6b3300c2b8c3c3f5efbcff9cacb40f60.ppt
- Количество слайдов: 21

Clinical Lab Private Limited R 374, TTC MIDC, Rabale Navi Mumbai - 400 701 Maharashtra Bio Availability & Bio Equivalence Studies

MISSION To be one of the most trusted partners of our clients through compliance to all regulatory standards in the development of drugs to improve the quality of human life. To build long-lasting relationships with our clients by providing quality data in a timely and cost-effective manner through our team of experienced and dedicated professionals. VISION To become a world class CRO ensuring conformance to the highest ethical and quality standards. Constantly endeavoring for growth and consolidation of clinical services.
Core values at Panexcell • Quality is our primary value. We ensure quality in our compliance in processes, credibility of data and excellence of deliverables. • Customer Focus Our management, processes and people are aligned towards satisfying the needs of our customers. We are dedicated to service excellence that gains us customer continuity. • Knowledge We acquire knowledge in order to lead in our field. We share our knowledge with our people and our customers. • Commitment We are committed to our mission and to our stakeholders. Our commitment is a promise that we will always perform to the highest of their expectations. • Trust We deliver what we promise with honesty and integrity, thus earning the trust that fosters long term relationships.
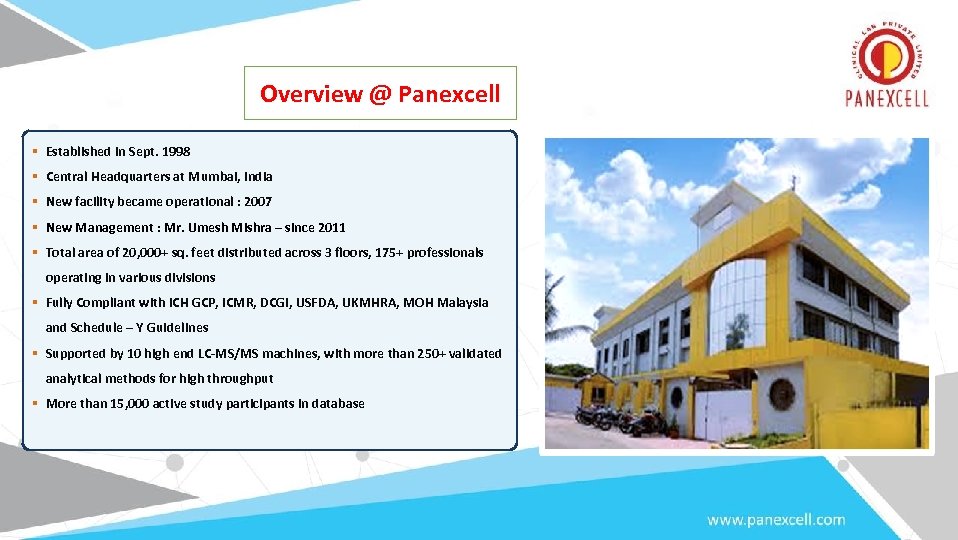
Overview @ Panexcell § Established in Sept. 1998 § Central Headquarters at Mumbai, India § New facility became operational : 2007 § New Management : Mr. Umesh Mishra – since 2011 § Total area of 20, 000+ sq. feet distributed across 3 floors, 175+ professionals operating in various divisions § Fully Compliant with ICH GCP, ICMR, DCGI, USFDA, UKMHRA, MOH Malaysia and Schedule – Y Guidelines § Supported by 10 high end LC-MS/MS machines, with more than 250+ validated analytical methods for high throughput § More than 15, 000 active study participants in database Overview

Overview Experience: § 18+ years of pharmaceutical experience for National & Global clients § 50+ Pharma and Biotech Sponsors all over the globe § 1000+ successful clinical studies Consistency: § Less than 10% attrition YOY § More than 80% Repeat Business Global Footprints : § Operations : India § Business Offices in India, USA, China and Australia
Regulatory Inspections & Study Approvals DCGI (2017) (Drug Controller General, India) US-FDA (2015) US-FDA (2014) Mo. H Kazakhstan (2012) Mo. H Turkey (2008) MCC, South Africa (2008) HPRA, Ireland 2017 MHRA, UK 2016 HSA, Singapore 2015 TGA, Australia 2014 Mo. H, Malaysia (2012)

Capabilities/Expertise@Panexcell PK / PD studies § Bioequivalence / Bioavailability studies § Pharmacokinetic / Pharmacodynamic studies in Healthy subjects § Special / Patient Population studies § Food effect / Drug – Drug Interaction studies. § Proof of Concept studies § End-to End Service § Protocol to Report § Project Management § Final submission Report (e CTD / ICH E 3)

Clinical Capabilities @ Panexcell Biostatistics & Medical Writing § Sample Size and Power Calculation § Medical writing and literature search § Providing Statistical Inputs during Protocol Designing § Randomization § Statistical Analysis Plan (SAP) § SAS Programming and Validation § Interim Analysis § Statistical Report

Standalone services at Panexcell Stand Alone Services § Regulatory Services § Bio Analytical Services § Bio-statistical & Medical Writing § Quality Assurance § Medical writing § Project Management

Clinical Unit at Panexcell India § 60 Beds arranged in 2 study areas spread over 8, 000 sq. ft. at central office § 2 Intensive Care beds with cardiac monitoring § Access controlled wards § Data base of 15, 000+ healthy volunteers including 900 + female volunteers and 14, 000+ male volunteers § Separate pharmacy with controlled access § World-class infrastructure to support clinical activities § Capacity to handle approx. 2000 Volunteers /Year (7500 dosing)

Clinical Unit: Phlebotomy • Phlebotomy area with separate matrix processing room • Separate dining area with in-house kitchen • Screening room • Counseling room with audio-video recording facility • Ambulatory room • Examination and monitoring rooms • Recreation hall • Archives

Clinical Equipments in CPU • • • ECG machine Pulse Oxy meter Defibrillator Suction machine Ambu bag and oxygen cylinder Digital synchronized clocks Pharmacy chambers Cold centrifuge Deep freezer Glucometer Biometric system Alcohol breath analyzer
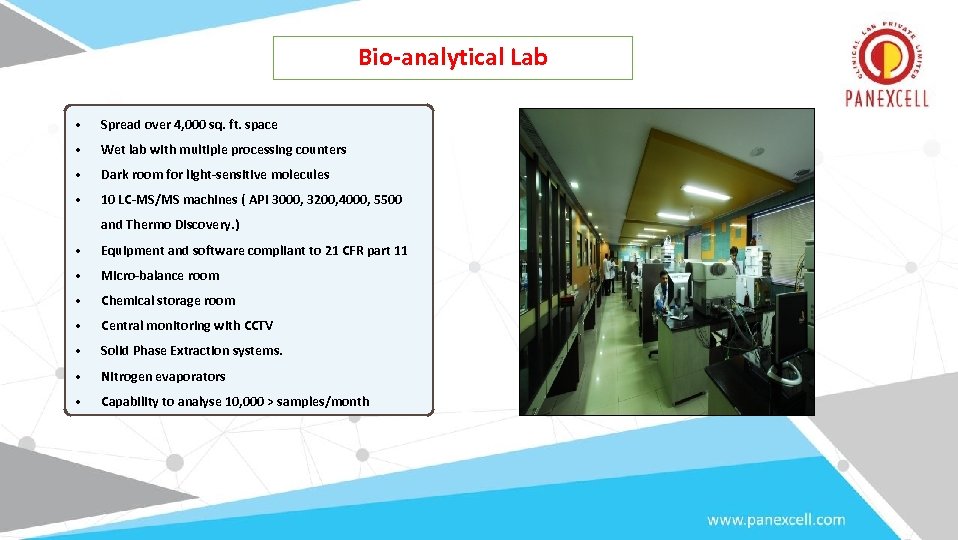
Bio-analytical Lab • Spread over 4, 000 sq. ft. space • Wet lab with multiple processing counters • Dark room for light-sensitive molecules • 10 LC-MS/MS machines ( API 3000, 3200, 4000, 5500 and Thermo Discovery. ) • Equipment and software compliant to 21 CFR part 11 • Micro-balance room • Chemical storage room • Central monitoring with CCTV • Solid Phase Extraction systems. • Nitrogen evaporators • Capability to analyse 10, 000 > samples/month

Bioanalytical facilities • LC-MS/MS: • API 5500 (3) • API 4000 (2) • API 3200 (2) • API 3000 (2) • Discovery (Thermo Finnigan) • • • UV Spectro/Micro-plate reader Fume hoods Automated sample evaporators Balance: Analytical and micro balance Cold centrifuge Deep freezers (-200 C & -700 C)
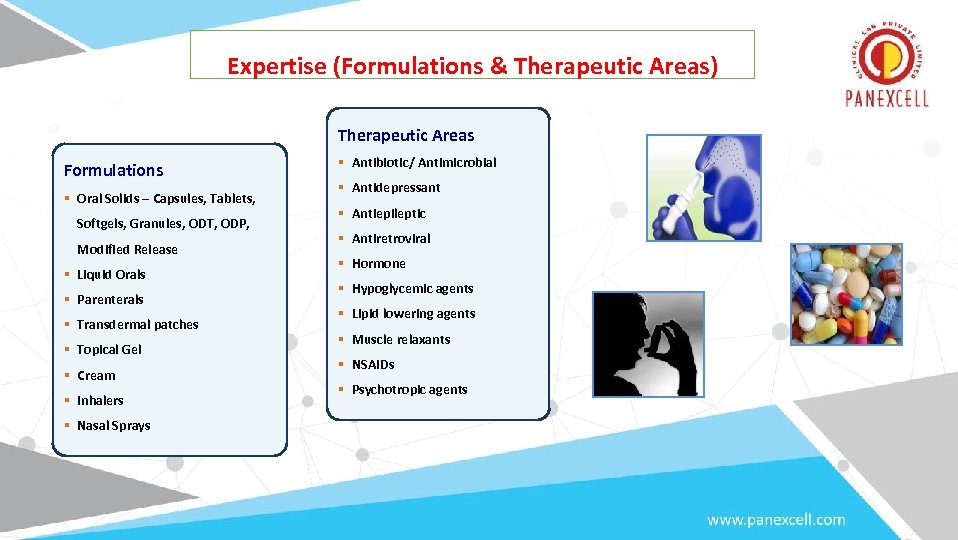
Expertise (Formulations & Therapeutic Areas) Therapeutic Areas Formulations § Oral Solids – Capsules, Tablets, Softgels, Granules, ODT, ODP, Modified Release § Liquid Orals § Parenterals § Transdermal patches § Topical Gel § Cream § Inhalers § Nasal Sprays § Antibiotic/ Antimicrobial § Antidepressant § Antiepileptic § Antiretroviral § Hormone § Hypoglycemic agents § Lipid lowering agents § Muscle relaxants § NSAIDs § Psychotropic agents

Quality Assurance ü Quality Assurance at Panexcell is an independent function reporting to CSO and ensures ethical conduct of clinical studies as per GCP, GLP & other applicable regulatory requirement ü Quality Control (QC) Procedures in place apart from Quality Assurance (QA) Procedures ü All activities are subjected to rigorous review for Quality Assurance including § Clinical (over 50 SOP’s), Bio-analytical, PK / Stats / Reporting Monitoring Visit and Audits Review of Project Plans Inspection of Documents Site Qualificati on Quality Assurance Training to Staff ü Internal Audits by QA team for Area Specific SOP compliance ü In-house capabilities to perform Site Audits, Systems / Process Audit, Vendor Audit ü All QA systems are compliant with all applicable local & international regulations Inspection Readiness CAPA Development & Maintenance of SOP
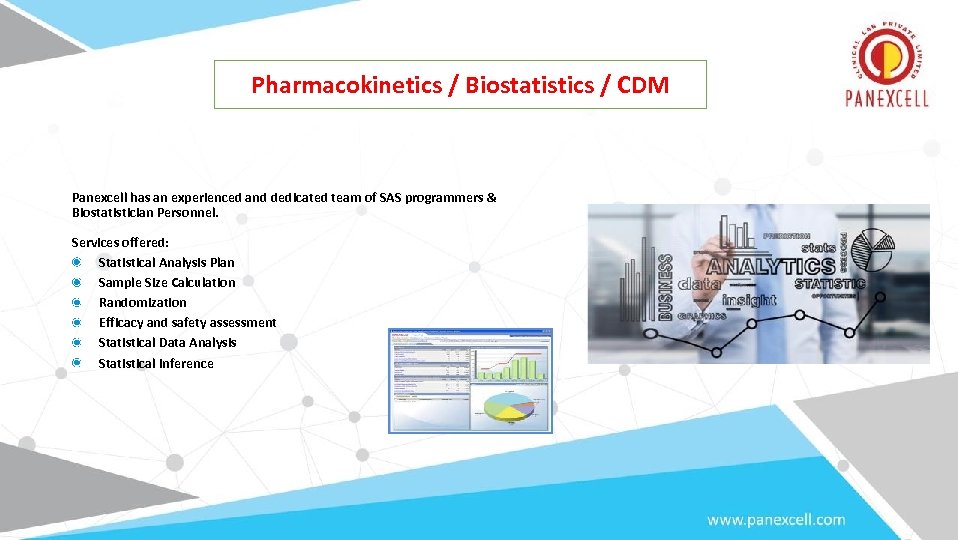
Biostatistics. Pharmacokinetics / Biostatistics / CDM Panexcell has an experienced and dedicated team of SAS programmers & Biostatistician Personnel. Services offered: Statistical Analysis Plan Sample Size Calculation Randomization Efficacy and safety assessment Statistical Data Analysis Statistical Inference
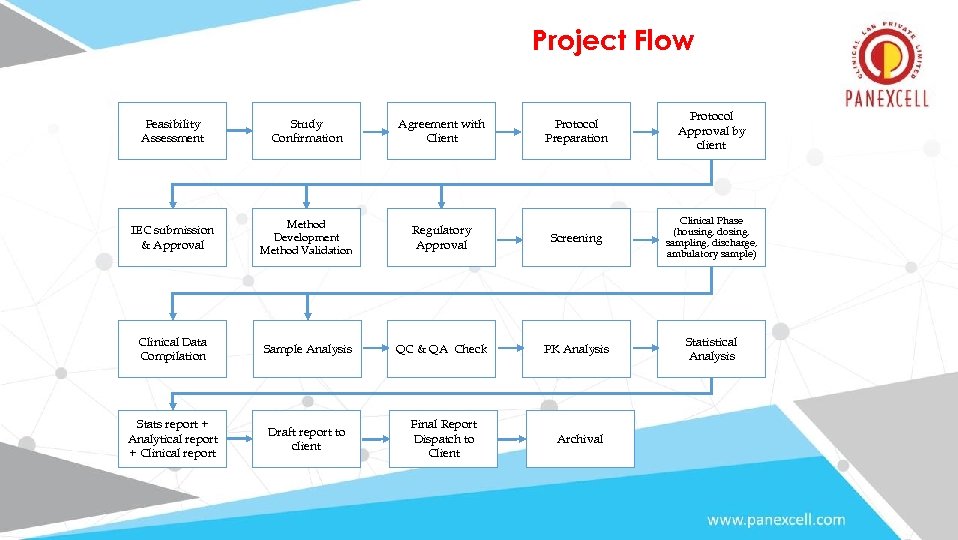
Project Flow Protocol Preparation Protocol Approval by client Regulatory Approval Screening Clinical Phase (housing, dosing, sampling, discharge, ambulatory sample) Sample Analysis QC & QA Check PK Analysis Statistical Analysis Draft report to client Final Report Dispatch to Client Archival Feasibility Assessment Study Confirmation Agreement with Client IEC submission & Approval Method Development Method Validation Clinical Data Compilation Stats report + Analytical report + Clinical report
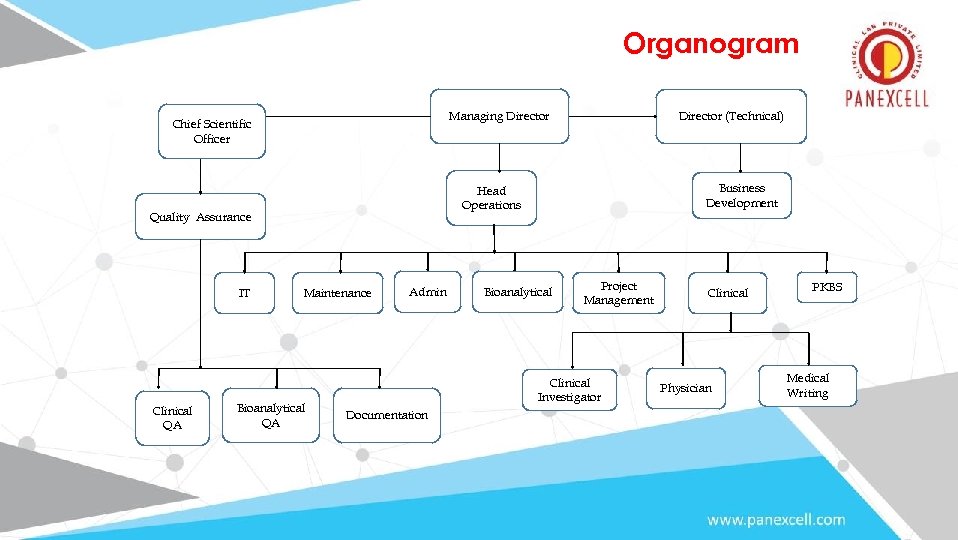
Organogram Managing Director Chief Scientific Officer Clinical QA Business Development Head Operations Quality Assurance IT Director (Technical) Maintenance Bioanalytical QA Admin Bioanalytical Project Management Clinical Investigator Documentation Clinical Physician PKBS Medical Writing

Key Personnel • Mr. Umesh Mishra (Managing Director) • Mr. Manojit Pathak (Director - Technical) • Dr. Yati Chugh (Chief Scientific Officer) • Mr. Manoj Shukla (Head - Operations) • Dr. Nitin Kulkarni (Head - Clinical & Principal Investigator) • Mr. Pankaj Kasar (Head - Bioanalytical) • Mr. Sriram Eswaran (Head - Quality Assurance) • Dr. Mamata Jadhav (Project Management ) • Dr. Yogesh Barde (Manager - Business Development)

Contact us: Panexcell Clinical Labs Pvt. Ltd. Panexcell Clinical Lab Private Limited R-374, TTC MIDC, Rabale, Navi Mumbai – 400701, India Tel: +91 22 3969 7979 Fax: +91 22 3969 7999 E-mail: info@panexcell. com www. panexcell. com Thank You
6b3300c2b8c3c3f5efbcff9cacb40f60.ppt