dc33ba9b315d38c6408eb3ccf3b9720f.ppt
- Количество слайдов: 29
 Clinical Registries in Cardiac Surgery Peter S. Greene, MD CMIO, Johns Hopkins Medicine Diane Alejo Information Systems Manager Division of Cardiac Surgery September 15, 2010 ICTR Clinical Registry Workshop
Clinical Registries in Cardiac Surgery Peter S. Greene, MD CMIO, Johns Hopkins Medicine Diane Alejo Information Systems Manager Division of Cardiac Surgery September 15, 2010 ICTR Clinical Registry Workshop
 Cardiac Surgery Data Management Cardiac Surgery Database spans 1944 - 2010 • Clinical and administrative data tracking • Supports IRB approved clinical research activities • Allows longitudinal outcome follow-up STS Adult Cardiac Surgery Data / STS Congenital Data Heart and Heart- Lung Transplant Database UNOS Registry ISHLT / INTERMACS VAD Registry Collaborative Transplant Research Database
Cardiac Surgery Data Management Cardiac Surgery Database spans 1944 - 2010 • Clinical and administrative data tracking • Supports IRB approved clinical research activities • Allows longitudinal outcome follow-up STS Adult Cardiac Surgery Data / STS Congenital Data Heart and Heart- Lung Transplant Database UNOS Registry ISHLT / INTERMACS VAD Registry Collaborative Transplant Research Database
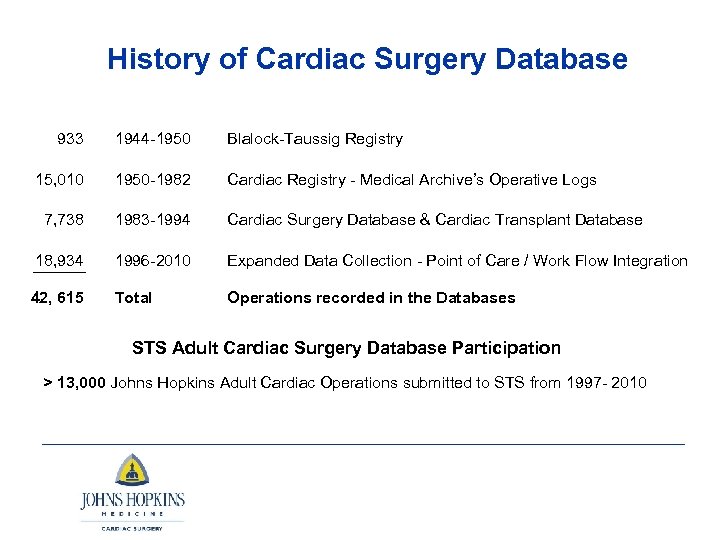 History of Cardiac Surgery Database 933 1944 -1950 Blalock-Taussig Registry 15, 010 1950 -1982 Cardiac Registry - Medical Archive’s Operative Logs 7, 738 1983 -1994 Cardiac Surgery Database & Cardiac Transplant Database 18, 934 1996 -2010 Expanded Data Collection - Point of Care / Work Flow Integration 42, 615 Total Operations recorded in the Databases STS Adult Cardiac Surgery Database Participation > 13, 000 Johns Hopkins Adult Cardiac Operations submitted to STS from 1997 - 2010
History of Cardiac Surgery Database 933 1944 -1950 Blalock-Taussig Registry 15, 010 1950 -1982 Cardiac Registry - Medical Archive’s Operative Logs 7, 738 1983 -1994 Cardiac Surgery Database & Cardiac Transplant Database 18, 934 1996 -2010 Expanded Data Collection - Point of Care / Work Flow Integration 42, 615 Total Operations recorded in the Databases STS Adult Cardiac Surgery Database Participation > 13, 000 Johns Hopkins Adult Cardiac Operations submitted to STS from 1997 - 2010
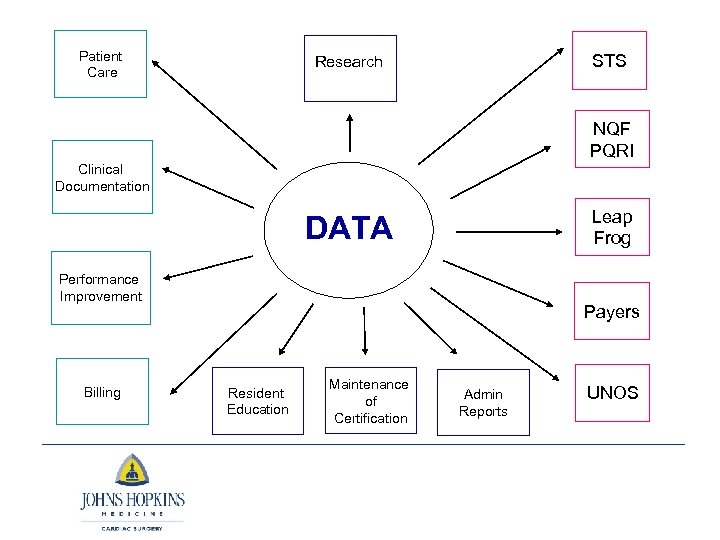 Patient Care STS Research NQF PQRI Clinical Documentation Leap Frog DATA Performance Improvement Billing Payers Resident Education Maintenance of Certification Admin Reports UNOS
Patient Care STS Research NQF PQRI Clinical Documentation Leap Frog DATA Performance Improvement Billing Payers Resident Education Maintenance of Certification Admin Reports UNOS
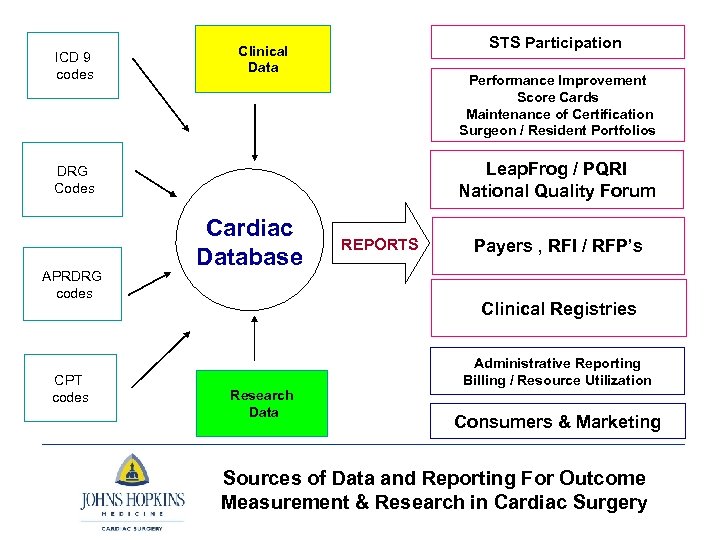 ICD 9 codes STS Participation Clinical Data Performance Improvement Score Cards Maintenance of Certification Surgeon / Resident Portfolios Leap. Frog / PQRI National Quality Forum DRG Codes APRDRG codes CPT codes Cardiac Database REPORTS Payers , RFI / RFP’s Clinical Registries Research Data Administrative Reporting Billing / Resource Utilization Consumers & Marketing Sources of Data and Reporting For Outcome Measurement & Research in Cardiac Surgery
ICD 9 codes STS Participation Clinical Data Performance Improvement Score Cards Maintenance of Certification Surgeon / Resident Portfolios Leap. Frog / PQRI National Quality Forum DRG Codes APRDRG codes CPT codes Cardiac Database REPORTS Payers , RFI / RFP’s Clinical Registries Research Data Administrative Reporting Billing / Resource Utilization Consumers & Marketing Sources of Data and Reporting For Outcome Measurement & Research in Cardiac Surgery
 Some Lessons Learned 1. Must have strong clinical leadership and pervasive buy-in 2. Must integrate with clinical workflow 3. Must provide net benefit to clinicians 4. Must stay within scope of readily known data 5. Must have a stable and capable clinical team 6. Must have a stable and capable data team 7. Must audit for completeness 8. Must give regular feedback 9. Must pre-stage submissions
Some Lessons Learned 1. Must have strong clinical leadership and pervasive buy-in 2. Must integrate with clinical workflow 3. Must provide net benefit to clinicians 4. Must stay within scope of readily known data 5. Must have a stable and capable clinical team 6. Must have a stable and capable data team 7. Must audit for completeness 8. Must give regular feedback 9. Must pre-stage submissions
 Outcome Data
Outcome Data
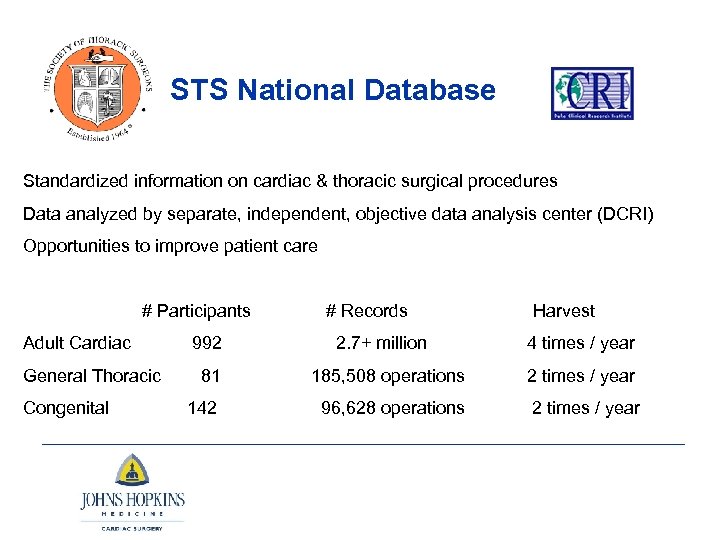 STS National Database Standardized information on cardiac & thoracic surgical procedures Data analyzed by separate, independent, objective data analysis center (DCRI) Opportunities to improve patient care # Participants # Records Harvest Adult Cardiac 992 2. 7+ million 4 times / year General Thoracic 81 185, 508 operations 2 times / year Congenital 96, 628 operations 2 times / year 142
STS National Database Standardized information on cardiac & thoracic surgical procedures Data analyzed by separate, independent, objective data analysis center (DCRI) Opportunities to improve patient care # Participants # Records Harvest Adult Cardiac 992 2. 7+ million 4 times / year General Thoracic 81 185, 508 operations 2 times / year Congenital 96, 628 operations 2 times / year 142
 STS National Database STS Pilot Pay For Performance (P 4 P) Program Incentive payments for achievement of thresholds in performance measures A model of quality improvement with 3 types of measures: Structureal: IT, database participation, volume Process: IMA use, discharge beta blockers Outcomes: Mortality, Morbidity: CVA, renal failure Blended STS NCD and financial (UB-92) database NQF performance measures 2007 PQRI Initiative – CMS New 2007 STS Composite Scoring System
STS National Database STS Pilot Pay For Performance (P 4 P) Program Incentive payments for achievement of thresholds in performance measures A model of quality improvement with 3 types of measures: Structureal: IT, database participation, volume Process: IMA use, discharge beta blockers Outcomes: Mortality, Morbidity: CVA, renal failure Blended STS NCD and financial (UB-92) database NQF performance measures 2007 PQRI Initiative – CMS New 2007 STS Composite Scoring System
 STS National Database DCRI – Data Warehouse and Analysis Center Data transmitted electronically National, Regional and “Like Institution” benchmarking Reports include site specific, risk adjusted, regional and national aggregate date including morbidity, mortality and LOS for CABG, Valves and CABG/Valve surgery Statistical Analysis – Risk Modeling- Logistic Regression, Hierarchical regression modeling
STS National Database DCRI – Data Warehouse and Analysis Center Data transmitted electronically National, Regional and “Like Institution” benchmarking Reports include site specific, risk adjusted, regional and national aggregate date including morbidity, mortality and LOS for CABG, Valves and CABG/Valve surgery Statistical Analysis – Risk Modeling- Logistic Regression, Hierarchical regression modeling
 STS National Database STS Auditing Risk factor model variables NQF measures Op log procedures Operative deaths and morbidity
STS National Database STS Auditing Risk factor model variables NQF measures Op log procedures Operative deaths and morbidity
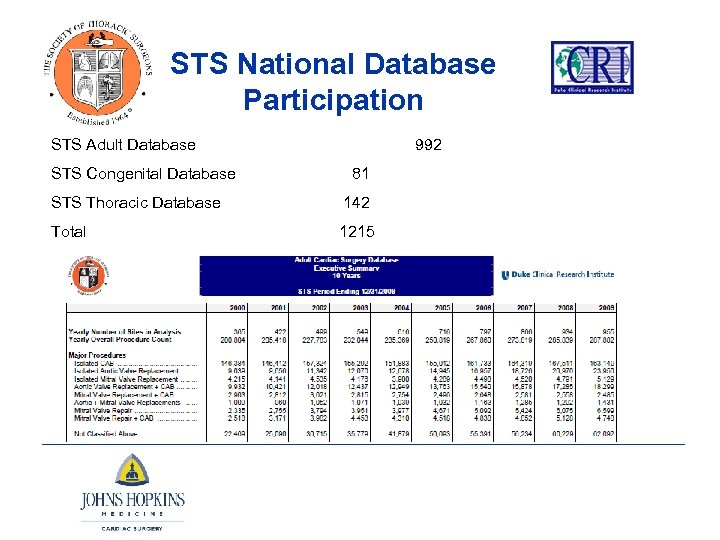 STS National Database Participation STS Adult Database 992 STS Congenital Database 81 STS Thoracic Database 142 Total 1215
STS National Database Participation STS Adult Database 992 STS Congenital Database 81 STS Thoracic Database 142 Total 1215
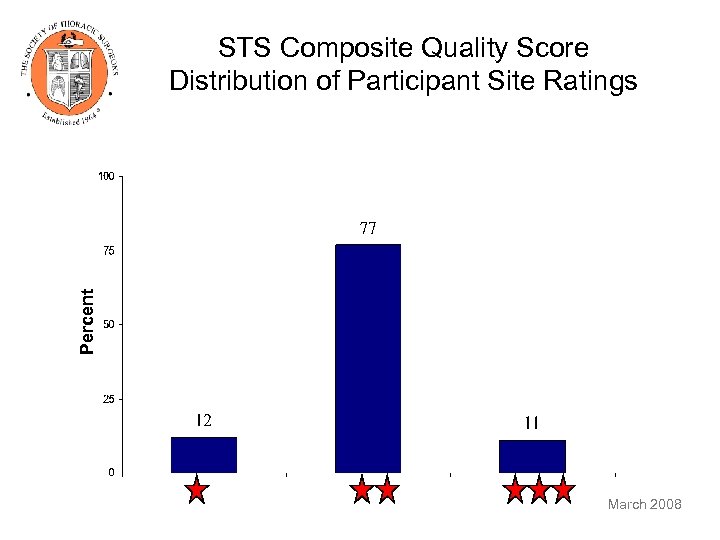 Percent STS Composite Quality Score Distribution of Participant Site Ratings March 2008
Percent STS Composite Quality Score Distribution of Participant Site Ratings March 2008
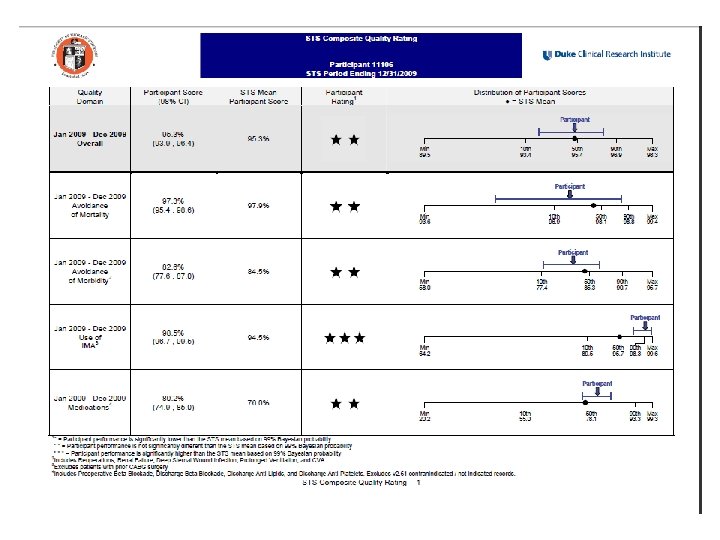
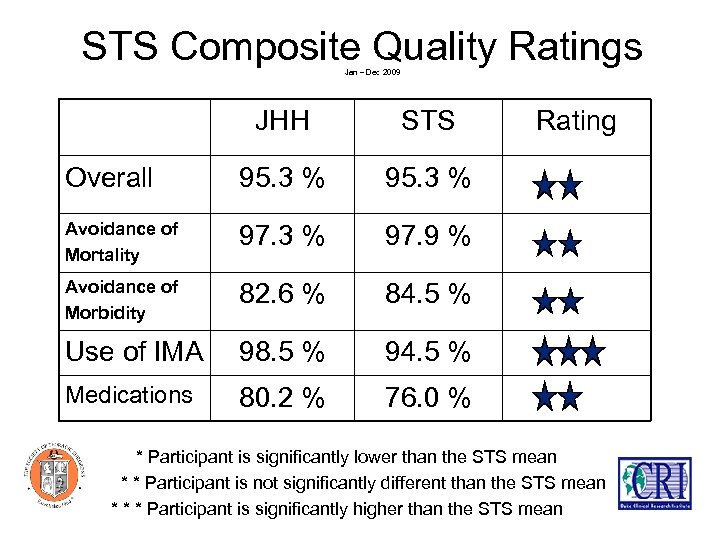 STS Composite Quality Ratings Jan – Dec 2009 JHH STS Overall 95. 3 % Avoidance of Mortality 97. 3 % 97. 9 % Avoidance of Morbidity 82. 6 % 84. 5 % Use of IMA 98. 5 % 94. 5 % Medications 80. 2 % Rating 76. 0 % * Participant is significantly lower than the STS mean * * Participant is not significantly different than the STS mean * * * Participant is significantly higher than the STS mean
STS Composite Quality Ratings Jan – Dec 2009 JHH STS Overall 95. 3 % Avoidance of Mortality 97. 3 % 97. 9 % Avoidance of Morbidity 82. 6 % 84. 5 % Use of IMA 98. 5 % 94. 5 % Medications 80. 2 % Rating 76. 0 % * Participant is significantly lower than the STS mean * * Participant is not significantly different than the STS mean * * * Participant is significantly higher than the STS mean

 Research Informatics Department of Surgery
Research Informatics Department of Surgery
 PREMISES OF THIS PROPOSAL FOR A SURGERY DATA CENTER • Almost every faculty member and research trainee has a need for accessing clinical data for research purposes • There is insufficient revenue to support a centralized research database – There is a modest amount of research database activity in the department • There is an extensive amount of clinical information within JHMI in an electronic format, but these exist in multiple sources • There is an extensive amount of surgical patient data being collected analyzed for non-research activities (e. g. : safety, accreditation, payers, training)
PREMISES OF THIS PROPOSAL FOR A SURGERY DATA CENTER • Almost every faculty member and research trainee has a need for accessing clinical data for research purposes • There is insufficient revenue to support a centralized research database – There is a modest amount of research database activity in the department • There is an extensive amount of clinical information within JHMI in an electronic format, but these exist in multiple sources • There is an extensive amount of surgical patient data being collected analyzed for non-research activities (e. g. : safety, accreditation, payers, training)
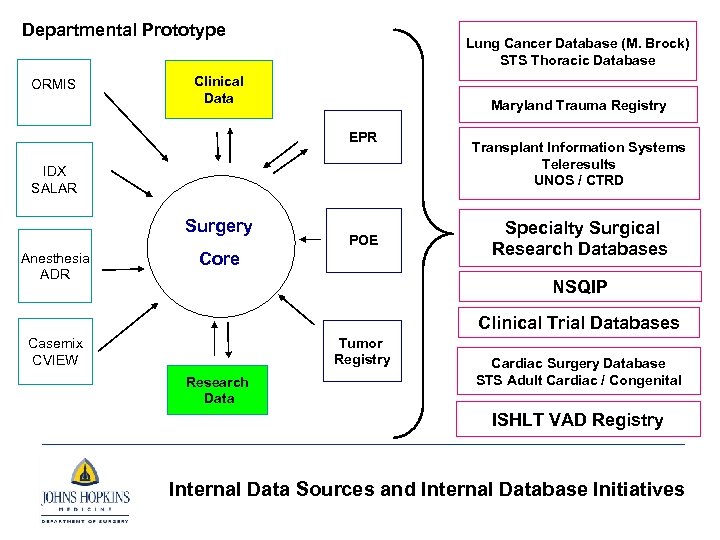 Departmental Prototype ORMIS Lung Cancer Database (M. Brock) STS Thoracic Database Clinical Data Maryland Trauma Registry EPR IDX SALAR Surgery Anesthesia ADR POE Core Transplant Information Systems Teleresults UNOS / CTRD Specialty Surgical Research Databases NSQIP Clinical Trial Databases Tumor Registry Casemix CVIEW Research Data Cardiac Surgery Database STS Adult Cardiac / Congenital ISHLT VAD Registry Internal Data Sources and Internal Database Initiatives
Departmental Prototype ORMIS Lung Cancer Database (M. Brock) STS Thoracic Database Clinical Data Maryland Trauma Registry EPR IDX SALAR Surgery Anesthesia ADR POE Core Transplant Information Systems Teleresults UNOS / CTRD Specialty Surgical Research Databases NSQIP Clinical Trial Databases Tumor Registry Casemix CVIEW Research Data Cardiac Surgery Database STS Adult Cardiac / Congenital ISHLT VAD Registry Internal Data Sources and Internal Database Initiatives

 Idea – Explore Influence of Randomness
Idea – Explore Influence of Randomness
 Idea – Quality Collaborative
Idea – Quality Collaborative
 Idea – Patient Registries From Dr. Adrian Puttgen Dept. Neurology, Critical Care http: //www. youtube. com/watch? v=WQ 2 PFo. Hpt. K 8
Idea – Patient Registries From Dr. Adrian Puttgen Dept. Neurology, Critical Care http: //www. youtube. com/watch? v=WQ 2 PFo. Hpt. K 8
 Clinical Registry Opportunities 1. Unique patient population 2. Unique patient tracking capability 3. Unique patient detail or comprehensiveness 4. Unique patient data integration 5. Regional quality programs 6. National quality programs
Clinical Registry Opportunities 1. Unique patient population 2. Unique patient tracking capability 3. Unique patient detail or comprehensiveness 4. Unique patient data integration 5. Regional quality programs 6. National quality programs


