2ef1033720710e07bfb34faa869009ce.ppt
- Количество слайдов: 33
 Co-development of Devices and Drugs in Taiwan Li-Ling Liu Deputy Director-General Bureau of Pharmaceutical Affairs Department of Health Taiwan
Co-development of Devices and Drugs in Taiwan Li-Ling Liu Deputy Director-General Bureau of Pharmaceutical Affairs Department of Health Taiwan
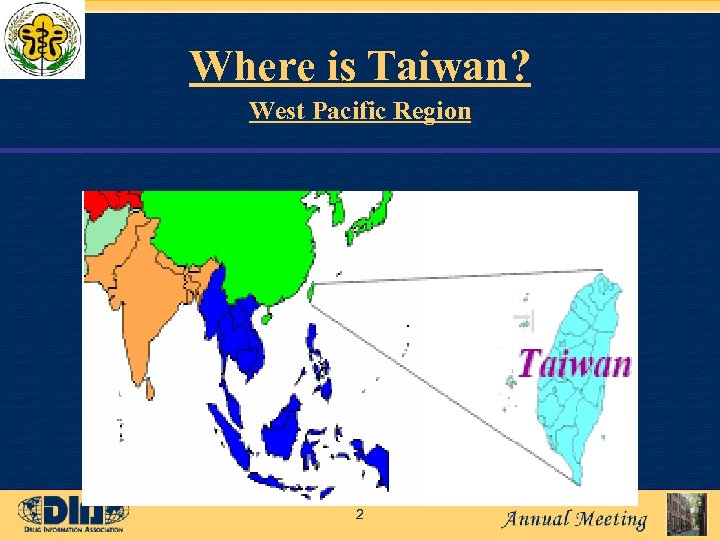 Where is Taiwan? West Pacific Region 2
Where is Taiwan? West Pacific Region 2
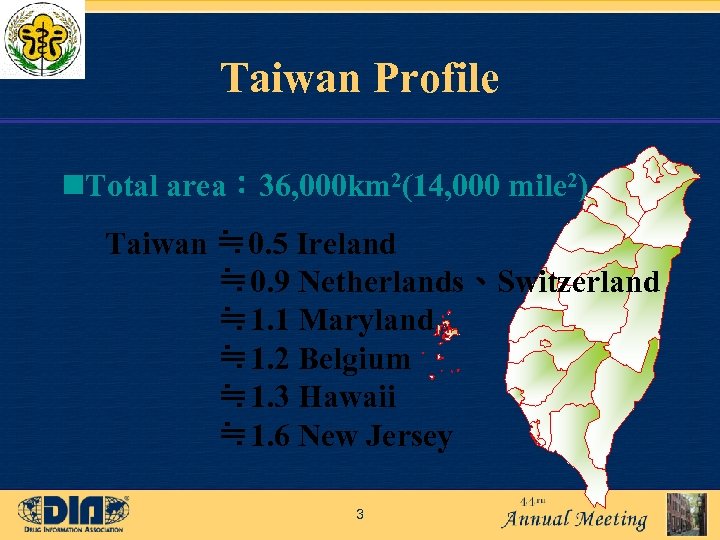 Taiwan Profile n. Total area: 36, 000 km 2(14, 000 mile 2) Taiwan ≒ 0. 5 Ireland ≒ 0. 9 Netherlands、Switzerland ≒ 1. 1 Maryland ≒ 1. 2 Belgium ≒ 1. 3 Hawaii ≒ 1. 6 New Jersey 3
Taiwan Profile n. Total area: 36, 000 km 2(14, 000 mile 2) Taiwan ≒ 0. 5 Ireland ≒ 0. 9 Netherlands、Switzerland ≒ 1. 1 Maryland ≒ 1. 2 Belgium ≒ 1. 3 Hawaii ≒ 1. 6 New Jersey 3
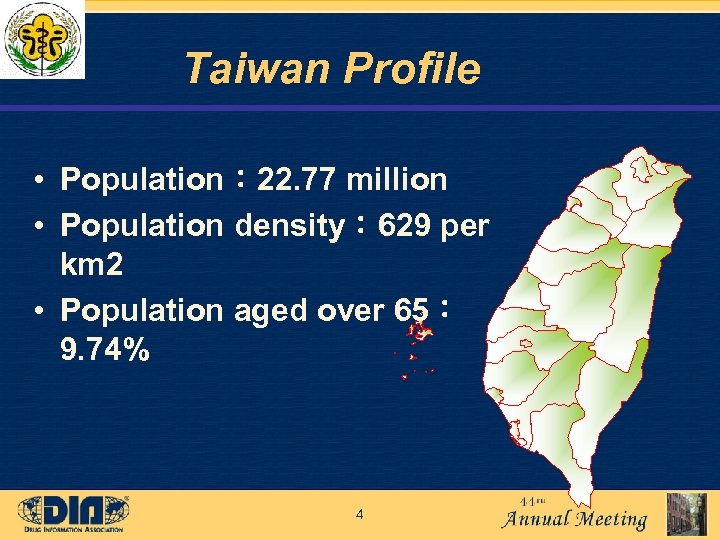 Taiwan Profile • Population: 22. 77 million • Population density: 629 per km 2 • Population aged over 65: 9. 74% 4
Taiwan Profile • Population: 22. 77 million • Population density: 629 per km 2 • Population aged over 65: 9. 74% 4
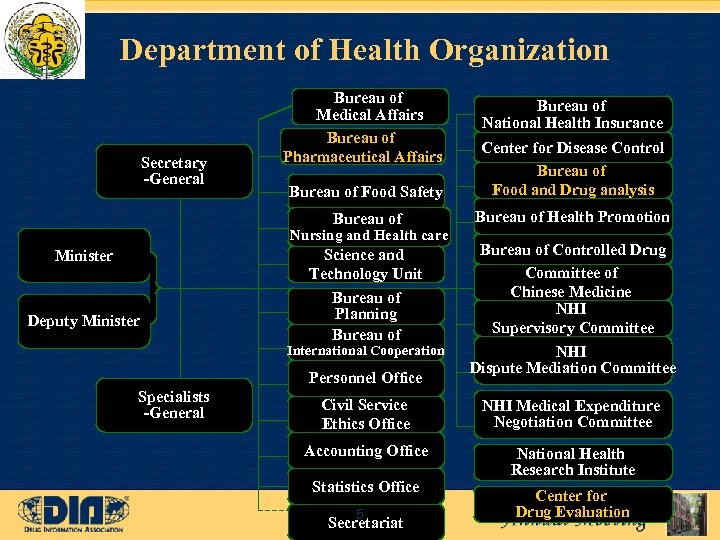 Department of Health Organization Bureau of Medical Affairs Bureau of Pharmaceutical Affairs Bureau of National Health Insurance Bureau of Food Safety Center for Disease Control Bureau of Food and Drug analysis Bureau of Secretary -General Bureau of Health Promotion Science and Technology Unit Bureau of Planning Bureau of Controlled Drug Committee of Chinese Medicine NHI Supervisory Committee NHI Dispute Mediation Committee Nursing and Health care Minister Deputy Minister International Cooperation Personnel Office Specialists -General Civil Service Ethics Office NHI Medical Expenditure Negotiation Committee Accounting Office National Health Research Institute Statistics Office 5 Secretariat Center for Drug Evaluation
Department of Health Organization Bureau of Medical Affairs Bureau of Pharmaceutical Affairs Bureau of National Health Insurance Bureau of Food Safety Center for Disease Control Bureau of Food and Drug analysis Bureau of Secretary -General Bureau of Health Promotion Science and Technology Unit Bureau of Planning Bureau of Controlled Drug Committee of Chinese Medicine NHI Supervisory Committee NHI Dispute Mediation Committee Nursing and Health care Minister Deputy Minister International Cooperation Personnel Office Specialists -General Civil Service Ethics Office NHI Medical Expenditure Negotiation Committee Accounting Office National Health Research Institute Statistics Office 5 Secretariat Center for Drug Evaluation
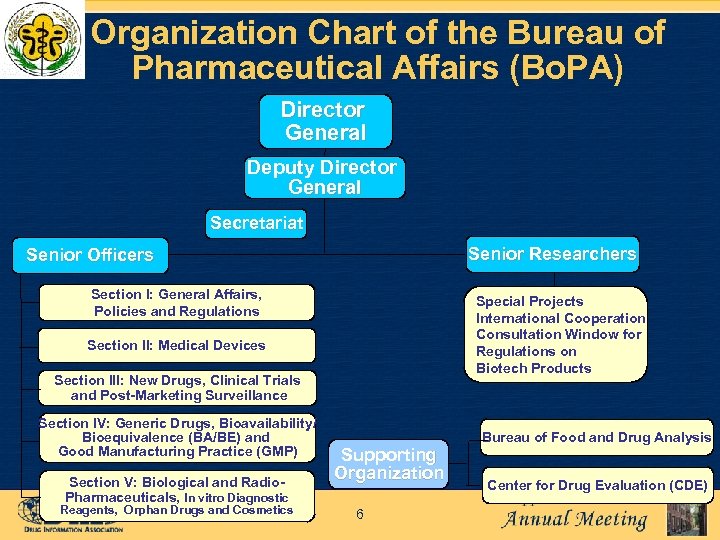 Organization Chart of the Bureau of Pharmaceutical Affairs (Bo. PA) Director General Deputy Director General Secretariat Senior Researchers Senior Officers Section I: General Affairs, Policies and Regulations Special Projects International Cooperation Consultation Window for Regulations on Biotech Products Section II: Medical Devices Section III: New Drugs, Clinical Trials and Post-Marketing Surveillance Section IV: Generic Drugs, Bioavailability/ Bioequivalence (BA/BE) and Good Manufacturing Practice (GMP) Section V: Biological and Radio. Pharmaceuticals, In vitro Diagnostic Reagents, Orphan Drugs and Cosmetics Supporting Organization 6 Bureau of Food and Drug Analysis Center for Drug Evaluation (CDE)
Organization Chart of the Bureau of Pharmaceutical Affairs (Bo. PA) Director General Deputy Director General Secretariat Senior Researchers Senior Officers Section I: General Affairs, Policies and Regulations Special Projects International Cooperation Consultation Window for Regulations on Biotech Products Section II: Medical Devices Section III: New Drugs, Clinical Trials and Post-Marketing Surveillance Section IV: Generic Drugs, Bioavailability/ Bioequivalence (BA/BE) and Good Manufacturing Practice (GMP) Section V: Biological and Radio. Pharmaceuticals, In vitro Diagnostic Reagents, Orphan Drugs and Cosmetics Supporting Organization 6 Bureau of Food and Drug Analysis Center for Drug Evaluation (CDE)
 Responsibilities of Bo. PA • Facilitator of Public Health • Educator for Health Knowledge • Promoter for Health Industry • Participator for International Collaboration 7
Responsibilities of Bo. PA • Facilitator of Public Health • Educator for Health Knowledge • Promoter for Health Industry • Participator for International Collaboration 7
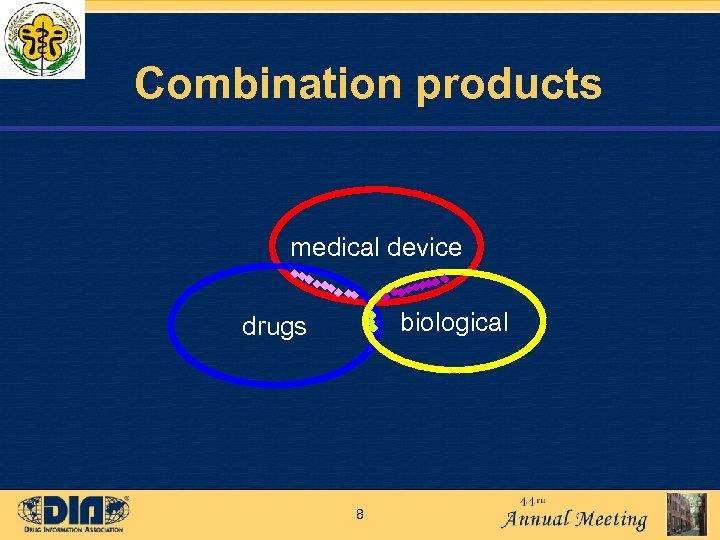 Combination products medical device biological drugs 8
Combination products medical device biological drugs 8
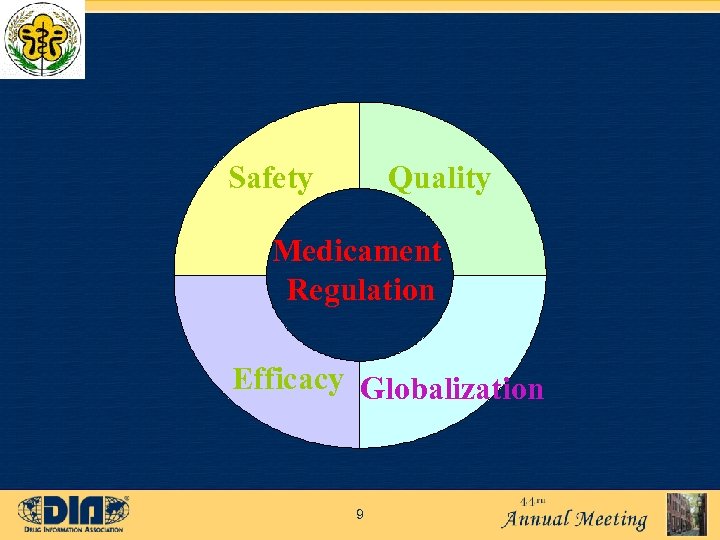 Safety Quality Medicament Regulation Efficacy Globalization 9
Safety Quality Medicament Regulation Efficacy Globalization 9
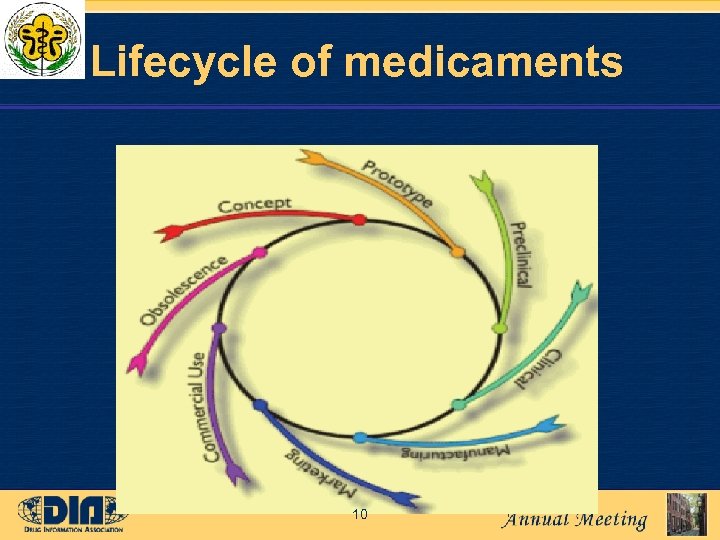 Lifecycle of medicaments 10
Lifecycle of medicaments 10
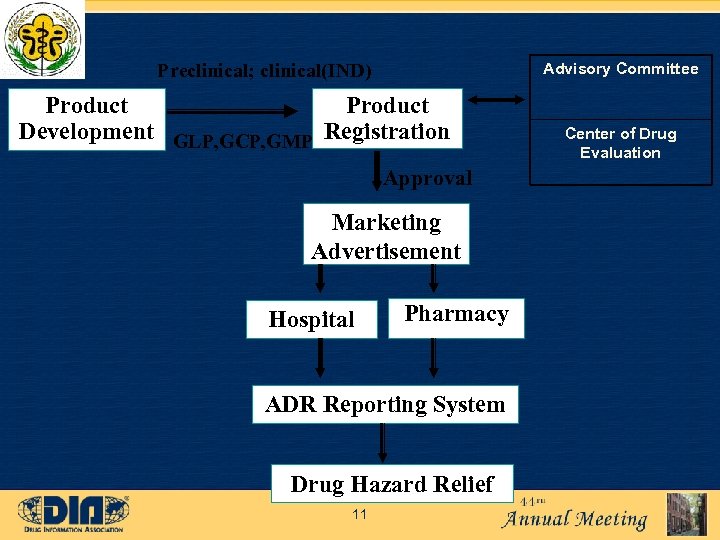 Advisory Committee Preclinical; clinical(IND) Product Development GLP, GCP, GMP Registration Approval Marketing Advertisement Hospital Pharmacy ADR Reporting System Drug Hazard Relief 11 Center of Drug Evaluation
Advisory Committee Preclinical; clinical(IND) Product Development GLP, GCP, GMP Registration Approval Marketing Advertisement Hospital Pharmacy ADR Reporting System Drug Hazard Relief 11 Center of Drug Evaluation
 Laws related with Pharmaceutical Affairs • Act of pharmaceutical affairs (drug, medical device, company, pharmacy) • Act of pharmacists • Act of drug hazard relief • Act of controlled substances • Act of orphan disease prevention and pharmaceuticals • Act of control of cosmetic hygiene 12
Laws related with Pharmaceutical Affairs • Act of pharmaceutical affairs (drug, medical device, company, pharmacy) • Act of pharmacists • Act of drug hazard relief • Act of controlled substances • Act of orphan disease prevention and pharmaceuticals • Act of control of cosmetic hygiene 12
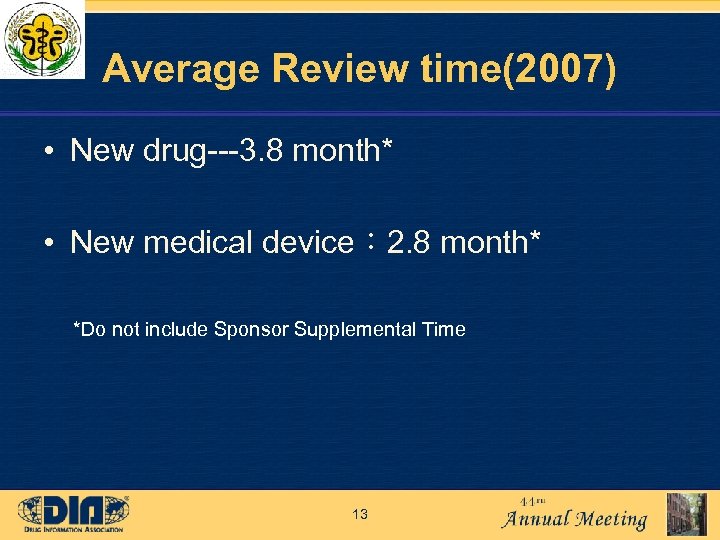 Average Review time(2007) • New drug---3. 8 month* • New medical device: 2. 8 month* *Do not include Sponsor Supplemental Time 13
Average Review time(2007) • New drug---3. 8 month* • New medical device: 2. 8 month* *Do not include Sponsor Supplemental Time 13
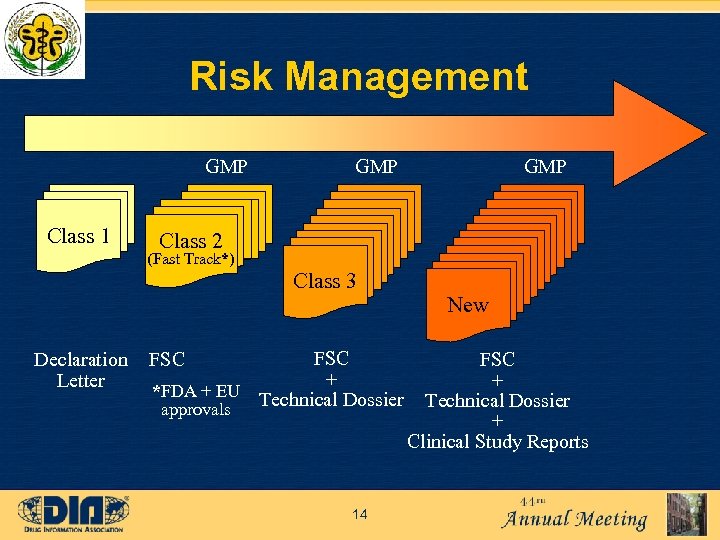 Risk Management GMP Class 1 GMP Class 2 (Fast Track*) Class 3 Declaration Letter FSC + *FDA + EU Technical Dossier approvals FSC 14 New FSC + Technical Dossier + Clinical Study Reports
Risk Management GMP Class 1 GMP Class 2 (Fast Track*) Class 3 Declaration Letter FSC + *FDA + EU Technical Dossier approvals FSC 14 New FSC + Technical Dossier + Clinical Study Reports
 Combination Products Marketed in Taiwan • Drug eluting cardiovascular stent • Spinal cage + recombinant BMP for spinal fusion • Artificial skin scaffold + cultured epithelial Cells • Antimicrobial dressing • Antimicrobial or heparin coated catheter • Photodynamic therapy with laser light source • Orthopedic implant with growth factors 15
Combination Products Marketed in Taiwan • Drug eluting cardiovascular stent • Spinal cage + recombinant BMP for spinal fusion • Artificial skin scaffold + cultured epithelial Cells • Antimicrobial dressing • Antimicrobial or heparin coated catheter • Photodynamic therapy with laser light source • Orthopedic implant with growth factors 15
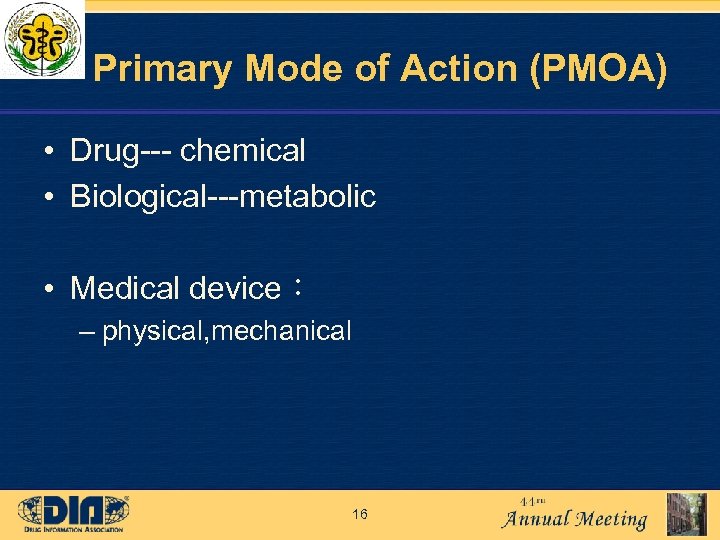 Primary Mode of Action (PMOA) • Drug--- chemical • Biological---metabolic • Medical device: – physical, mechanical 16
Primary Mode of Action (PMOA) • Drug--- chemical • Biological---metabolic • Medical device: – physical, mechanical 16
 Primary Mode of Action (PMOA) • Drug Eluting Stent – PMOA is stent opening the artery – Regulated as a device • Drug Eluting Disk – PMOA is cancer chemotherapy for brain tumor – Regulated as a drug • Contraceptive Sponge – PMOA is the contraceptive drug activity – Regulated as a drug 17
Primary Mode of Action (PMOA) • Drug Eluting Stent – PMOA is stent opening the artery – Regulated as a device • Drug Eluting Disk – PMOA is cancer chemotherapy for brain tumor – Regulated as a drug • Contraceptive Sponge – PMOA is the contraceptive drug activity – Regulated as a drug 17
 Combination product evaluation 1. Technical review ---PMOA ---Substantial equivalent/Generic ---Nonclinical study ---Clinical study/Bridge study 2. Joint advisory committee of drug and medical device 18
Combination product evaluation 1. Technical review ---PMOA ---Substantial equivalent/Generic ---Nonclinical study ---Clinical study/Bridge study 2. Joint advisory committee of drug and medical device 18
 Combination Products Marketed in Taiwan • Prefilled syringe • Meter dose Inhaler • Transdermal delivery system 19
Combination Products Marketed in Taiwan • Prefilled syringe • Meter dose Inhaler • Transdermal delivery system 19
 New Tools for Personalized Medicine Global and high throughput analysis http: //www. sciencemag. org 20
New Tools for Personalized Medicine Global and high throughput analysis http: //www. sciencemag. org 20
 Re-Labeling of Carbamazepine (Department of Health, Taiwan) l Research team leaded by Dr. Yuan-Tsong Chen in Taiwan l Warning : “Retrospective studies have shown that the human leukocyte antigen HLA-B*1502 has a very significant association to the carbamazepine induced SJS/TEN. The odds ratio is about 1357 with 95% confidence interval of 193 -8838. This means a patient who carries the HLA-B*1502 gene will have at least 193 times higher risk of developing ADR than a patient who ios not a HLA-B*15802 carrier. Approximately 5% of the Taiwan population carry the HLA-B*1502 gene” 21
Re-Labeling of Carbamazepine (Department of Health, Taiwan) l Research team leaded by Dr. Yuan-Tsong Chen in Taiwan l Warning : “Retrospective studies have shown that the human leukocyte antigen HLA-B*1502 has a very significant association to the carbamazepine induced SJS/TEN. The odds ratio is about 1357 with 95% confidence interval of 193 -8838. This means a patient who carries the HLA-B*1502 gene will have at least 193 times higher risk of developing ADR than a patient who ios not a HLA-B*15802 carrier. Approximately 5% of the Taiwan population carry the HLA-B*1502 gene” 21
 Re-Labeling of Carbamazepine FDA Recommendations Healthcare professionals who prescribe carbamazepine products should be fully aware of new prescribing information in the product label and in the revised boxed warning. “ …These reactions are estimated to occur in 1 to 6 per 10, 000 new users in countries with mainly Caucasian populations, but the risk in some asian countries is estimated to be about 10 times higher. . . . ……Patients with ancestry in genetically at-risk populations should be screened for the presence of HLA-B*1502 prior to initiating treatment with carbatrol……” 22
Re-Labeling of Carbamazepine FDA Recommendations Healthcare professionals who prescribe carbamazepine products should be fully aware of new prescribing information in the product label and in the revised boxed warning. “ …These reactions are estimated to occur in 1 to 6 per 10, 000 new users in countries with mainly Caucasian populations, but the risk in some asian countries is estimated to be about 10 times higher. . . . ……Patients with ancestry in genetically at-risk populations should be screened for the presence of HLA-B*1502 prior to initiating treatment with carbatrol……” 22
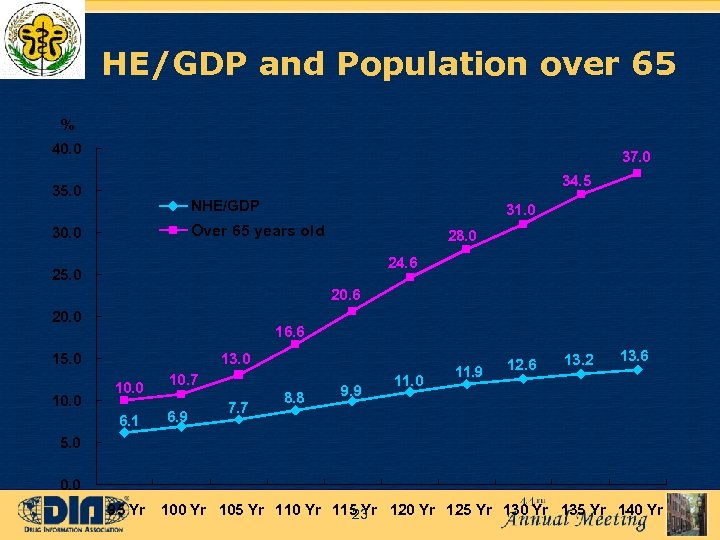 HE/GDP and Population over 65 % 40. 0 37. 0 34. 5 35. 0 NHE/GDP 31. 0 Over 65 years old 30. 0 28. 0 24. 6 25. 0 20. 6 20. 0 16. 6 15. 0 13. 0 10. 0 6. 1 10. 0 10. 7 6. 9 7. 7 8. 8 9. 9 11. 0 11. 9 12. 6 13. 2 13. 6 5. 0 0. 0 95 Yr 100 Yr 105 Yr 110 Yr 115 Yr 120 Yr 125 Yr 130 Yr 135 Yr 140 Yr 23
HE/GDP and Population over 65 % 40. 0 37. 0 34. 5 35. 0 NHE/GDP 31. 0 Over 65 years old 30. 0 28. 0 24. 6 25. 0 20. 6 20. 0 16. 6 15. 0 13. 0 10. 0 6. 1 10. 0 10. 7 6. 9 7. 7 8. 8 9. 9 11. 0 11. 9 12. 6 13. 2 13. 6 5. 0 0. 0 95 Yr 100 Yr 105 Yr 110 Yr 115 Yr 120 Yr 125 Yr 130 Yr 135 Yr 140 Yr 23
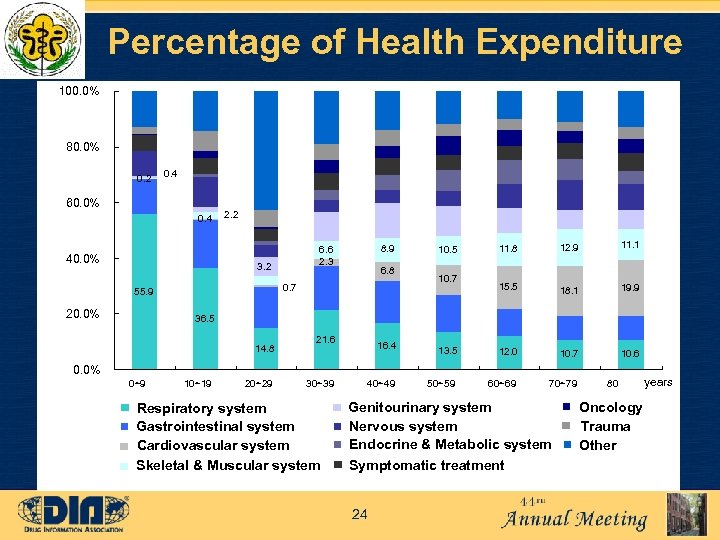 Percentage of Health Expenditure 100. 0% 80. 0% 0. 2 0. 4 60. 0% 0. 4 40. 0% 2. 2 3. 2 6. 8 0. 7 55. 9 20. 0% 8. 9 6. 6 2. 3 10. 5 10. 7 11. 8 12. 9 11. 1 15. 5 18. 1 19. 9 10. 6 36. 5 14. 8 21. 6 16. 4 13. 5 12. 0 10. 7 40~49 50~59 60~69 70~79 0. 0% 0~9 10~19 20~29 30~39 Respiratory system Gastrointestinal system Cardiovascular system Skeletal & Muscular system Genitourinary system Nervous system Endocrine & Metabolic system Symptomatic treatment 24 80 Oncology Trauma Other years
Percentage of Health Expenditure 100. 0% 80. 0% 0. 2 0. 4 60. 0% 0. 4 40. 0% 2. 2 3. 2 6. 8 0. 7 55. 9 20. 0% 8. 9 6. 6 2. 3 10. 5 10. 7 11. 8 12. 9 11. 1 15. 5 18. 1 19. 9 10. 6 36. 5 14. 8 21. 6 16. 4 13. 5 12. 0 10. 7 40~49 50~59 60~69 70~79 0. 0% 0~9 10~19 20~29 30~39 Respiratory system Gastrointestinal system Cardiovascular system Skeletal & Muscular system Genitourinary system Nervous system Endocrine & Metabolic system Symptomatic treatment 24 80 Oncology Trauma Other years
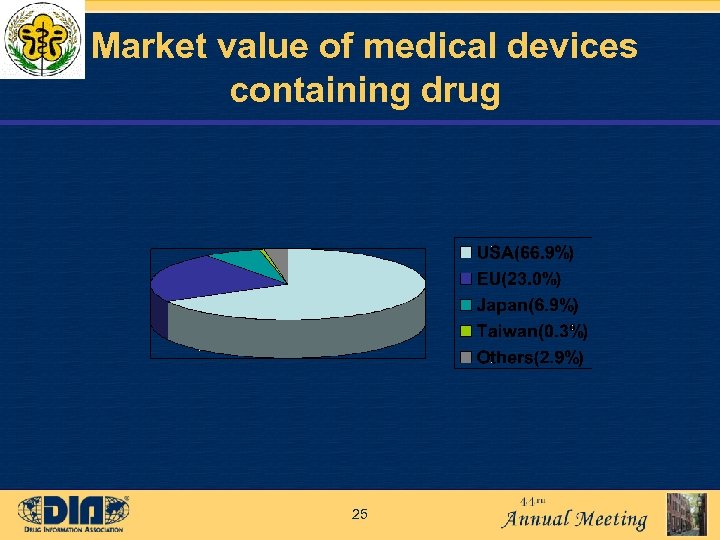 Market value of medical devices containing drug 25
Market value of medical devices containing drug 25
 Four Centers of Excellence for Clinical Trials • • National Taiwan University Hospital Tri-service Hospital National Chung-Kung University Hospital Wan Fang Hospital 26
Four Centers of Excellence for Clinical Trials • • National Taiwan University Hospital Tri-service Hospital National Chung-Kung University Hospital Wan Fang Hospital 26
 14 Government Funded General Clinical Research Centers Chang Gung Memorial Hospital Linkou Branch 長庚紀念醫院林口總院 Buddhist Tzu Chi General Hospital 佛教慈濟綜合醫院 Koo Foundation Sun Yat-Sen Cancer Center 辜公亮基金會和信治癌中心 Taichung Veterans General Hospital 台中榮民總醫院 Mackay Memorial Hospital 馬偕紀念醫院 Changhua Christian Hospital 彰化基督教醫院 Jianan Mental Hospital 衛生署嘉南療養院 Yuli Hospital 衛生署玉里醫院 Bali Psychiatric Center 衛生署八里療養院 Taipei Medical University Hospital 臺北醫學大學附設醫院 Chung Shan Medical University Hospital 中山醫學大學附設醫院 China Medical University Hospital 中國醫藥大學附設醫院 Chung-Ho Memorial Hospital, Kaohsiung Medical University 高醫中和醫院 Chi Medical Center 奇美醫院 27
14 Government Funded General Clinical Research Centers Chang Gung Memorial Hospital Linkou Branch 長庚紀念醫院林口總院 Buddhist Tzu Chi General Hospital 佛教慈濟綜合醫院 Koo Foundation Sun Yat-Sen Cancer Center 辜公亮基金會和信治癌中心 Taichung Veterans General Hospital 台中榮民總醫院 Mackay Memorial Hospital 馬偕紀念醫院 Changhua Christian Hospital 彰化基督教醫院 Jianan Mental Hospital 衛生署嘉南療養院 Yuli Hospital 衛生署玉里醫院 Bali Psychiatric Center 衛生署八里療養院 Taipei Medical University Hospital 臺北醫學大學附設醫院 Chung Shan Medical University Hospital 中山醫學大學附設醫院 China Medical University Hospital 中國醫藥大學附設醫院 Chung-Ho Memorial Hospital, Kaohsiung Medical University 高醫中和醫院 Chi Medical Center 奇美醫院 27
 Post Market Surveillance • • • GMP/QSD inspection every 2 -3 years Product license is valid for 5 years. Reporting of Adverse Events Recall Post market inspection and product testing 28
Post Market Surveillance • • • GMP/QSD inspection every 2 -3 years Product license is valid for 5 years. Reporting of Adverse Events Recall Post market inspection and product testing 28
 Post Market Surveillance Pharmacovigilancein Taiwan http: //adr. doh. gov. tw/ 29
Post Market Surveillance Pharmacovigilancein Taiwan http: //adr. doh. gov. tw/ 29
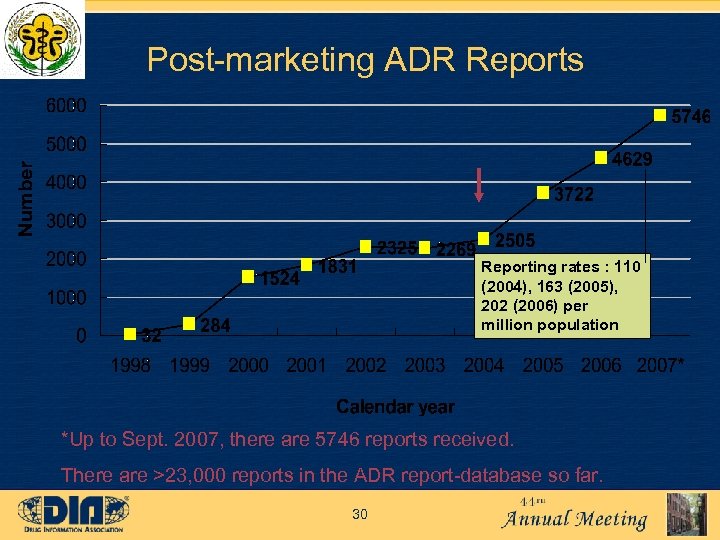 Post-marketing ADR Reports Reporting rates : 110 (2004), 163 (2005), 202 (2006) per million population *Up to Sept. 2007, there are 5746 reports received. There are >23, 000 reports in the ADR report-database so far. 30
Post-marketing ADR Reports Reporting rates : 110 (2004), 163 (2005), 202 (2006) per million population *Up to Sept. 2007, there are 5746 reports received. There are >23, 000 reports in the ADR report-database so far. 30
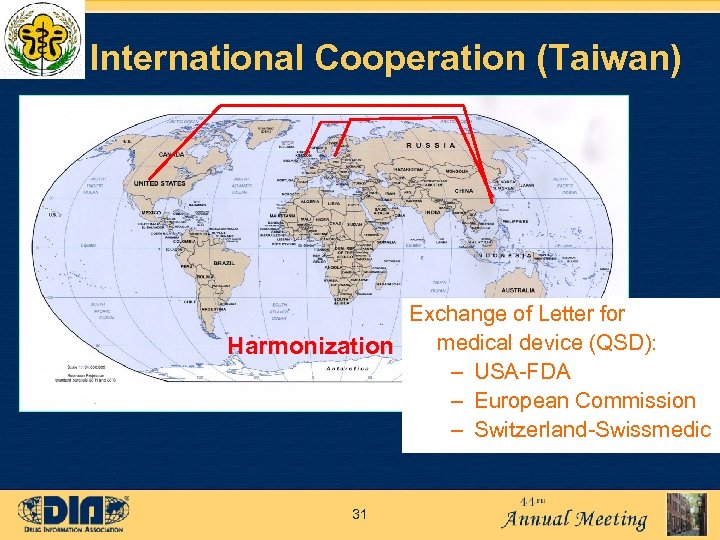 International Cooperation (Taiwan) Exchange of Letter for medical device (QSD): Harmonization – USA-FDA – European Commission – Switzerland-Swissmedic 31
International Cooperation (Taiwan) Exchange of Letter for medical device (QSD): Harmonization – USA-FDA – European Commission – Switzerland-Swissmedic 31
 Global Harmonization Drug---ICH Medial Device---GHTF Combination Product---? Harmonization 32
Global Harmonization Drug---ICH Medial Device---GHTF Combination Product---? Harmonization 32
 MORE International Cooperation Projects are Welcome! Welcome to Taipei for the “ 2008 Symposium of LSIF on CPP and Related Regulatory Issues” in Taipei, Nov. 20, 2008 33
MORE International Cooperation Projects are Welcome! Welcome to Taipei for the “ 2008 Symposium of LSIF on CPP and Related Regulatory Issues” in Taipei, Nov. 20, 2008 33


