51fc2f56082c04b37a98ff15144d6441.ppt
- Количество слайдов: 23
 Clinical Safety & Effectiveness Reducing TAT in the Clinical Cytogenetics Laboratory- Phase 2 DATE 1
Clinical Safety & Effectiveness Reducing TAT in the Clinical Cytogenetics Laboratory- Phase 2 DATE 1
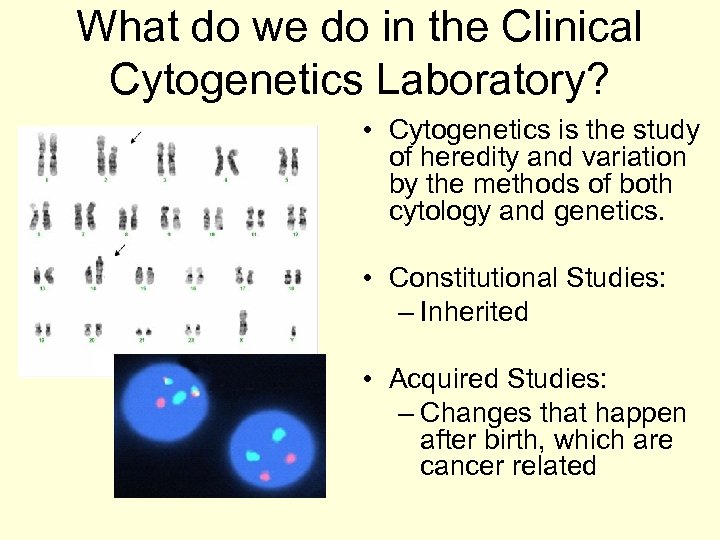 What do we do in the Clinical Cytogenetics Laboratory? • Cytogenetics is the study of heredity and variation by the methods of both cytology and genetics. • Constitutional Studies: – Inherited • Acquired Studies: – Changes that happen after birth, which are cancer related
What do we do in the Clinical Cytogenetics Laboratory? • Cytogenetics is the study of heredity and variation by the methods of both cytology and genetics. • Constitutional Studies: – Inherited • Acquired Studies: – Changes that happen after birth, which are cancer related
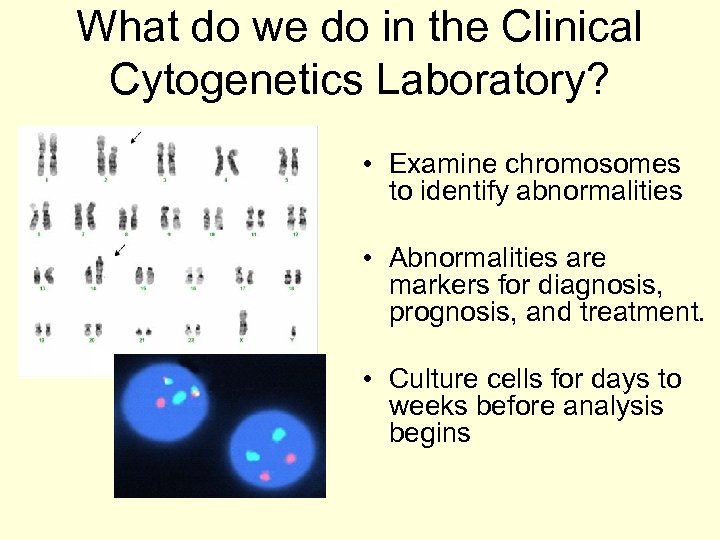 What do we do in the Clinical Cytogenetics Laboratory? • Examine chromosomes to identify abnormalities • Abnormalities are markers for diagnosis, prognosis, and treatment. • Culture cells for days to weeks before analysis begins
What do we do in the Clinical Cytogenetics Laboratory? • Examine chromosomes to identify abnormalities • Abnormalities are markers for diagnosis, prognosis, and treatment. • Culture cells for days to weeks before analysis begins
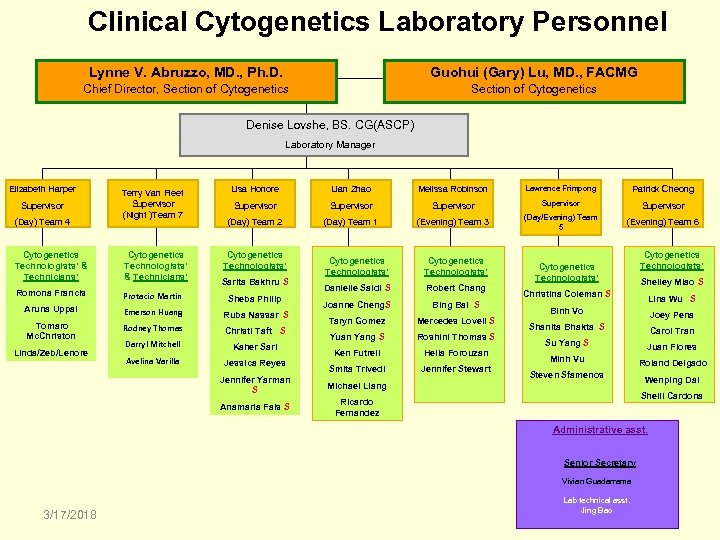 Clinical Cytogenetics Laboratory Personnel Lynne V. Abruzzo, MD. , Ph. D. Guohui (Gary) Lu, MD. , FACMG Chief Director, Section of Cytogenetics Denise Lovshe, BS. CG(ASCP) Laboratory Manager Elizabeth Harper Supervisor (Day) Team 4 Terry Van Fleet Supervisor (Night )Team 7 Cytogenetics Technologists’ & Technicians’ Romona Francis Protacio Martin Aruna Uppal Emerson Huang Tomaro Mc. Christon Rodney Thomas Linda/Zeb/Lenore Darryl Mitchell Avelina Varilla Lisa Honore Supervisor (Day) Team 2 Cytogenetics Technologists’ Sarita Bakhru S Sheba Philip Ruba Nassar S Christi Taft S Kaher Sari Jessica Reyes Lian Zhao Supervisor (Day) Team 1 Melissa Robinson Lawrence Frimpong Patrick Cheong Supervisor (Evening) Team 3 (Day/Evening) Team 5 (Evening) Team 6 Cytogenetics Technologists’ Danielle Saldi S Robert Chang Joanne Cheng. S Bing Bai S Taryn Gomez Mercedes Lovell S Yuan Yang S Roshini Thomas S Ken Futrell Helia Forouzan Smita Trivedi Jennifer Stewart Jennifer Yarman S Cytogenetics Technologists’ Shelley Miao S Christina Coleman S Lina Wu S Binh Vo Joey Pena Shanita Bhakta S Carol Tran Su Yang S Juan Flores Minh Vu Roland Delgado Steven Sfamenos Wenping Dai Michael Liang Anamaria Fals S Cytogenetics Technologists’ Ricardo Fernandez Shelli Cardona Administrative asst. Senior Secretary Vivian Guadarrama 3/17/2018 Lab technical asst. Jing Bao
Clinical Cytogenetics Laboratory Personnel Lynne V. Abruzzo, MD. , Ph. D. Guohui (Gary) Lu, MD. , FACMG Chief Director, Section of Cytogenetics Denise Lovshe, BS. CG(ASCP) Laboratory Manager Elizabeth Harper Supervisor (Day) Team 4 Terry Van Fleet Supervisor (Night )Team 7 Cytogenetics Technologists’ & Technicians’ Romona Francis Protacio Martin Aruna Uppal Emerson Huang Tomaro Mc. Christon Rodney Thomas Linda/Zeb/Lenore Darryl Mitchell Avelina Varilla Lisa Honore Supervisor (Day) Team 2 Cytogenetics Technologists’ Sarita Bakhru S Sheba Philip Ruba Nassar S Christi Taft S Kaher Sari Jessica Reyes Lian Zhao Supervisor (Day) Team 1 Melissa Robinson Lawrence Frimpong Patrick Cheong Supervisor (Evening) Team 3 (Day/Evening) Team 5 (Evening) Team 6 Cytogenetics Technologists’ Danielle Saldi S Robert Chang Joanne Cheng. S Bing Bai S Taryn Gomez Mercedes Lovell S Yuan Yang S Roshini Thomas S Ken Futrell Helia Forouzan Smita Trivedi Jennifer Stewart Jennifer Yarman S Cytogenetics Technologists’ Shelley Miao S Christina Coleman S Lina Wu S Binh Vo Joey Pena Shanita Bhakta S Carol Tran Su Yang S Juan Flores Minh Vu Roland Delgado Steven Sfamenos Wenping Dai Michael Liang Anamaria Fals S Cytogenetics Technologists’ Ricardo Fernandez Shelli Cardona Administrative asst. Senior Secretary Vivian Guadarrama 3/17/2018 Lab technical asst. Jing Bao
 Workload • ~22, 215 specimens per year – 98% hematologic malignancies – 2% solid tumors • Conventional karyotypic analysis – ~50% of tests – 3 hours per case (after specimen culture & processing) • FISH (Fluorescence In Situ Hybridization) – ~50% of tests (~100 different probes) – 1. 5 hours per case (after specimen culture & processing)
Workload • ~22, 215 specimens per year – 98% hematologic malignancies – 2% solid tumors • Conventional karyotypic analysis – ~50% of tests – 3 hours per case (after specimen culture & processing) • FISH (Fluorescence In Situ Hybridization) – ~50% of tests (~100 different probes) – 1. 5 hours per case (after specimen culture & processing)
 The Team – Martha Johnson-Hamilton Pathology and Laboratory Medicine Quality Technologist – Denise Lovshe Cytogenetics Laboratory Manager – Elizabeth Harper Cytogenetics Supervisor – Soo Ha Cheong Cytogenetics Supervisor – Sarita Bakhru Sr. Cytogenetics Technologist – Lisa Honore’ Cytogenetics Supervisor 6
The Team – Martha Johnson-Hamilton Pathology and Laboratory Medicine Quality Technologist – Denise Lovshe Cytogenetics Laboratory Manager – Elizabeth Harper Cytogenetics Supervisor – Soo Ha Cheong Cytogenetics Supervisor – Sarita Bakhru Sr. Cytogenetics Technologist – Lisa Honore’ Cytogenetics Supervisor 6
 What We Are Trying to Accomplish? AIM STATEMENT The aim of this project is to reduce the TAT for resulting neoplastic bone marrow specimens submitted for Cytogenetics analysis to 21 calendar days for 90% of specimens by June 2011. 7
What We Are Trying to Accomplish? AIM STATEMENT The aim of this project is to reduce the TAT for resulting neoplastic bone marrow specimens submitted for Cytogenetics analysis to 21 calendar days for 90% of specimens by June 2011. 7
 Background The Cytogenetics Laboratory and Lab Quality Improvement group wanted to improve on its 2008 CS&E Project (Phase 1) which had 85% of its neoplastic bone marrow specimens completed within a 21 day period. Strategic Alignment: We will continue to increase the quality, safety and value of our clinical care. 8
Background The Cytogenetics Laboratory and Lab Quality Improvement group wanted to improve on its 2008 CS&E Project (Phase 1) which had 85% of its neoplastic bone marrow specimens completed within a 21 day period. Strategic Alignment: We will continue to increase the quality, safety and value of our clinical care. 8
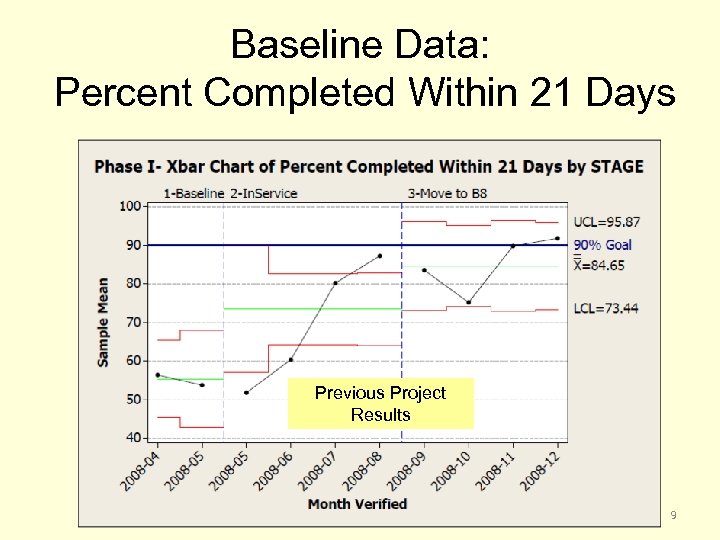 Baseline Data: Percent Completed Within 21 Days Previous Project Results 9
Baseline Data: Percent Completed Within 21 Days Previous Project Results 9
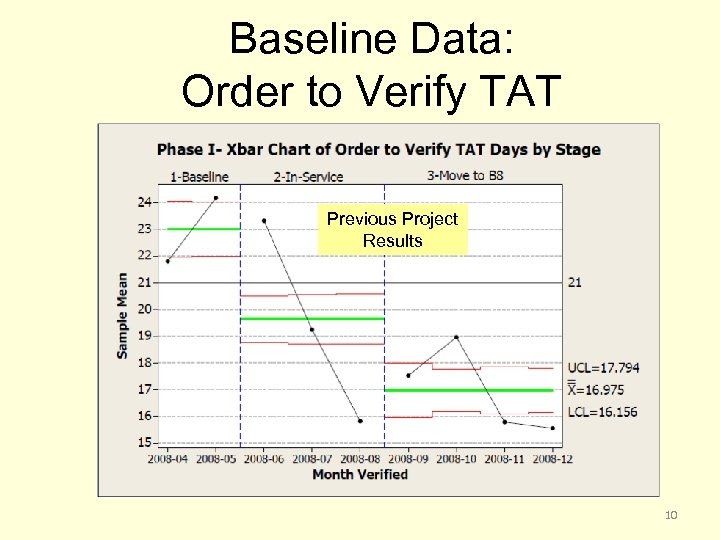 Baseline Data: Order to Verify TAT Previous Project Results 10
Baseline Data: Order to Verify TAT Previous Project Results 10
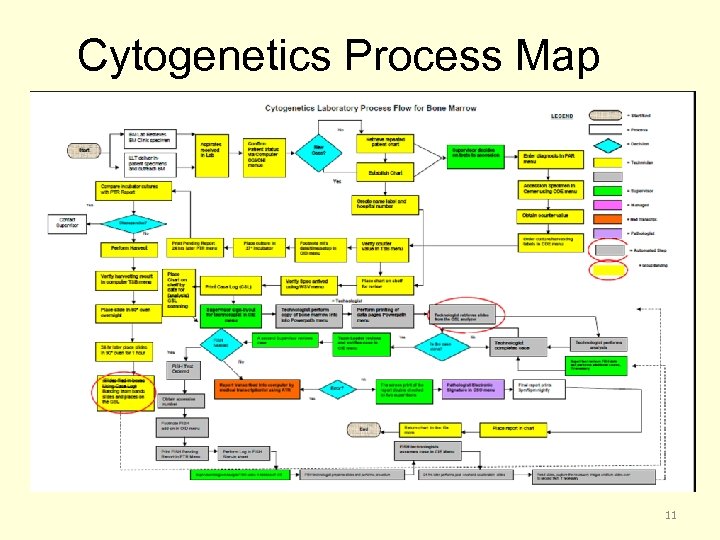 Cytogenetics Process Map 11
Cytogenetics Process Map 11
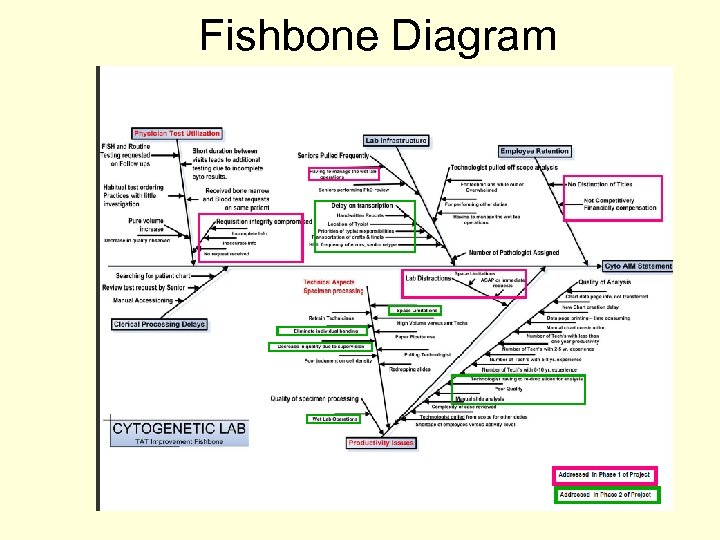 Fishbone Diagram
Fishbone Diagram
 Major Identified Issues • Overwhelmed Cytogenetics Technologists • Manual Slide Analysis • Decrease in Efficiency • Delay in the transcription of cases • Increase in the workload • Space Limitations
Major Identified Issues • Overwhelmed Cytogenetics Technologists • Manual Slide Analysis • Decrease in Efficiency • Delay in the transcription of cases • Increase in the workload • Space Limitations
 Implemented Solutions • No Patient Left Behind (Jan 2009)
Implemented Solutions • No Patient Left Behind (Jan 2009)
 Implemented Solutions • Banding Process Change (May 2009) • Hired Full Time Secretary (Sept 2009) • Genetix Slide Loader (GSL) (March 2010)
Implemented Solutions • Banding Process Change (May 2009) • Hired Full Time Secretary (Sept 2009) • Genetix Slide Loader (GSL) (March 2010)
 Implemented Solutions • Cross Trained Cytogenetics Technologists (Mar 2010) • Changed the sample media (Sept 2010) • Changed the staffing hours (Jan 2011) • Acquired space for the Breast pathologists to sign out remotely (Jan 2011)
Implemented Solutions • Cross Trained Cytogenetics Technologists (Mar 2010) • Changed the sample media (Sept 2010) • Changed the staffing hours (Jan 2011) • Acquired space for the Breast pathologists to sign out remotely (Jan 2011)
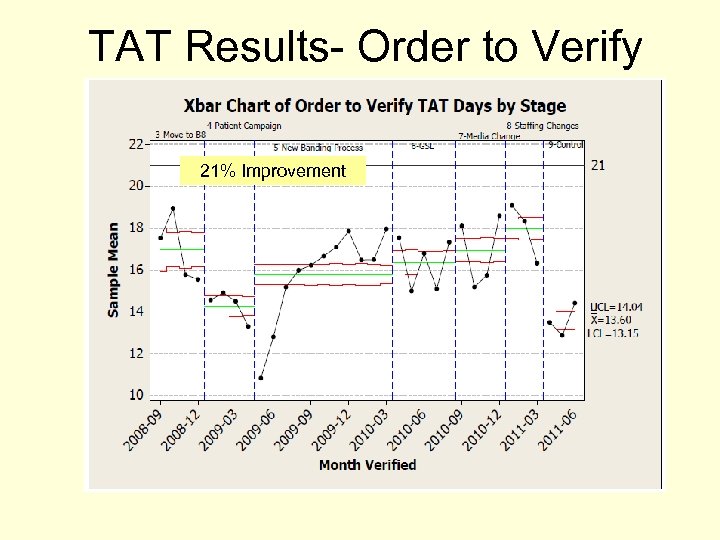 TAT Results- Order to Verify 21% Improvement
TAT Results- Order to Verify 21% Improvement
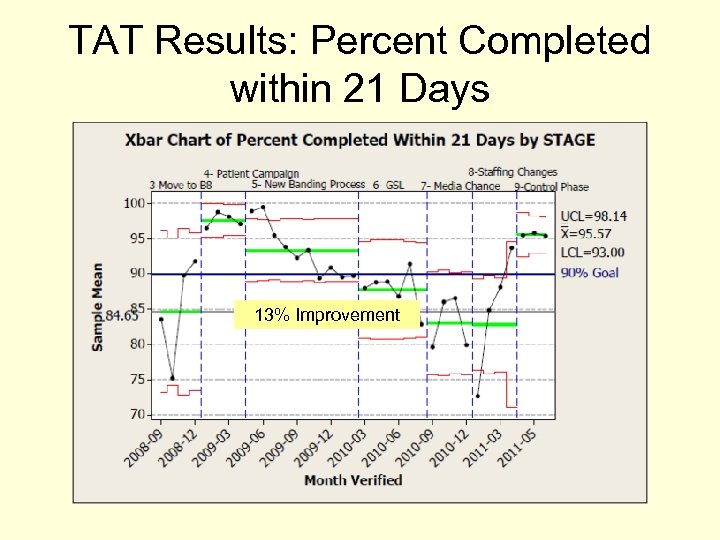 TAT Results: Percent Completed within 21 Days 13% Improvement
TAT Results: Percent Completed within 21 Days 13% Improvement
 Return on Investment Hard Savings: • Annualized margin increase of $547, 200 • Jan 2008 - Apr 2010 vs. May 2010 - Jun 2011 • The lab began keeping over 61% of the cases that they were previously sending out to a reference lab. 19
Return on Investment Hard Savings: • Annualized margin increase of $547, 200 • Jan 2008 - Apr 2010 vs. May 2010 - Jun 2011 • The lab began keeping over 61% of the cases that they were previously sending out to a reference lab. 19
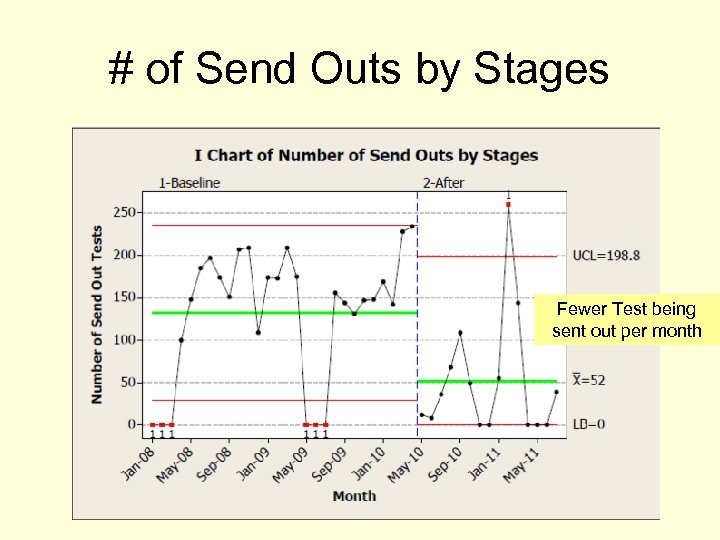 # of Send Outs by Stages Fewer Test being sent out per month
# of Send Outs by Stages Fewer Test being sent out per month
 Next Steps… • Go paperless with the updated version of our Cerner computer system. (Dec 2011) • Interface the GSL analyzer with the updated Cerner system. (Dec 2011) • Train the Lab Technicians to do more tasks
Next Steps… • Go paperless with the updated version of our Cerner computer system. (Dec 2011) • Interface the GSL analyzer with the updated Cerner system. (Dec 2011) • Train the Lab Technicians to do more tasks
 Lessons Learned • Careful planning pays off • Change Management is not as simple as it sounds • You can’t communicate too much! • Data-driven decisions work • Collaboration improves buy-in • Input & feedback from stakeholders are important • Automation can create capacity 22
Lessons Learned • Careful planning pays off • Change Management is not as simple as it sounds • You can’t communicate too much! • Data-driven decisions work • Collaboration improves buy-in • Input & feedback from stakeholders are important • Automation can create capacity 22
 Thank you!
Thank you!


