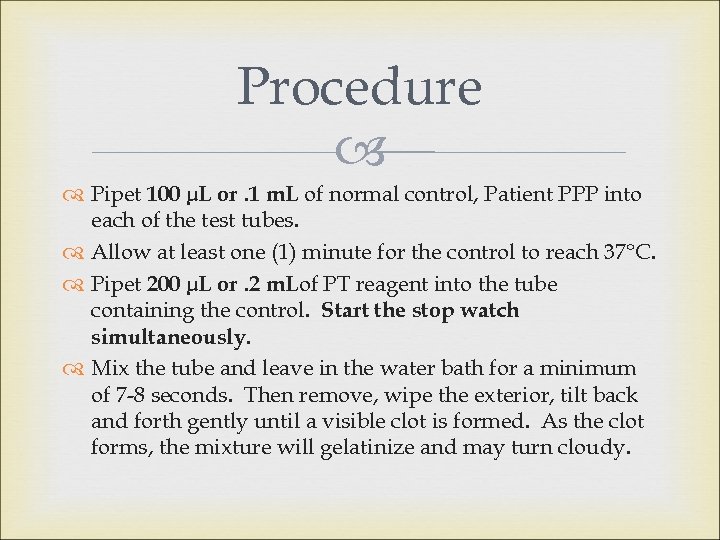
Procedure Pipet 100 µL or. 1 m. L of normal control, Patient PPP into each of the test tubes. Allow at least one (1) minute for the control to reach 37°C. Pipet 200 µL or. 2 m. Lof PT reagent into the tube containing the control. Start the stop watch simultaneously. Mix the tube and leave in the water bath for a minimum of 7 -8 seconds. Then remove, wipe the exterior, tilt back and forth gently until a visible clot is formed. As the clot forms, the mixture will gelatinize and may turn cloudy.

Procedure Stop the stop watch immediately when the clot begins to form and record the time in seconds. Carry out 1 significant figure passed the decimal point. For example, if your result is 12. 23 seconds, report as 12. 2 seconds. Repeat the procedure for the second run of normal control. Record the time.

Procedure If the results from run 1 and run 2 are within + 1 second from each other, average the two results and report with appropriate units. For manual PT, results should match within 1. 0 second (if result is less than 20 seconds). Results over 20 seconds should match within 2. 0 seconds. If results are not within required limits, a third run should be performed and average the two that match within acceptable limits. Be sure and cross out any values you are not using for the final calculation. Include measurement unit of seconds on report sheet.
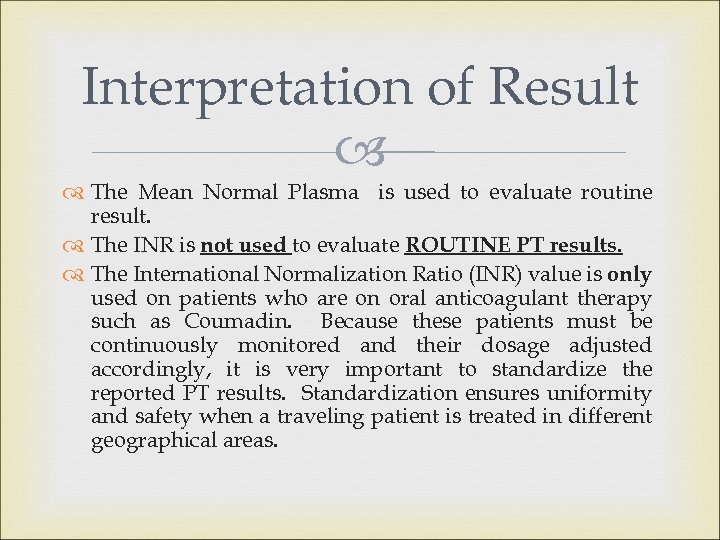
Interpretation of Result The Mean Normal Plasma is used to evaluate routine result. The INR is not used to evaluate ROUTINE PT results. The International Normalization Ratio (INR) value is only used on patients who are on oral anticoagulant therapy such as Coumadin. Because these patients must be continuously monitored and their dosage adjusted accordingly, it is very important to standardize the reported PT results. Standardization ensures uniformity and safety when a traveling patient is treated in different geographical areas.
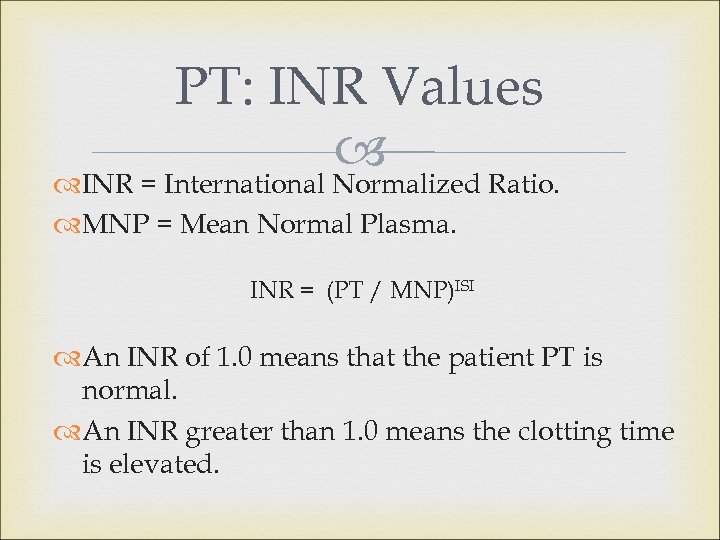
PT: INR Values INR = International Normalized Ratio. MNP = Mean Normal Plasma. INR = (PT / MNP)ISI An INR of 1. 0 means that the patient PT is normal. An INR greater than 1. 0 means the clotting time is elevated.
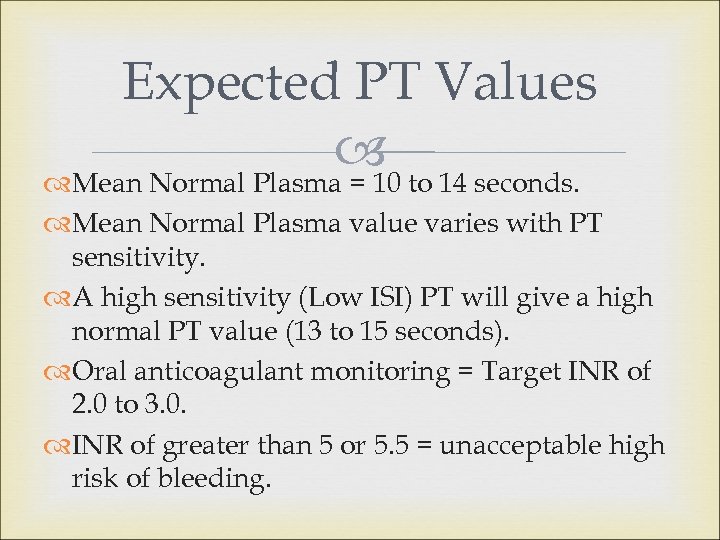
Expected PT Values to 14 seconds. Mean Normal Plasma = 10 Mean Normal Plasma value varies with PT sensitivity. A high sensitivity (Low ISI) PT will give a high normal PT value (13 to 15 seconds). Oral anticoagulant monitoring = Target INR of 2. 0 to 3. 0. INR of greater than 5 or 5. 5 = unacceptable high risk of bleeding.
Sources of Error 1. Associated with specimen a. Inappropropriate ratio of anticoagulant to blood b. Failure to correct citrate volume if hematocrit > 55% c. Clotted, hemolyzed or lipemic samples d. Lack of PPP e. Delay in testing or processing f. Inappropriate storage

Sources of Error 1. Associated with storage a. Incorrect preparation of reagents b. Failure to properly store reagents c. Use of reagents beyond reconstituted stability time or expiration date d. Contaminated reagents

Sources of Error 1. Associated with procedure a. Incorrect temperature b. Incorrect incubation times c. Incorrect volumes of sample, reagents or both
5f01b0d423ef10fdc0bcc75e5813eda7.ppt