c5b2eb115eaaa4f21ba2550971860fd7.ppt
- Количество слайдов: 22
 CLopidogrel as Adjunctive Reperfus. Ion Therap. Y – Thrombolysis In Myocardial Infarction (TIMI) 28 Disclosure statement: Dr. Sabatine is supported in part by grants (R 01 HL 072879 and R 01 HL 072872) from the NHLBI, and receives research grant support from and serves as a consultant to Bristol-Myers Squibb, Sanofi. Aventis, and Astra. Zeneca.
CLopidogrel as Adjunctive Reperfus. Ion Therap. Y – Thrombolysis In Myocardial Infarction (TIMI) 28 Disclosure statement: Dr. Sabatine is supported in part by grants (R 01 HL 072879 and R 01 HL 072872) from the NHLBI, and receives research grant support from and serves as a consultant to Bristol-Myers Squibb, Sanofi. Aventis, and Astra. Zeneca.
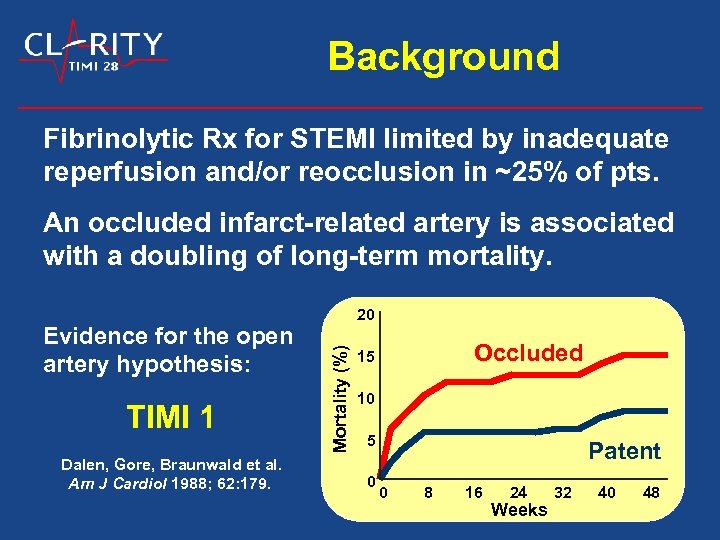 Background Fibrinolytic Rx for STEMI limited by inadequate reperfusion and/or reocclusion in ~25% of pts. An occluded infarct-related artery is associated with a doubling of long-term mortality. TIMI 1 Dalen, Gore, Braunwald et al. Am J Cardiol 1988; 62: 179. Mortality (%) Evidence for the open artery hypothesis: 20 Occluded 15 10 5 0 Patent 0 8 16 24 Weeks 32 40 48
Background Fibrinolytic Rx for STEMI limited by inadequate reperfusion and/or reocclusion in ~25% of pts. An occluded infarct-related artery is associated with a doubling of long-term mortality. TIMI 1 Dalen, Gore, Braunwald et al. Am J Cardiol 1988; 62: 179. Mortality (%) Evidence for the open artery hypothesis: 20 Occluded 15 10 5 0 Patent 0 8 16 24 Weeks 32 40 48
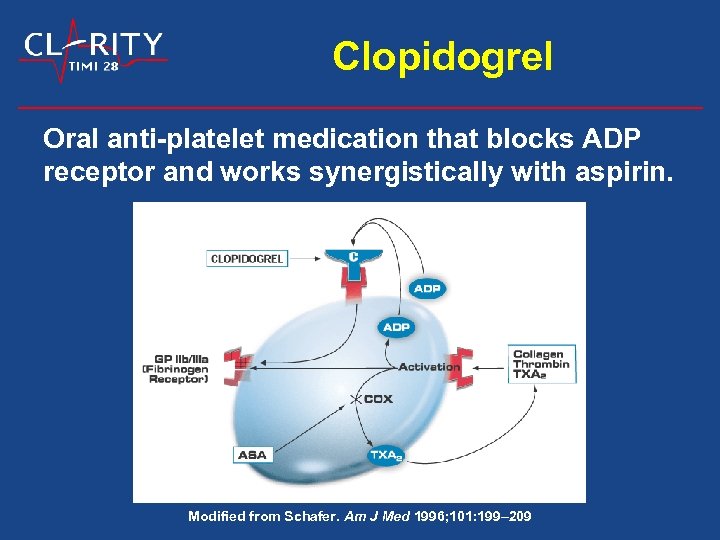 Clopidogrel Oral anti-platelet medication that blocks ADP receptor and works synergistically with aspirin. Modified from Schafer. Am J Med 1996; 101: 199– 209
Clopidogrel Oral anti-platelet medication that blocks ADP receptor and works synergistically with aspirin. Modified from Schafer. Am J Med 1996; 101: 199– 209
 Hypothesis The addition of clopidogrel to standard fibrinolytic regimens that include aspirin would: • Improve infarct-related artery patency • Decrease ischemic complications
Hypothesis The addition of clopidogrel to standard fibrinolytic regimens that include aspirin would: • Improve infarct-related artery patency • Decrease ischemic complications
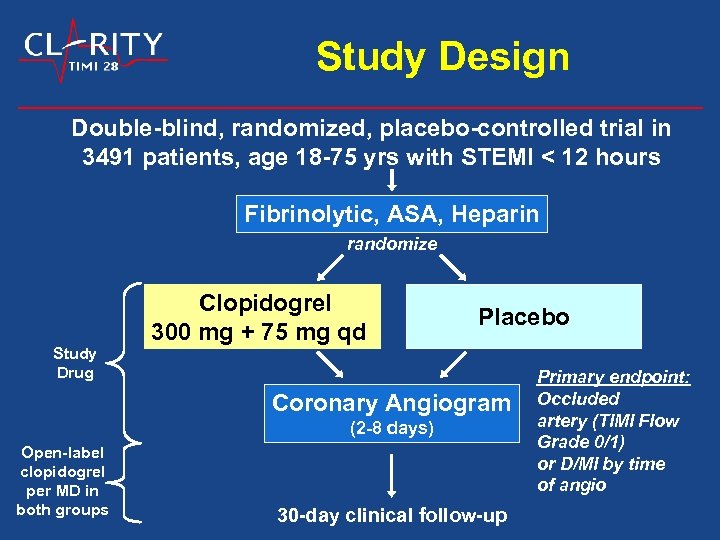 Study Design Double-blind, randomized, placebo-controlled trial in 3491 patients, age 18 -75 yrs with STEMI < 12 hours Fibrinolytic, ASA, Heparin randomize Study Drug Clopidogrel 300 mg + 75 mg qd Placebo Coronary Angiogram (2 -8 days) Open-label clopidogrel per MD in both groups 30 -day clinical follow-up Primary endpoint: Occluded artery (TIMI Flow Grade 0/1) or D/MI by time of angio
Study Design Double-blind, randomized, placebo-controlled trial in 3491 patients, age 18 -75 yrs with STEMI < 12 hours Fibrinolytic, ASA, Heparin randomize Study Drug Clopidogrel 300 mg + 75 mg qd Placebo Coronary Angiogram (2 -8 days) Open-label clopidogrel per MD in both groups 30 -day clinical follow-up Primary endpoint: Occluded artery (TIMI Flow Grade 0/1) or D/MI by time of angio
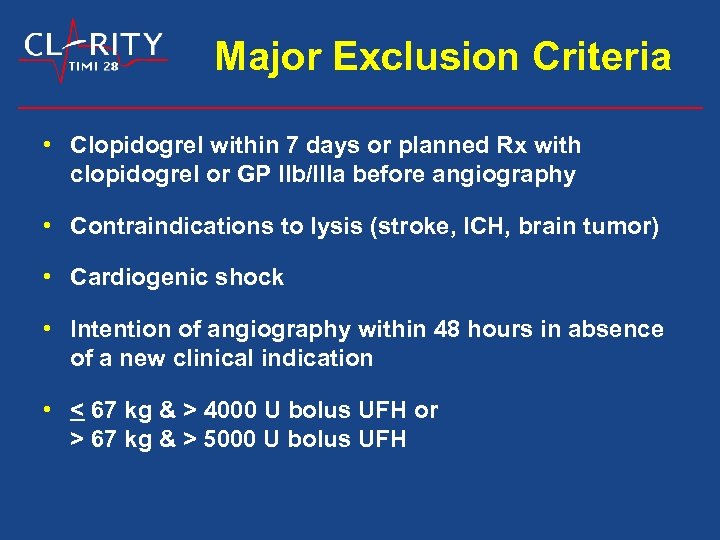 Major Exclusion Criteria • Clopidogrel within 7 days or planned Rx with clopidogrel or GP IIb/IIIa before angiography • Contraindications to lysis (stroke, ICH, brain tumor) • Cardiogenic shock • Intention of angiography within 48 hours in absence of a new clinical indication • < 67 kg & > 4000 U bolus UFH or > 67 kg & > 5000 U bolus UFH
Major Exclusion Criteria • Clopidogrel within 7 days or planned Rx with clopidogrel or GP IIb/IIIa before angiography • Contraindications to lysis (stroke, ICH, brain tumor) • Cardiogenic shock • Intention of angiography within 48 hours in absence of a new clinical indication • < 67 kg & > 4000 U bolus UFH or > 67 kg & > 5000 U bolus UFH
 Trial Organization TIMI Study Group Brigham and Women’s Hospital Harvard Medical School Eugene Braunwald, MD Christopher P. Cannon, MD Marc S. Sabatine, MD, MPH Amy C. Mc. Cagg, MBA TIMI Angio Core Lab C. Michael Gibson, MD, MS Data Coordinating Center Allan M. Skene, Ph. D Karen A. Hill, BS Nottingham Clinical Research Sponsors: Sanofi-Aventis & Bristol-Myers Squibb Bernard Job, MD Christophe Gaudin, MD Ravinder Saini, MD Leigh Townes, BS, RN
Trial Organization TIMI Study Group Brigham and Women’s Hospital Harvard Medical School Eugene Braunwald, MD Christopher P. Cannon, MD Marc S. Sabatine, MD, MPH Amy C. Mc. Cagg, MBA TIMI Angio Core Lab C. Michael Gibson, MD, MS Data Coordinating Center Allan M. Skene, Ph. D Karen A. Hill, BS Nottingham Clinical Research Sponsors: Sanofi-Aventis & Bristol-Myers Squibb Bernard Job, MD Christophe Gaudin, MD Ravinder Saini, MD Leigh Townes, BS, RN
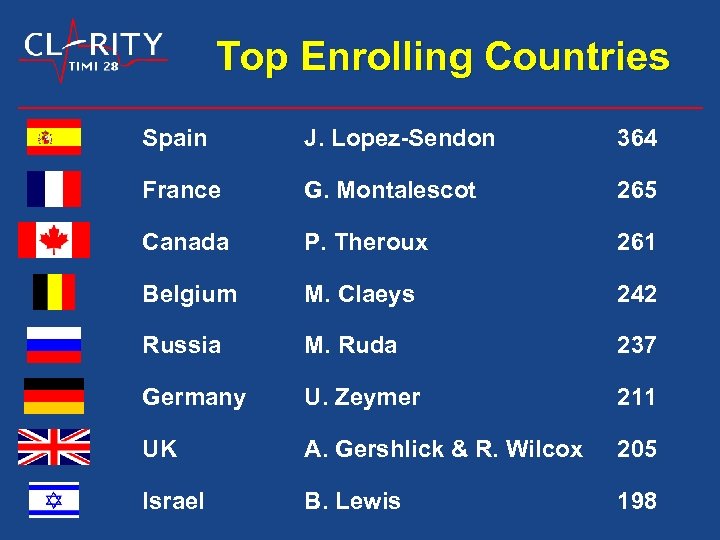 Top Enrolling Countries Spain J. Lopez-Sendon 364 France G. Montalescot 265 Canada P. Theroux 261 Belgium M. Claeys 242 Russia M. Ruda 237 Germany U. Zeymer 211 UK A. Gershlick & R. Wilcox 205 Israel B. Lewis 198
Top Enrolling Countries Spain J. Lopez-Sendon 364 France G. Montalescot 265 Canada P. Theroux 261 Belgium M. Claeys 242 Russia M. Ruda 237 Germany U. Zeymer 211 UK A. Gershlick & R. Wilcox 205 Israel B. Lewis 198
 Top Enrolling Centers Hospital Principal Investigator Research Coordinator AZ Klina, Belgium F. Cools S. Vanhagendoren Canisius-Wih. Ziek. , NL D. P. Hertzberger A. Schut Hosp. de Cabueñes, Spain A. Batalla Centre Hosp. , France A. Bonneau Scarborough Card. Res. , CA Kassam/Halperin P. Parsons Celso da Puccamp, Brazil J. F. Kerr Saraiva C. Travaini Garcia Centre Hospitalier, France Y. Lambert J. M. Caussanel Szpital Miejski, Poland J. Gessel L. Pawlowicz St. Petersburg Med Acad, RU S. Boldueva Royal Victoria Hospital, UK J. Adgey L. Soulat T. Mc. Allister
Top Enrolling Centers Hospital Principal Investigator Research Coordinator AZ Klina, Belgium F. Cools S. Vanhagendoren Canisius-Wih. Ziek. , NL D. P. Hertzberger A. Schut Hosp. de Cabueñes, Spain A. Batalla Centre Hosp. , France A. Bonneau Scarborough Card. Res. , CA Kassam/Halperin P. Parsons Celso da Puccamp, Brazil J. F. Kerr Saraiva C. Travaini Garcia Centre Hospitalier, France Y. Lambert J. M. Caussanel Szpital Miejski, Poland J. Gessel L. Pawlowicz St. Petersburg Med Acad, RU S. Boldueva Royal Victoria Hospital, UK J. Adgey L. Soulat T. Mc. Allister
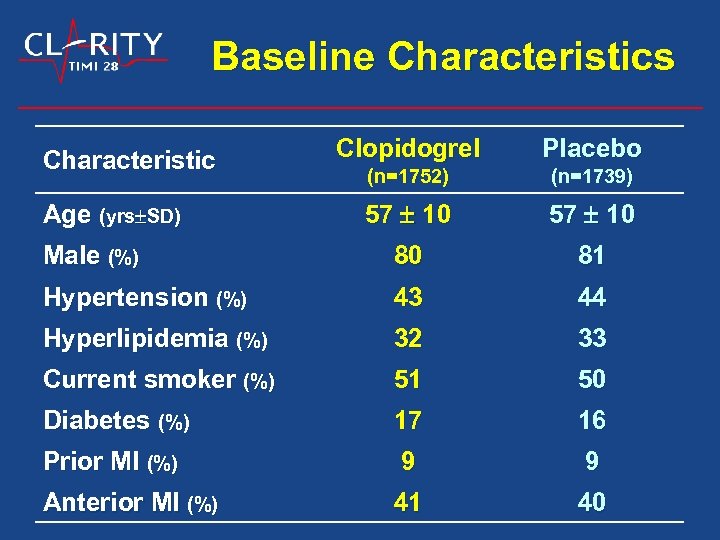 Baseline Characteristics Clopidogrel Placebo (n=1752) (n=1739) 57 10 Male (%) 80 81 Hypertension (%) 43 44 Hyperlipidemia (%) 32 33 Current smoker (%) 51 50 Diabetes (%) 17 16 Prior MI (%) 9 9 Anterior MI (%) 41 40 Characteristic Age (yrs SD)
Baseline Characteristics Clopidogrel Placebo (n=1752) (n=1739) 57 10 Male (%) 80 81 Hypertension (%) 43 44 Hyperlipidemia (%) 32 33 Current smoker (%) 51 50 Diabetes (%) 17 16 Prior MI (%) 9 9 Anterior MI (%) 41 40 Characteristic Age (yrs SD)
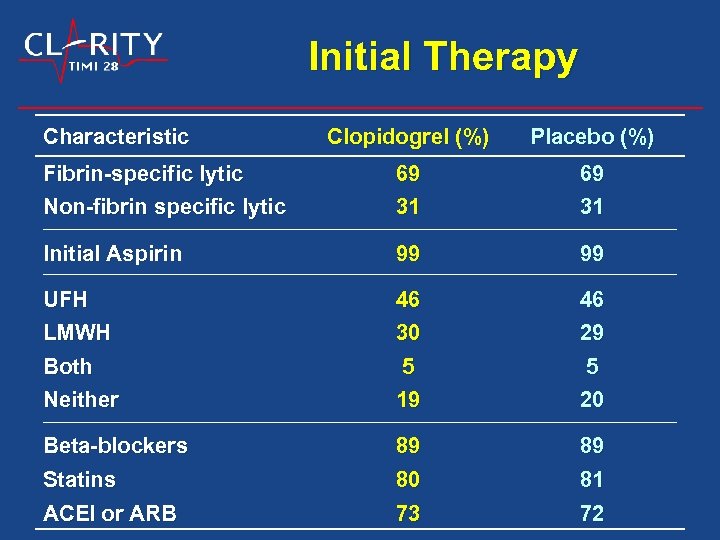 Initial Therapy Characteristic Clopidogrel (%) Placebo (%) Fibrin-specific lytic Non-fibrin specific lytic 69 31 Initial Aspirin 99 99 UFH LMWH 46 30 46 29 Both Neither 5 19 5 20 Beta-blockers Statins 89 80 89 81 ACEI or ARB 73 72
Initial Therapy Characteristic Clopidogrel (%) Placebo (%) Fibrin-specific lytic Non-fibrin specific lytic 69 31 Initial Aspirin 99 99 UFH LMWH 46 30 46 29 Both Neither 5 19 5 20 Beta-blockers Statins 89 80 89 81 ACEI or ARB 73 72
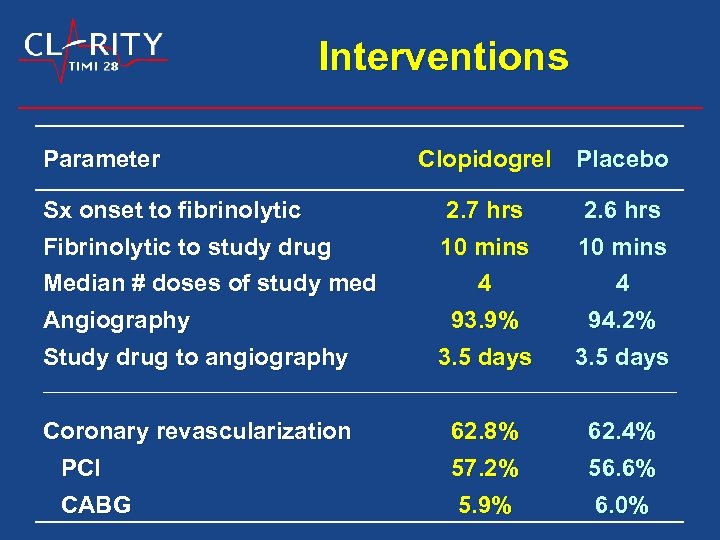 Interventions Parameter Clopidogrel Placebo 2. 7 hrs 10 mins 2. 6 hrs 10 mins 4 93. 9% 4 94. 2% Study drug to angiography 3. 5 days Coronary revascularization PCI 62. 8% 57. 2% 62. 4% 56. 6% 5. 9% 6. 0% Sx onset to fibrinolytic Fibrinolytic to study drug Median # doses of study med Angiography CABG
Interventions Parameter Clopidogrel Placebo 2. 7 hrs 10 mins 2. 6 hrs 10 mins 4 93. 9% 4 94. 2% Study drug to angiography 3. 5 days Coronary revascularization PCI 62. 8% 57. 2% 62. 4% 56. 6% 5. 9% 6. 0% Sx onset to fibrinolytic Fibrinolytic to study drug Median # doses of study med Angiography CABG
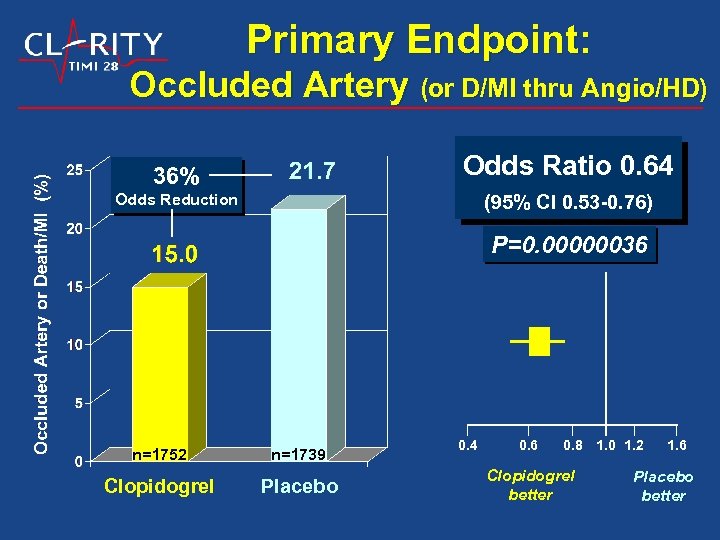 Primary Endpoint: Occluded Artery (or D/MI thru Angio/HD) 36% Odds Ratio 0. 64 Odds Reduction (95% CI 0. 53 -0. 76) P=0. 00000036 n=1752 n=1739 Clopidogrel Placebo 0. 4 0. 6 0. 8 1. 0 1. 2 Clopidogrel better 1. 6 Placebo better
Primary Endpoint: Occluded Artery (or D/MI thru Angio/HD) 36% Odds Ratio 0. 64 Odds Reduction (95% CI 0. 53 -0. 76) P=0. 00000036 n=1752 n=1739 Clopidogrel Placebo 0. 4 0. 6 0. 8 1. 0 1. 2 Clopidogrel better 1. 6 Placebo better
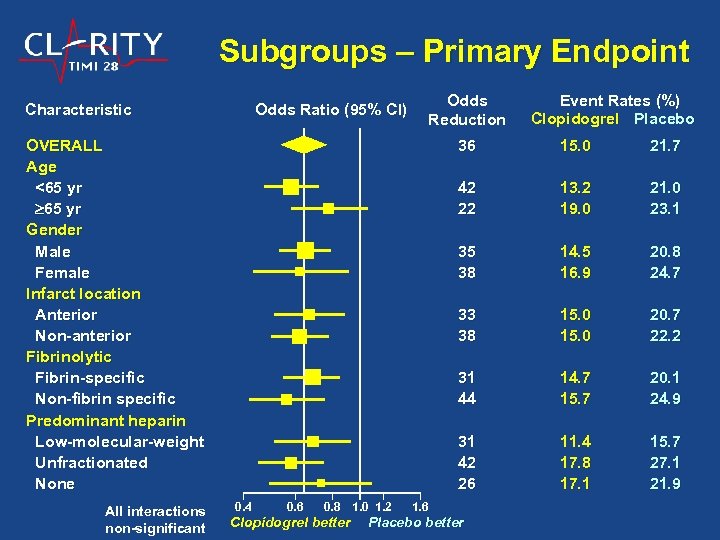 Subgroups – Primary Endpoint Characteristic Odds Ratio (95% CI) Odds Reduction OVERALL Age <65 yr Gender Male Female Infarct location Anterior Non-anterior Fibrinolytic Fibrin-specific Non-fibrin specific Predominant heparin Low-molecular-weight Unfractionated None All interactions non-significant Event Rates (%) Clopidogrel Placebo 36 14. 5 16. 9 20. 8 24. 7 15. 0 20. 7 22. 2 31 44 14. 7 15. 7 20. 1 24. 9 31 42 26 Clopidogrel better 21. 0 23. 1 33 38 0. 8 1. 0 1. 2 13. 2 19. 0 35 38 0. 6 21. 7 42 22 0. 4 15. 0 11. 4 17. 8 17. 1 15. 7 27. 1 21. 9 1. 6 Placebo better
Subgroups – Primary Endpoint Characteristic Odds Ratio (95% CI) Odds Reduction OVERALL Age <65 yr Gender Male Female Infarct location Anterior Non-anterior Fibrinolytic Fibrin-specific Non-fibrin specific Predominant heparin Low-molecular-weight Unfractionated None All interactions non-significant Event Rates (%) Clopidogrel Placebo 36 14. 5 16. 9 20. 8 24. 7 15. 0 20. 7 22. 2 31 44 14. 7 15. 7 20. 1 24. 9 31 42 26 Clopidogrel better 21. 0 23. 1 33 38 0. 8 1. 0 1. 2 13. 2 19. 0 35 38 0. 6 21. 7 42 22 0. 4 15. 0 11. 4 17. 8 17. 1 15. 7 27. 1 21. 9 1. 6 Placebo better
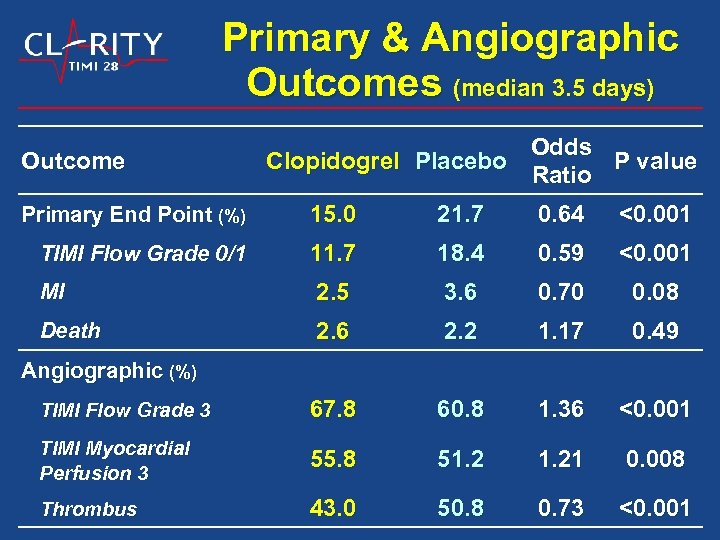 Primary & Angiographic Outcomes (median 3. 5 days) Outcome Odds Clopidogrel Placebo P value Ratio Primary End Point (%) 15. 0 21. 7 0. 64 <0. 001 TIMI Flow Grade 0/1 11. 7 18. 4 0. 59 <0. 001 MI 2. 5 3. 6 0. 70 0. 08 Death 2. 6 2. 2 1. 17 0. 49 TIMI Flow Grade 3 67. 8 60. 8 1. 36 <0. 001 TIMI Myocardial Perfusion 3 55. 8 51. 21 0. 008 Thrombus 43. 0 50. 8 0. 73 <0. 001 Angiographic (%)
Primary & Angiographic Outcomes (median 3. 5 days) Outcome Odds Clopidogrel Placebo P value Ratio Primary End Point (%) 15. 0 21. 7 0. 64 <0. 001 TIMI Flow Grade 0/1 11. 7 18. 4 0. 59 <0. 001 MI 2. 5 3. 6 0. 70 0. 08 Death 2. 6 2. 2 1. 17 0. 49 TIMI Flow Grade 3 67. 8 60. 8 1. 36 <0. 001 TIMI Myocardial Perfusion 3 55. 8 51. 21 0. 008 Thrombus 43. 0 50. 8 0. 73 <0. 001 Angiographic (%)
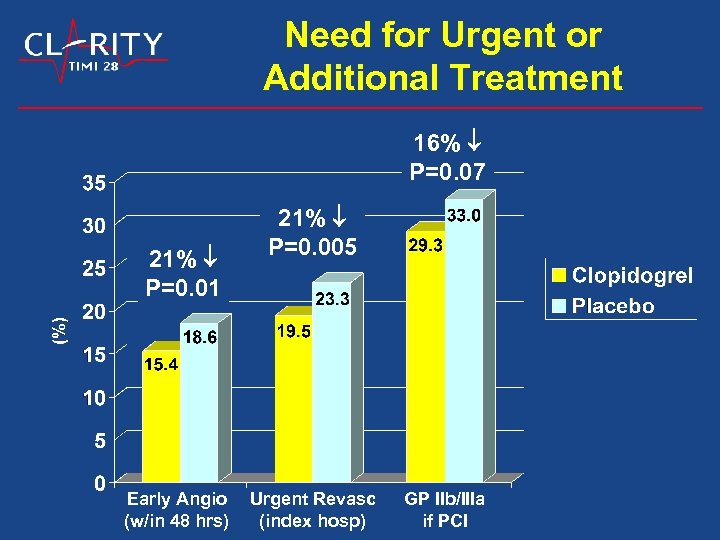 Need for Urgent or Additional Treatment 16% P=0. 07 21% P=0. 01 Early Angio (w/in 48 hrs) 21% P=0. 005 Urgent Revasc (index hosp) GP IIb/IIIa if PCI
Need for Urgent or Additional Treatment 16% P=0. 07 21% P=0. 01 Early Angio (w/in 48 hrs) 21% P=0. 005 Urgent Revasc (index hosp) GP IIb/IIIa if PCI
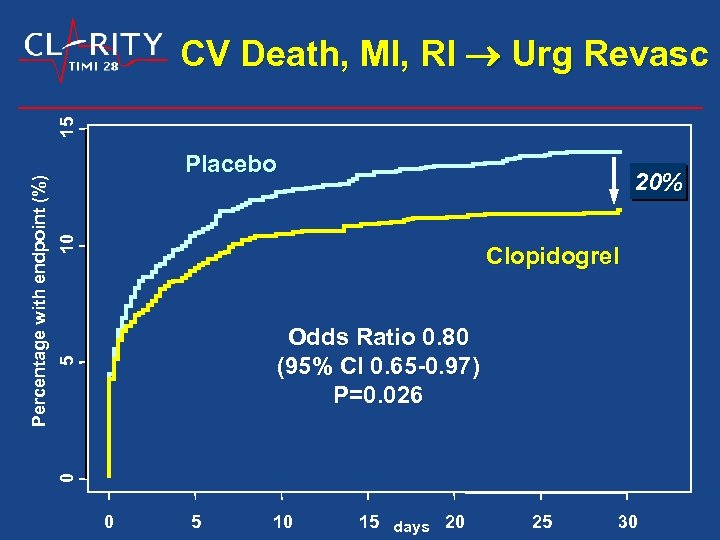 Placebo 10 20% Clopidogrel 5 Odds Ratio 0. 80 (95% CI 0. 65 -0. 97) P=0. 026 0 Percentage with endpoint (%) 15 CV Death, MI, RI Urg Revasc 0 5 10 15 days 20 25 30
Placebo 10 20% Clopidogrel 5 Odds Ratio 0. 80 (95% CI 0. 65 -0. 97) P=0. 026 0 Percentage with endpoint (%) 15 CV Death, MI, RI Urg Revasc 0 5 10 15 days 20 25 30
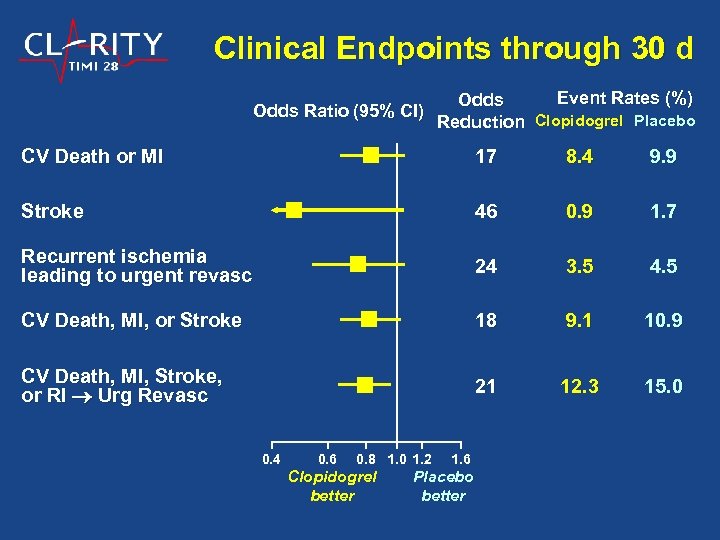 Clinical Endpoints through 30 d Odds Ratio (95% CI) Event Rates (%) Odds Reduction Clopidogrel Placebo CV Death or MI 17 8. 4 9. 9 Stroke 46 0. 9 1. 7 Recurrent ischemia leading to urgent revasc 24 3. 5 4. 5 CV Death, MI, or Stroke 18 9. 1 10. 9 CV Death, MI, Stroke, or RI Urg Revasc 21 12. 3 15. 0 0. 4 0. 6 0. 8 1. 0 1. 2 Clopidogrel better 1. 6 Placebo better
Clinical Endpoints through 30 d Odds Ratio (95% CI) Event Rates (%) Odds Reduction Clopidogrel Placebo CV Death or MI 17 8. 4 9. 9 Stroke 46 0. 9 1. 7 Recurrent ischemia leading to urgent revasc 24 3. 5 4. 5 CV Death, MI, or Stroke 18 9. 1 10. 9 CV Death, MI, Stroke, or RI Urg Revasc 21 12. 3 15. 0 0. 4 0. 6 0. 8 1. 0 1. 2 Clopidogrel better 1. 6 Placebo better
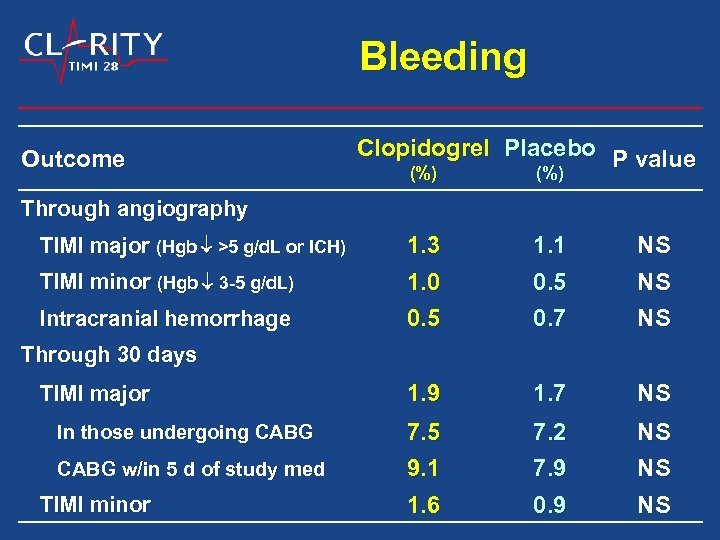 Bleeding Outcome Clopidogrel Placebo P value (%) Through angiography TIMI minor (Hgb 3 -5 g/d. L) 1. 3 1. 0 1. 1 0. 5 NS NS Intracranial hemorrhage 0. 5 0. 7 NS 1. 9 1. 7 NS 7. 5 9. 1 7. 2 7. 9 NS NS 1. 6 0. 9 NS TIMI major (Hgb >5 g/d. L or ICH) Through 30 days TIMI major In those undergoing CABG w/in 5 d of study med TIMI minor
Bleeding Outcome Clopidogrel Placebo P value (%) Through angiography TIMI minor (Hgb 3 -5 g/d. L) 1. 3 1. 0 1. 1 0. 5 NS NS Intracranial hemorrhage 0. 5 0. 7 NS 1. 9 1. 7 NS 7. 5 9. 1 7. 2 7. 9 NS NS 1. 6 0. 9 NS TIMI major (Hgb >5 g/d. L or ICH) Through 30 days TIMI major In those undergoing CABG w/in 5 d of study med TIMI minor
 Summary In patients with STEMI 75 yrs, receiving a standard fibrinolytic regimen, a loading dose of 300 mg of clopidogrel followed by 75 mg daily resulted in: • 36% reduction in the odds of an occluded infarctrelated artery, or death/MI by angio (NNT = 16) • Highly consistent benefit across all major subgroups • 20% reduction in CV death, MI, or recurrent ischemia leading to urgent revasc through 30 days (NNT = 36) • No excess in TIMI major or minor bleeding (including in those undergoing CABG) or in ICH
Summary In patients with STEMI 75 yrs, receiving a standard fibrinolytic regimen, a loading dose of 300 mg of clopidogrel followed by 75 mg daily resulted in: • 36% reduction in the odds of an occluded infarctrelated artery, or death/MI by angio (NNT = 16) • Highly consistent benefit across all major subgroups • 20% reduction in CV death, MI, or recurrent ischemia leading to urgent revasc through 30 days (NNT = 36) • No excess in TIMI major or minor bleeding (including in those undergoing CABG) or in ICH
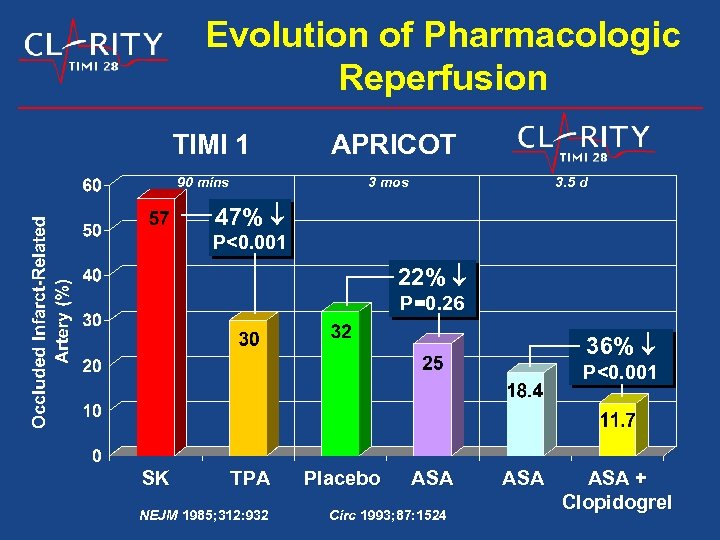 Evolution of Pharmacologic Reperfusion TIMI 1 90 mins APRICOT 3 mos 3. 5 d 47% P<0. 001 22% P=0. 26 36% P<0. 001 SK TPA NEJM 1985; 312: 932 Placebo ASA Circ 1993; 87: 1524 ASA + Clopidogrel
Evolution of Pharmacologic Reperfusion TIMI 1 90 mins APRICOT 3 mos 3. 5 d 47% P<0. 001 22% P=0. 26 36% P<0. 001 SK TPA NEJM 1985; 312: 932 Placebo ASA Circ 1993; 87: 1524 ASA + Clopidogrel
 M A R C H 9, 2 0 0 5 Conclusion Clopidogrel offers an effective, simple, inexpensive, and safe means by which to improve infarct-related artery patency and reduce ischemic complications. Sabatine MS, Cannon CP, Gibson CM, Lopez-Sendon JL, Montalescot G, Theroux P, Claeys MJ, Cools F, Hill KA, Skene AM, Mc. Cabe CH and Braunwald E for the CLARITY-TIMI 28 Investigators. N Engl J Med 2005; 352 www. nejm. org. ACC 2005 LBCT Slide Set available at www. timi. org.
M A R C H 9, 2 0 0 5 Conclusion Clopidogrel offers an effective, simple, inexpensive, and safe means by which to improve infarct-related artery patency and reduce ischemic complications. Sabatine MS, Cannon CP, Gibson CM, Lopez-Sendon JL, Montalescot G, Theroux P, Claeys MJ, Cools F, Hill KA, Skene AM, Mc. Cabe CH and Braunwald E for the CLARITY-TIMI 28 Investigators. N Engl J Med 2005; 352 www. nejm. org. ACC 2005 LBCT Slide Set available at www. timi. org.


