386dd7a63e3bf305b86d75ff3b6cf545.ppt
- Количество слайдов: 19

Clinical Observations Interoperability: A Use Case Scenario Rachel Richesson, Ph. D, MPH* University of South Florida College of Medicine Clinical Observations Interoperability Session HCLSIG Face to Face, November 8, 2007 http: //esw. w 3. org/topic/HCLS/Clinical. Observations. Interoperability * Acknowledgements to the members of the COI Task Force

Outline • Motivation and Background • Need • Use Case Scenario – Eligibility Criteria – Sample Protocols • Challenges • Next Steps


Clinical Sites Quebec Canada Toronto, Canada Tokyo Japan Paris, France Lyon, France London Edinburgh, UK Melbourne, Australia Sao Paulo, Brazil Bad Bramstedt, Groningen, Germany Netherlands Cambridge, UK

Motivation and Background • Identification & recruitment of eligible subjects is an obstacle for the conduct of clinical research. • Current screening mostly manual. • Unlikely that all of the data required to assess eligibility for a given protocol will be available in the EMR. • Final eligibility determined by the clinical research staff with F 2 F assessment. • Applications that identify likely candidates (“probably eligible”) would help researchers target recruitment efforts.

Need for Patient Recruitment • Ability to rapidly identify and recruit children for the right Clinical Trial – Children get access to the latest advances in medicine – Clinical researchers get cohorts to conduct studies • Use Case Scenario: – Can we leverage existing EMR data to identify and recruit appropriate patients for Clinical Trials?

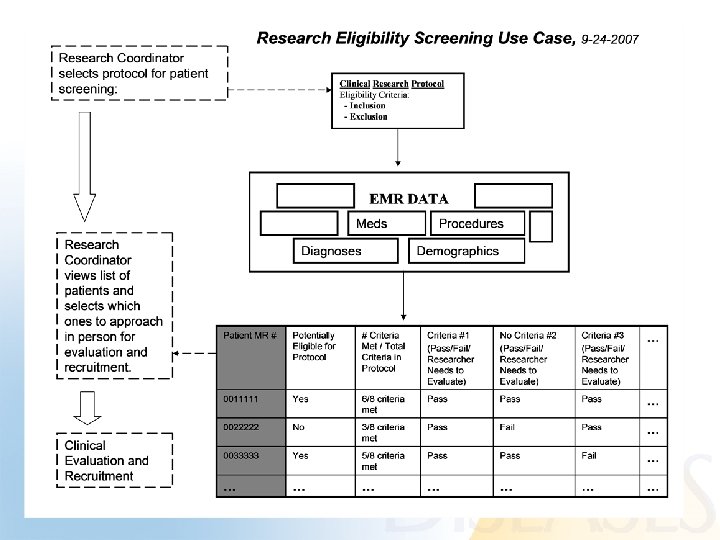
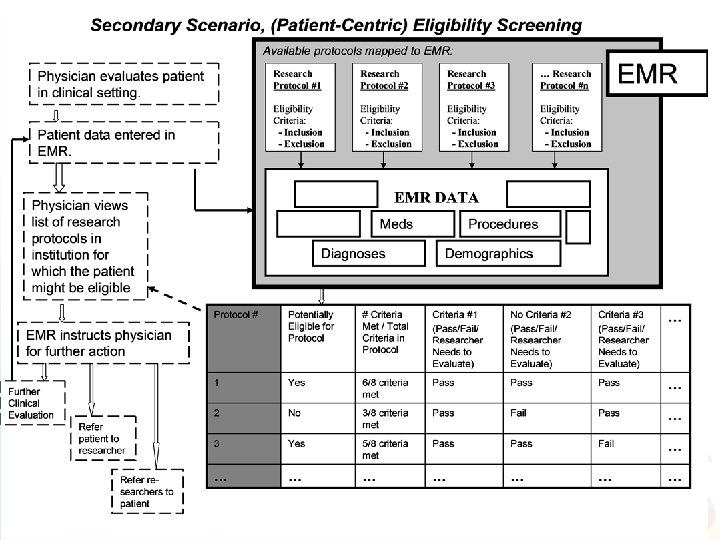
Use Case • • Patient Recruitment for Clinical Trials using EMR data Team effort Several iterations Final use-case posted to wiki (URL below): http: //esw. w 3. org/topic/HCLS/Clinical. Observations. Intero perability? action=Attach. File&do=get&target=Eligibilit y+Screening_FINAL_10 -8 -2007. doc
Variations • EMR data-driven triggers – Certain values/clinical scenarios in the EMR data for a patient would trigger retrieval and analysis of more EMR data – This could lead to a dynamic identification of the patient as a recruit for an ongoing clinical trial. • Physician-directed recruitment – Identify appropriate clinical trials for which a patient is eligible, based on his/her data.
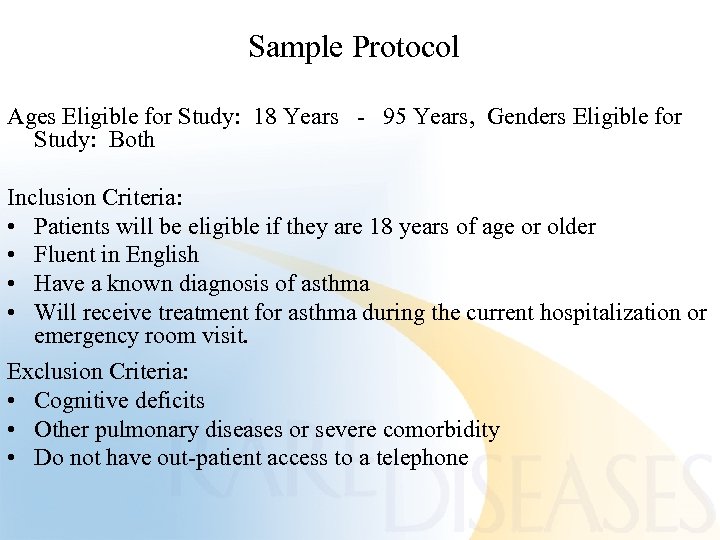
Sample Protocol Ages Eligible for Study: 18 Years - 95 Years, Genders Eligible for Study: Both Inclusion Criteria: • Patients will be eligible if they are 18 years of age or older • Fluent in English • Have a known diagnosis of asthma • Will receive treatment for asthma during the current hospitalization or emergency room visit. Exclusion Criteria: • Cognitive deficits • Other pulmonary diseases or severe comorbidity • Do not have out-patient access to a telephone
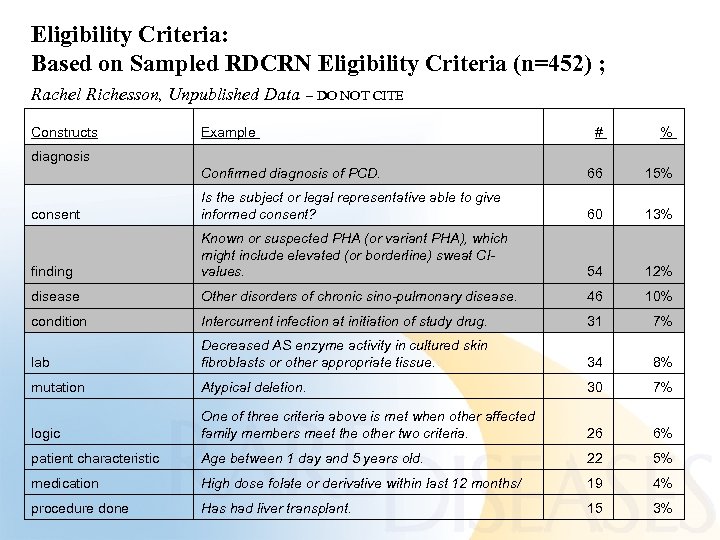
Eligibility Criteria: Based on Sampled RDCRN Eligibility Criteria (n=452) ; Rachel Richesson, Unpublished Data – DO NOT CITE Constructs Example # % diagnosis Confirmed diagnosis of PCD. 66 15% consent Is the subject or legal representative able to give informed consent? 60 13% finding Known or suspected PHA (or variant PHA), which might include elevated (or borderline) sweat Cl- values. 54 12% disease Other disorders of chronic sino-pulmonary disease. 46 10% condition Intercurrent infection at initiation of study drug. 31 7% lab Decreased AS enzyme activity in cultured skin fibroblasts or other appropriate tissue. 34 8% mutation Atypical deletion. 30 7% logic One of three criteria above is met when other affected family members meet the other two criteria. 26 6% patient characteristic Age between 1 day and 5 years old. 22 5% medication High dose folate or derivative within last 12 months/ 19 4% procedure done Has had liver transplant. 15 3%
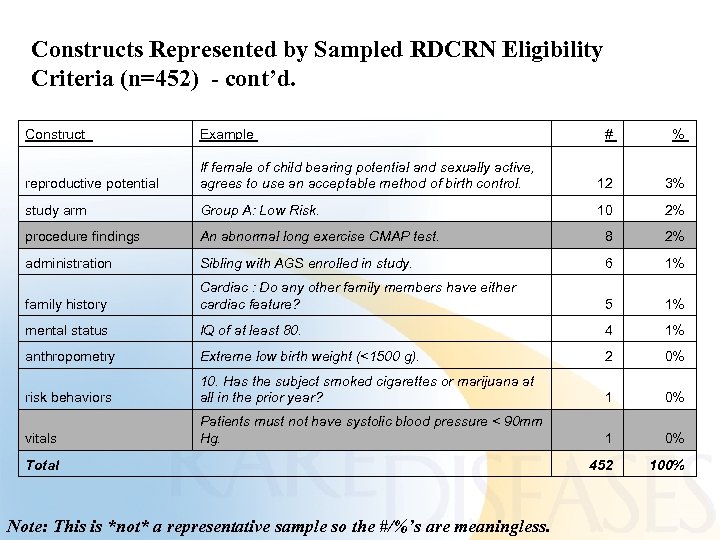
Constructs Represented by Sampled RDCRN Eligibility Criteria (n=452) - cont’d. Construct Example # % reproductive potential If female of child bearing potential and sexually active, agrees to use an acceptable method of birth control. 12 3% study arm Group A: Low Risk. 10 2% procedure findings An abnormal long exercise CMAP test. 8 2% administration Sibling with AGS enrolled in study. 6 1% family history Cardiac : Do any other family members have either cardiac feature? 5 1% mental status IQ of at least 80. 4 1% anthropometry Extreme low birth weight (<1500 g). 2 0% risk behaviors 10. Has the subject smoked cigarettes or marijuana at all in the prior year? 1 0% vitals Patients must not have systolic blood pressure < 90 mm Hg. 1 0% 452 100% Total Note: This is *not* a representative sample so the #/%’s are meaningless.
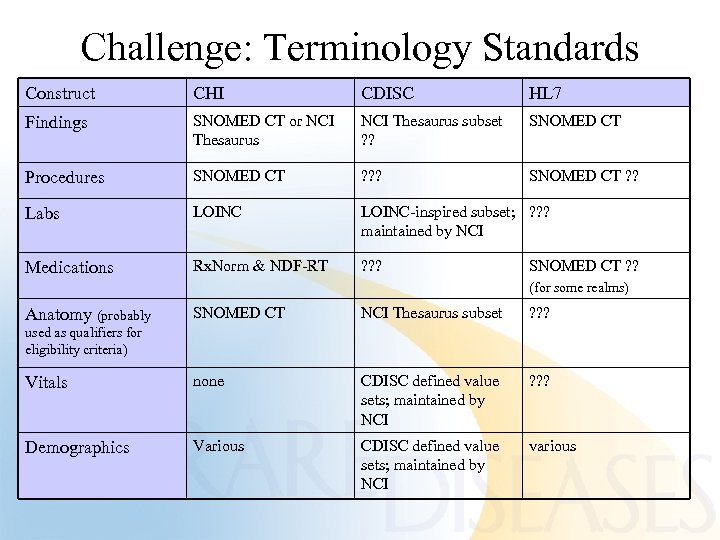
Challenge: Terminology Standards Construct CHI CDISC HL 7 Findings SNOMED CT or NCI Thesaurus subset ? ? SNOMED CT Procedures SNOMED CT ? ? ? SNOMED CT ? ? Labs LOINC-inspired subset; ? ? ? maintained by NCI Medications Rx. Norm & NDF-RT ? ? ? SNOMED CT ? ? (for some realms) SNOMED CT NCI Thesaurus subset ? ? ? Vitals none CDISC defined value sets; maintained by NCI ? ? ? Demographics Various CDISC defined value sets; maintained by NCI various Anatomy (probably used as qualifiers for eligibility criteria)
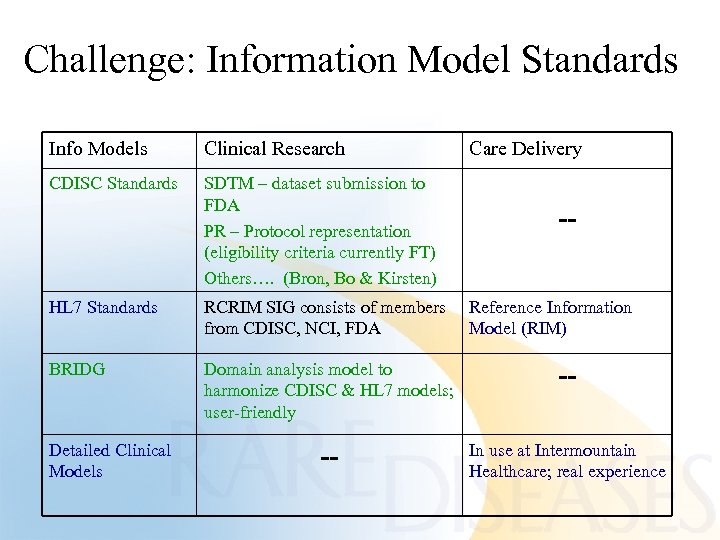
Challenge: Information Model Standards Info Models Clinical Research Care Delivery CDISC Standards SDTM – dataset submission to FDA PR – Protocol representation (eligibility criteria currently FT) Others…. (Bron, Bo & Kirsten) HL 7 Standards RCRIM SIG consists of members from CDISC, NCI, FDA Reference Information Model (RIM) BRIDG Domain analysis model to harmonize CDISC & HL 7 models; user-friendly -- -- In use at Intermountain Healthcare; real experience Detailed Clinical Models --


Next Steps • Seek buy-in for Use Case that represents a real world need and provides value to a wide variety of stakeholders in the Healthcare and Life Sciences • Develop a collaborative framework comprising of Providers, Pharma and Vendors • Work towards a POC that demonstrates the feasibility of using EMR data for Clinical Research Next Attraction: Detailed Clinical Models by Tom Oniki

Acknowledgements • Jeff Krischer, Ph. D, U. of South Florida • Office of Rare Diseases • National Center for Research Resources (RR 019259) • DOD - Advanced Cancer Detection Systems (DAMD 17 -01 -2 -0056 ) This content does not necessarily represent the official views of NCRR or NIH or DOD.
386dd7a63e3bf305b86d75ff3b6cf545.ppt