75e564f3bcbdaf11fa55fb393a539eb6.ppt
- Количество слайдов: 34

Clinical Research Bridging the Gap between Basic Science and Improved Human Health FASEB Clinical Research Subcommittee

What Is Clinical Research? • Research conducted on humans or human tissues that makes use of patient data. • Includes study of disease mechanisms, therapeutic interventions, epidemiology, and clinical trials. • Aimed at understanding human disease and improving human health. • Interaction between researchers and patient data is a key feature. Definitions of Clinical Research

Why is Clinical Research Important? • Enhances our understanding of human physiology and pathophysiology. • Translates basic research into medical care. • Informs and drives basic research. • Improves diagnostic tools and preventive care. • Improves human health!

What Can Clinical Research Accomplish? • Research in human physiology and pathophysiology translates basic research into knowledge of disease mechanisms and medical therapeutics. • Clinical trials establish safety and efficacy of new interventions. • Epidemiological and behavioral research identify high risk populations with potential to benefit from prevention, early detection, or therapeutic intervention. • Outcomes and health services research assess the health impact and cost-effectiveness of interventions.

Physiological Research • Carefully controlled studies in patients or normal subjects that are intended to understand basic physiologic mechanisms and their disruption in human disease. • Translation of basic research findings (in vitro and preclinical animal investigation) to human subjects to confirm the human relevance of proposed disease mechanisms, which will inform scientifically targeted medical therapeutics. • This work is usually done through General Clinical Research Centers (GCRC) and informs clinical trials.

Clinical Trials • Research that prospectively assigns human subjects to intervention and concurrent comparison/control groups to study the cause-and-effect relationship between a medical intervention and a health outcome (International Committee of Medical Journal Editors). http: //clinicaltrials. gov/ct/info/whatis#types

Clinical Trial Phases • Phase I trials: First-time test of intervention in a small group of people (20 -80) to evaluate safety, determine appropriate dosage, and identify side effects. • Phase II trials: Intervention given to a larger group (100 -300) to evaluate effectiveness and safety. • Phase III trials: Intervention given to large groups (1, 000 -3, 000) to confirm effectiveness, monitor side effects, compare to other treatments, and collect information that will allow it to be used safely. • Phase IV trials: Post marketing studies determine additional information including risks, benefits, and optimal use of an intervention.

Population Research • Epidemiology and behavioral research to identify high risk populations with potential to benefit from prevention, early detection, or intervention of established disease processes. • Outcomes and health services research to assess the health impact and cost-effectiveness of an effective intervention in a population or community setting.
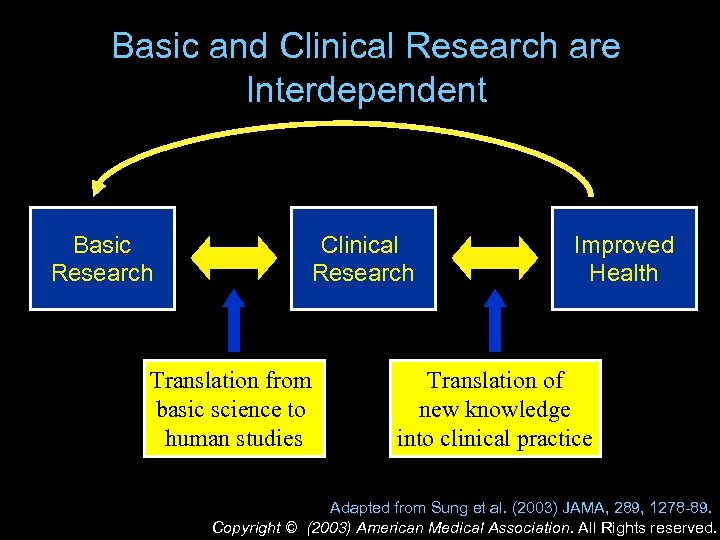
Basic and Clinical Research are Interdependent Basic Research Clinical Research Translation from basic science to human studies Improved Health Translation of new knowledge into clinical practice Adapted from Sung et al. (2003) JAMA, 289, 1278 -89. Copyright © (2003) American Medical Association. All Rights reserved.

Linking HIV to AIDS • Basic research into DNA, viruses, and retroviruses paved the way for the identification of the first human retrovirus in cancer patients. • Clinical studies showed that cancer-causing retroviruses exert similar effects to AIDS on the human immune system. • This helped scientists to identify HIV, a retrovirus in the same family as the cancer-causing retroviruses, as the one responsible for the development of AIDS. http: //opa. faseb. org/pdf/Finding_Chinks. pdf

Helping Newborns Breathe • Tens of thousands of babies died annually from a mysterious respiratory ailment. • Clinical investigators characterized the patients and studied infant respiration and human lung physiology. • Separately, basic studies of surface tension and pulmonary physiology allowed researchers to identify and characterize pulmonary surfactant. • With this information in mind, clinical and basic scientists determined that the deaths were due to a lack of surfactant, which caused the alveoli, or air sacs in the lungs, to collapse. • Treatments were then developed in the lab and tested in and applied to patients with great clinical success. http: //opa. faseb. org/pdf/babies_bubbles. pdf

Combating Breast Cancer • Basic research findings demonstrated the anti-tumor effects of tamoxifen in lab animals. • Clinical researchers showed that tamoxifen shrank breast tumors in afflicted women and reduced the risk of tumor recurrence. • Clinical researchers also demonstrated that healthy women treated with tamoxifen were less likely to develop breast cancer. • Tamoxifen is now widely used as a breast cancer treatment and preventive. http: //opa. faseb. org/pdf/Breast_Cancer_Breakthru. pdf

Who Funds Clinical Research? • Federal Government – – – National Institutes of Health Department of Veterans Affairs Centers for Disease Control and Prevention Agency for Health Care Research and Quality Department of Defense • State and Local Governments • Private Organizations – Foundations and other not-for-profits – Industry
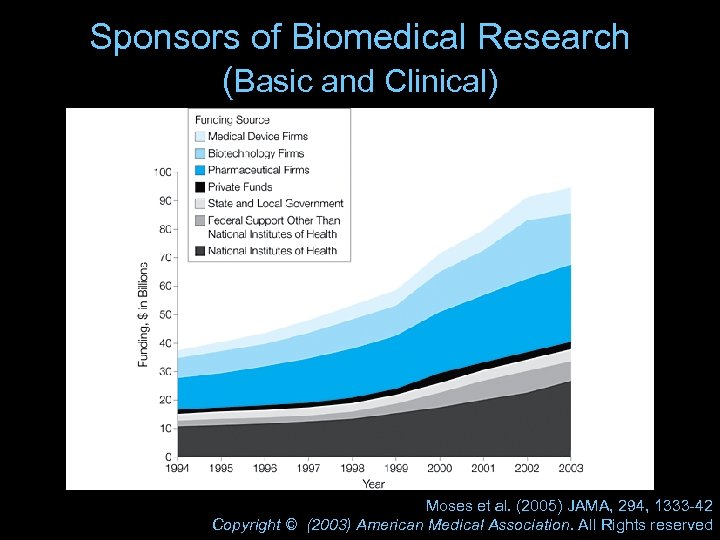
Sponsors of Biomedical Research (Basic and Clinical) Moses et al. (2005) JAMA, 294, 1333 -42 Copyright © (2003) American Medical Association. All Rights reserved

National Institutes of Health • NIH Mission: Science in pursuit of fundamental knowledge about the nature and behavior of living systems and the application of that knowledge to extend healthy life and reduce the burdens of illness and disability. • NIH is a major sponsor of clinical research. Former NIH Director, • Clinical Research Initiatives: Dr. Elias Zerhouni – NIH Roadmap: Reengineering the Clinical Research Enterprise – Clinical and Translational Science Awards
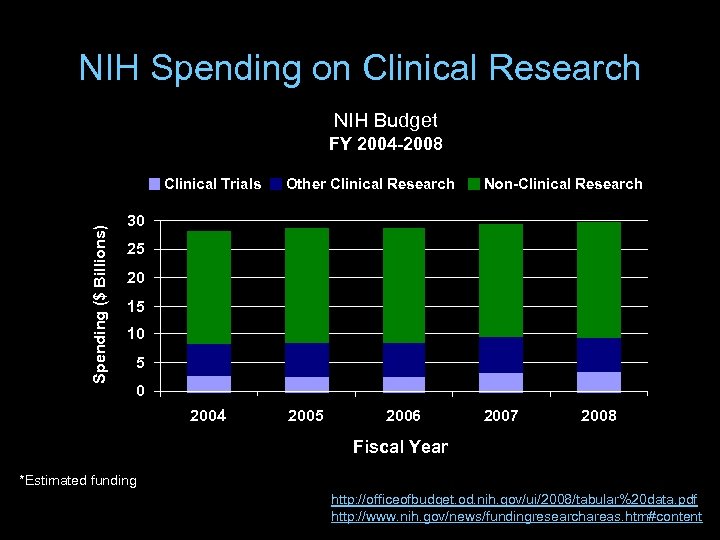
NIH Spending on Clinical Research NIH Budget FY 2004 -2008 Spending ($ Billions) Clinical Trials Other Clinical Research Non-Clinical Research 2005 2007 30 25 20 15 10 5 0 2004 2006 2008 Fiscal Year *Estimated funding http: //officeofbudget. od. nih. gov/ui/2008/tabular%20 data. pdf http: //www. nih. gov/news/fundingresearchareas. htm#content
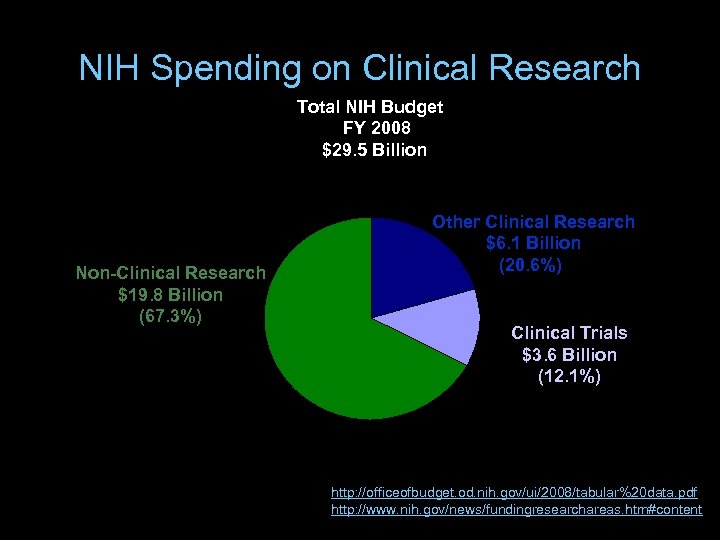
NIH Spending on Clinical Research Total NIH Budget FY 2008 $29. 5 Billion Non-Clinical Research $19. 8 Billion (67. 3%) Other Clinical Research $6. 1 Billion (20. 6%) Clinical Trials $3. 6 Billion (12. 1%) http: //officeofbudget. od. nih. gov/ui/2008/tabular%20 data. pdf http: //www. nih. gov/news/fundingresearchareas. htm#content

Reengineering the Clinical Research Enterprise • Improve medical diagnostics and treatment. • Enhance access to and sharing of data through integrated clinical research networks. • Enhance clinical outcomes assessment by developing a computerized system to collect and store patient-reported outcomes data. http: //nihroadmap. nih. gov/clinicalresearch/

Reengineering the Clinical Research Enterprise • Expand diversify the clinical research workforce by optimizing training and career development programs. • Harmonize federal requirements regarding conduct of clinical research to facilitate compliance with regulations and policies. • Promote translational research by improving clinical research infrastructure (Clinical and Translational Science Award program). http: //nihroadmap. nih. gov/clinicalresearch/
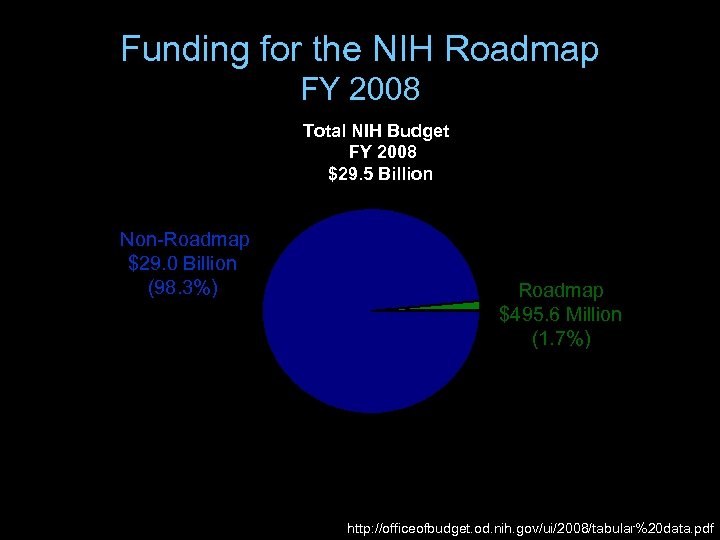
Funding for the NIH Roadmap FY 2008 Total NIH Budget FY 2008 $29. 5 Billion 2% Non-Roadmap $29. 0 Billion (98. 3%) Roadmap $495. 6 Million (1. 7%) 98% http: //officeofbudget. od. nih. gov/ui/2008/tabular%20 data. pdf

Clinical and Translational Science Awards • Transforms academic health centers into definable academic homes for clinical and translational science, which work together to improve the conduct of clinical research. • Subsumes some General Clinical Research Centers (GCRC). • 38 academic health centers currently participating. • Funded from existing clinical research programs and Roadmap dollars. • No funds are taken from basic research. http: //ctsaweb. org/about. html

Department of Veterans Affairs • Medical and Prosthetics Research Program—Core of the VA research and development program. • VA has four major R&D areas: – – Clinical Science Research and Development Service Health Services Research and Development Service Rehabilitation Research and Development Service Biomedical Laboratory Research and Development Service • 75% of supported researchers are clinician scientists. • More than half of all VA research is for investigator-initiated projects. • All R&D investment is intramural. http: //www. aaas. org/spp/rd/va 08 p. htm
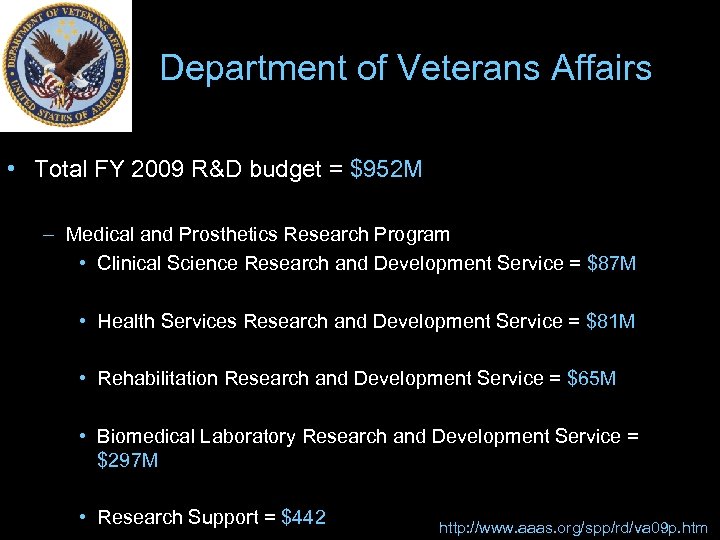
Department of Veterans Affairs • Total FY 2009 R&D budget = $952 M – Medical and Prosthetics Research Program • Clinical Science Research and Development Service = $87 M • Health Services Research and Development Service = $81 M • Rehabilitation Research and Development Service = $65 M • Biomedical Laboratory Research and Development Service = $297 M • Research Support = $442 http: //www. aaas. org/spp/rd/va 09 p. htm

Other Federal Sponsors of Clinical Research • Agency for Healthcare Research and Quality – Clinical practice, patient safety, health IT, primary and priority populations care, and evidence-based medicine. • Centers for Disease Control and Prevention – Infectious disease, disability, environmental health, health statistics. • Department of Defense – Congressional Special Interest Research Programs: Includes research on breast, prostate, and ovarian cancer and other diseases. – Deployment Health-Related Research: Research on conditions affecting deployed or previously deployed military (e. g. , PTSD). – Defense Advanced Research Projects Agency: Includes funding for applied biological research (e. g. , effects of bioagent exposure).

Private Sponsors of Clinical Research • Not-for-profits – Foundations, voluntary health organizations, and research institutes. – Examples: Bill and Melinda Gates Foundation, Burroughs-Wellcome Foundation, Juvenile Diabetes Research Foundation. • Industry – Pharmaceutical, biotechnology, and medical device firms. • Pharmaceutical Research and Manufacturers of America (Ph. RMA). • Biotechnology Industry Organization (BIO). – Total R&D spending by industry in 2006 estimated at $56. 1 B billion – Total Ph. RMA spending on R&D in 2006 estimated at $43. 4 billion
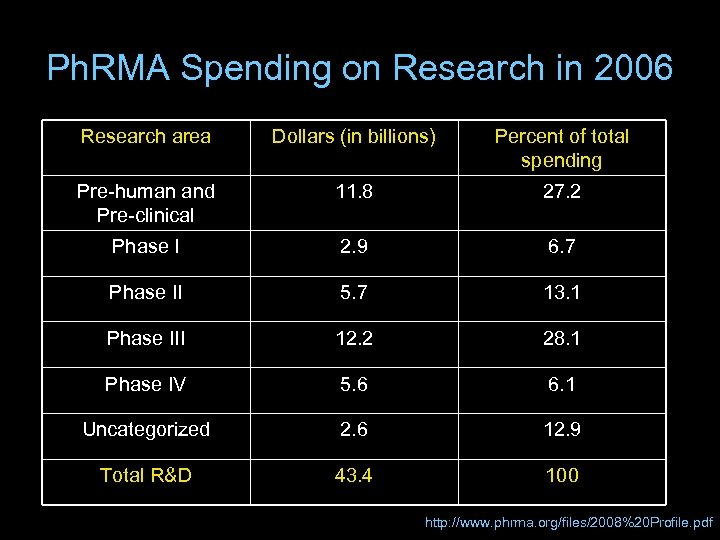
Ph. RMA Spending on Research in 2006 Research area Dollars (in billions) Percent of total spending Pre-human and Pre-clinical 11. 8 27. 2 Phase I 2. 9 6. 7 Phase II 5. 7 13. 1 Phase III 12. 2 28. 1 Phase IV 5. 6 6. 1 Uncategorized 2. 6 12. 9 Total R&D 43. 4 100 http: //www. phrma. org/files/2008%20 Profile. pdf

How is Clinical Research Regulated? • Office of Human Research Protections – Protects the rights, welfare, and well-being of subjects involved in research conducted or supported by the Department of Health and Human Services. • Food and Drug Administration – Regulates the approval process and assures the safety, efficacy, and security of drugs, biological products, medical devices. • Local Institutional Review Boards – Conducts initial review of research proposals. – Ensures participants are not exposed to unreasonable risks and that they give informed consent. – Conducts continuing review of approved research to ensure that human -subjects protections remain in force.

Challenges Faced By Clinical Researchers • Difficulty balancing patient care and research responsibilities. • Expensive medical training; lower salaries compared to private clinicians. • Long training time compared to medical school peers. • Burdensome regulations and paperwork. • Insufficient mentoring due to competing pressures. • Low success rate for clinical R 01 s.

Addressing the Challenges • Assess clinical research career development awards to optimize retention of clinical researchers. • Promote effective mentoring by senior clinical researchers. • Implement loan forgiveness programs to boost retention in the research track. • Streamline, standardize, and simplify regulatory requirements. • Include reasonable “protected time” requirements in grants to ensure sufficient time for research. • Ensure adequate representation of clinical researchers on review panels for clinical research grants. For additional information: Kotchen et al. (2006) J. Investigative Medicine; Murillo et al. (2006) Academic Medicine; Sung et al. (2003) JAMA.

Why Improve the Landscape for Clinical Research? • Enhance recruitment and retention of clinical researchers. • Prevent future shortage of clinical researchers. • Optimize bench to bedside translation of research. • Drive basic science discoveries. • Improve human health!

Resources: Clinical Research Organizations • Association of American Medical Colleges • Association for Patient-Oriented Research • Clinical Research Forum • Health Research Alliance • Institute of Medicine: Clinical Research Roundtable • National Institutes of Health

Resources: Clinical Research Reports • The NIH Director’s Panel on Clinical Research Report to the Advisory Committee to the NIH Director (The Nathan Report, 1997, NIH). • The Physician Scientist: Career Issues and Challenges at the Year 2000 (Zemlo et al. , 2000, FASEB Journal). • Central Challenges Facing the Clinical Research Enterprise (Sung et al. , 2005, JAMA). • Meeting the Challenges Facing Clinical Research: Solutions Proposed by Leaders of Medical Specialty and Clinical Research Societies (Murillo et al. , 2006, Academic Medicine) • Outcomes of National Institutes of Health Peer Review of Clinical Grant Applications (Kotchen et al. , 2006, J. Investigative Medicine). • Promoting Translational and Clinical Science: The Critical Role of Medical Schools and Teaching Hospitals (2006, AAMC). • New Physician-Investigators Receiving National Institutes of Health Research Project Grants (Dickler et al. , 2007, JAMA).

Resources: FASEB’s NIH Advocacy Tool • FASEB has created a customizable slide presentation for scientists, university deans, and professors to use locally to demonstrate NIH's impact on human health. For more information: http: //opa. faseb. org/pages/Publications/NIH_PPT. htm

Interested in Learning More? Please visit FASEB’s Office of Public Affairs at: http: //opa. faseb. org The Federation of American Societies for Experimental Biology
75e564f3bcbdaf11fa55fb393a539eb6.ppt