33820781cb40bb79459f727dce600b4e.ppt
- Количество слайдов: 13

Clinical process for properly prescribe allergen immunotherapy Noel Rodriguez-Perez, MD Professor of pediatrics, Allergy& Immunology State University of Tamaulipas, School of Medicine

Clinical process for properly prescribe allergen immunotherapy Summary Statement 80: The efficacy of immunotherapy depends on achieving an optimal therapeutic dose of each of the constituents in the allergen immunotherapy extract. A Cox L, Nelson HS, Lockey RF. Journal of Allergen immunotherapy: A practice parameter third update Allergy Clin Immunol 2011; 127: S 1 -S 46
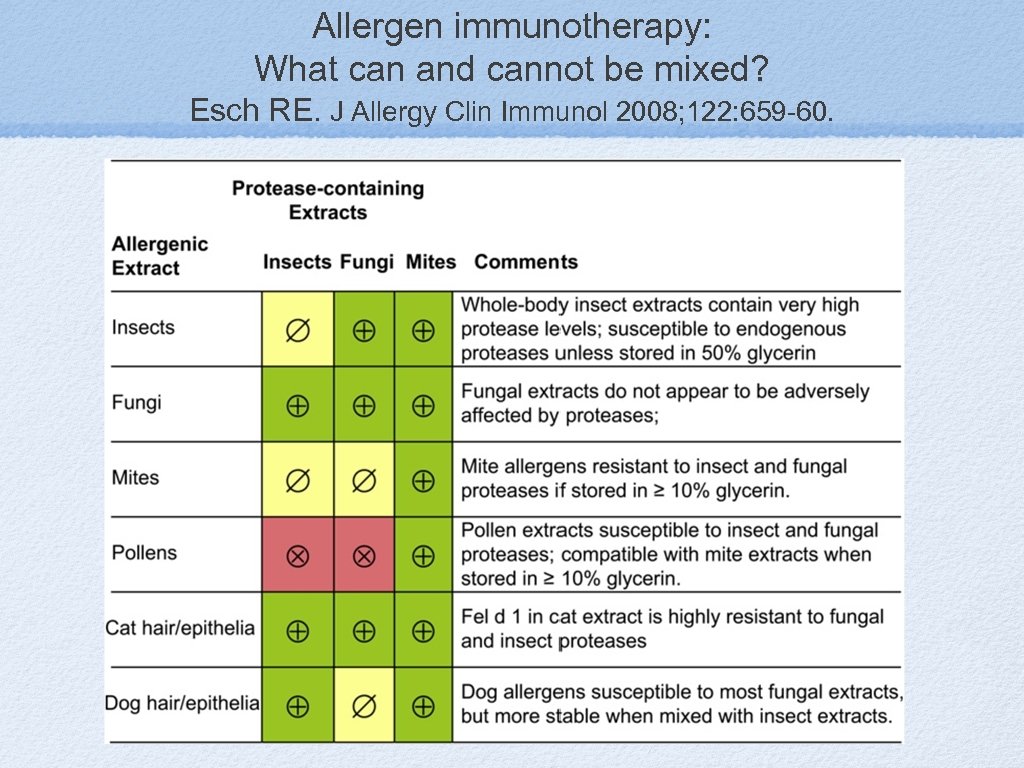
Allergen immunotherapy: What can and cannot be mixed? Esch RE. J Allergy Clin Immunol 2008; 122: 659 -60.

Clinical process for properly prescribe allergen immunotherapy CASE 1: M. D. K. Male. 16 years of age Previous history: Cow’s milk allergy in infancy. Hystory: 2 years, recurrent episodes of coriza, nasal congestion, acuous rhinorrea, epifora, fotofobia, intermitent dry cough. Symptoms, perenial with exacerbations in Winter, Spring and summer.
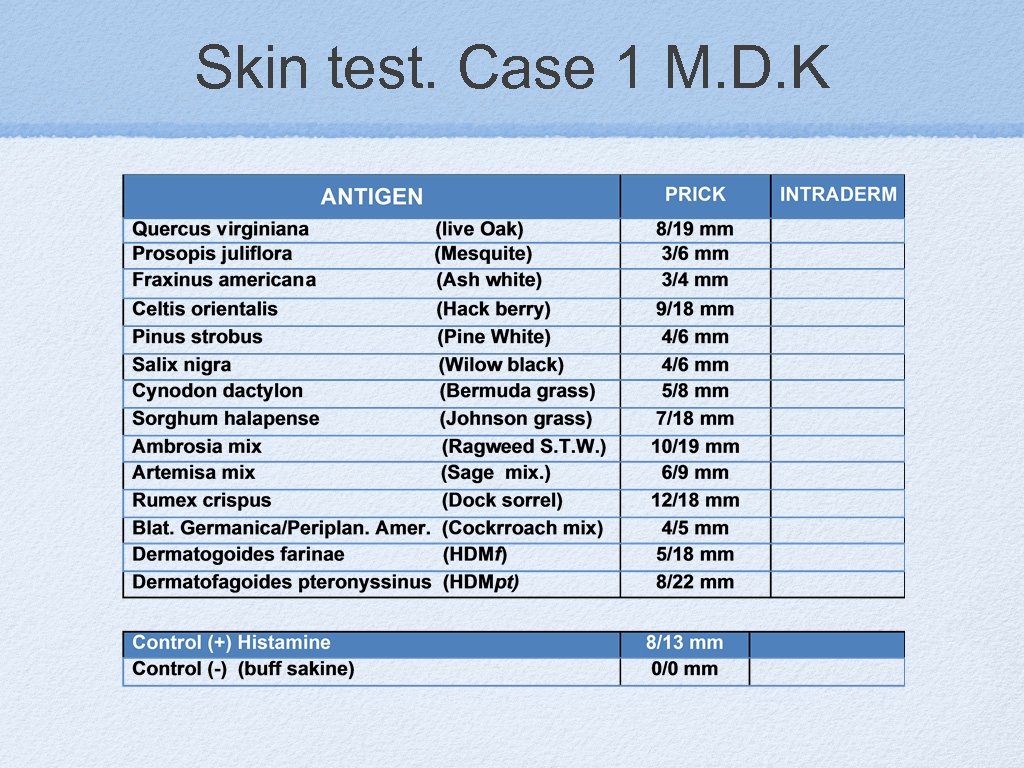
Skin test. Case 1 M. D. K

Case 1: M. D. K. 1. What allergens to be included? 2. How many in one vial? 3. Can we mix this unrelated allergens?

Zuberbier T, Bachert C, Bousquet PJ, Passalacqua G, Walter Canonica G, Merk H, Worm M, Wahn U, Bousquet J. GA 2 LEN/EAACI pocket guide for allergen-specific immunotherapy for allergic rhinitis and asthma. Allergy 2010; 65: 1525– 1530. Decision must be based on the allergen which causes: 1. The longest duration of symptoms per year 2. The most severe symptoms 3. A major impact on quality of life 4. Which is more difficult to avoid

CASE 1: M. D. K. House dust mites…Related to perennial symptoms Tree pollens… Late winter to early Spring Grass pollens…Late Spring to early Summer Weed pollens…Summer to early Autumn

CASE 1: M. D. K. Pollen SIT Quercus, Celtis, Sorgum, Ambrosia, Rumex Pollens probably effective dose range: 5 – 20 mcg (1: 100 – 1: 200 w/v) 5 pollen mix will dilute each other times 5. Maintenance dose Vial: 1: 200 V 1 X C 1 = V 2 X C 2 Were: V 1 = Final volume to prepare C 1 = Desired concentration of extract V 2 = Volume of extract needed (unknown) C 2 = Concentration of extract you will use (manufacturer concentrate) JACI. 2011; 127: S 1 -S 46
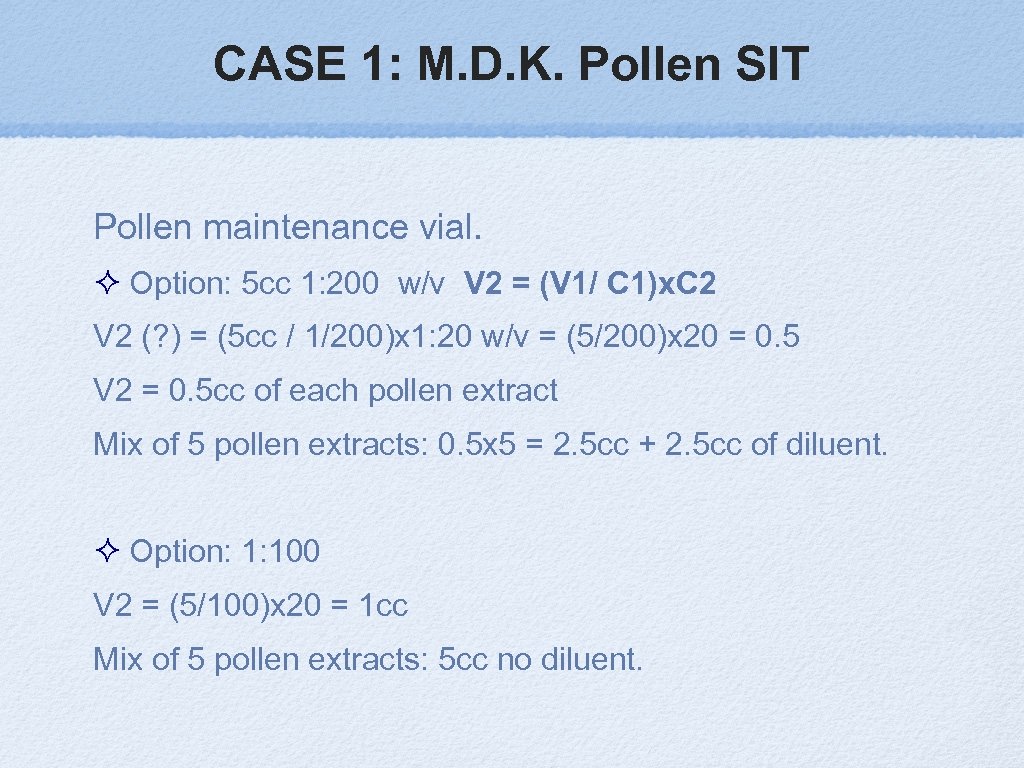
CASE 1: M. D. K. Pollen SIT Pollen maintenance vial. Option: 5 cc 1: 200 w/v V 2 = (V 1/ C 1)x. C 2 V 2 (? ) = (5 cc / 1/200)x 1: 20 w/v = (5/200)x 20 = 0. 5 V 2 = 0. 5 cc of each pollen extract Mix of 5 pollen extracts: 0. 5 x 5 = 2. 5 cc + 2. 5 cc of diluent. Option: 1: 100 V 2 = (5/100)x 20 = 1 cc Mix of 5 pollen extracts: 5 cc no diluent.

CASE 1: M. D. K: H. D. Mites or standarized Immunotherapy Mites maintenance vial F/Pt mix 50/50. Manufacturer concentrate: 10, 000; 30, 000 AU/m. L Effective maintenance dose: 500 – 2000 AU or 10 mcg/m. L (Mf: 10 mcg; MPt: 7 – 12 mcg/m. L) Maintenance vial: 2000 AU/m. L. V 2 = (V 1 x C 1)/C 2 V 2 (? ) = (5 cc x 2000)/10, 000 AU = 1. 0 cc V 2 = 1. 0 cc + 4 cc Diluent ( 12 mcg/m. L; 6 mcg/dose)
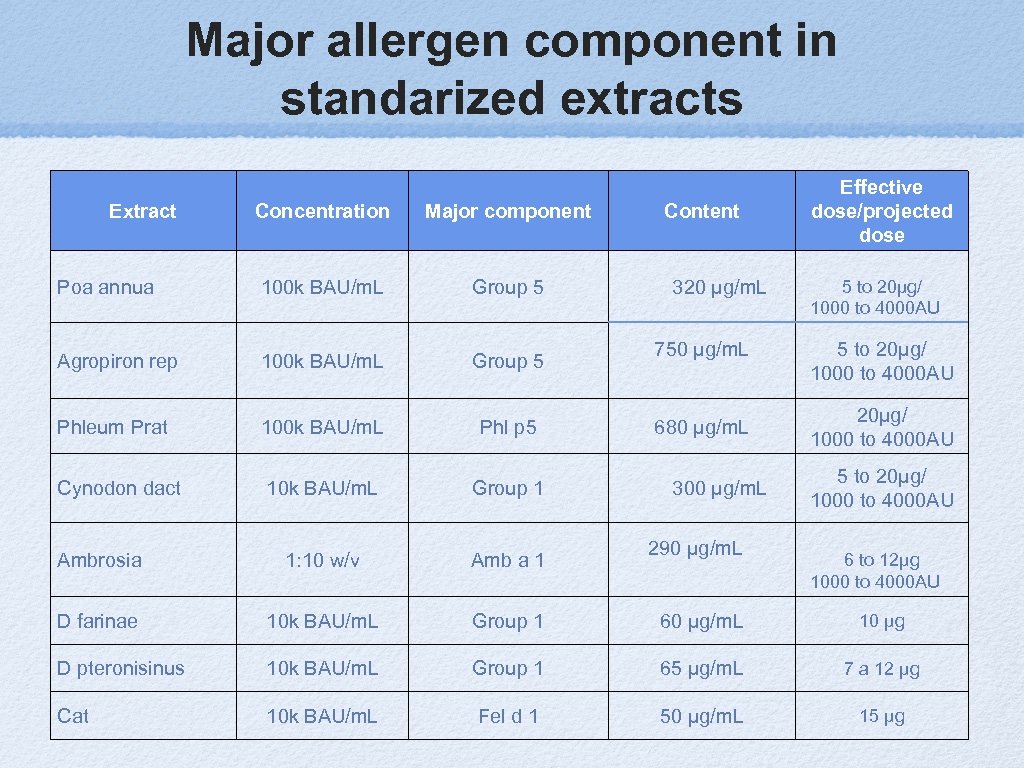
Major allergen component in standarized extracts Extract Content Effective dose/projected dose Concentration Major component Poa annua 100 k BAU/m. L Group 5 Agropiron rep 100 k BAU/m. L Group 5 Phleum Prat 100 k BAU/m. L Phl p 5 Cynodon dact 10 k BAU/m. L Group 1 Ambrosia 1: 10 w/v Amb a 1 D farinae 10 k BAU/m. L Group 1 60 μg/m. L 10 μg D pteronisinus 10 k BAU/m. L Group 1 65 μg/m. L 7 a 12 μg Cat 10 k BAU/m. L Fel d 1 50 μg/m. L 15 μg 320 μg/m. L 750 μg/m. L 680 μg/m. L 300 μg/m. L 290 μg/m. L 5 to 20µg/ 1000 to 4000 AU 5 to 20µg/ 1000 to 4000 AU 6 to 12µg 1000 to 4000 AU

Dose of Major component in mcg/m. L Suppose 5 cc maintenance dose vial for 10 doses of 0. 5 cc with Timothy grass pollen. 5 cc must contain 10 times the maintenance dose. Example: Timothy grass maintenance effective dose 20 mcg/m. L Phl p 5. 5 cc maintenance vial must contain 200 mcg. Manufacturer label 100000 AU/m. L = 680 mcg of Phl p 5/m. L. 200 divided by 680 = 0. 3 cc + 4. 7 cc of diluent.
33820781cb40bb79459f727dce600b4e.ppt