42eb8d5810a36984a9f70a370d965fa9.ppt
- Количество слайдов: 15
 Clinical Considerations for Managing Iron Overload in MDS: Analysis From EHA Aristoteles Giagounidis, MD, Ph. D Associate Professor of Medicine Head, Hematology/Oncology Clinical Research Unit St. Johannes Hospital Duisburg, Germany
Clinical Considerations for Managing Iron Overload in MDS: Analysis From EHA Aristoteles Giagounidis, MD, Ph. D Associate Professor of Medicine Head, Hematology/Oncology Clinical Research Unit St. Johannes Hospital Duisburg, Germany
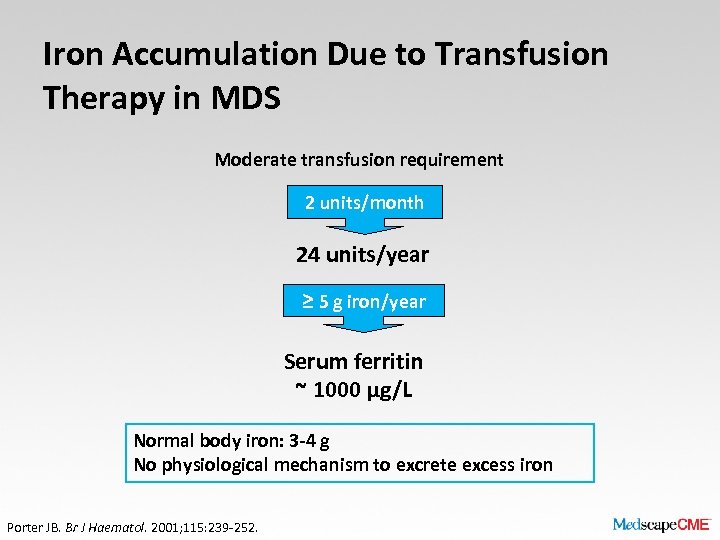 Iron Accumulation Due to Transfusion Therapy in MDS Moderate transfusion requirement 2 units/month 24 units/year ≥ 5 g iron/year Serum ferritin ~ 1000 μg/L Normal body iron: 3 -4 g No physiological mechanism to excrete excess iron Porter JB. Br J Haematol. 2001; 115: 239 -252.
Iron Accumulation Due to Transfusion Therapy in MDS Moderate transfusion requirement 2 units/month 24 units/year ≥ 5 g iron/year Serum ferritin ~ 1000 μg/L Normal body iron: 3 -4 g No physiological mechanism to excrete excess iron Porter JB. Br J Haematol. 2001; 115: 239 -252.
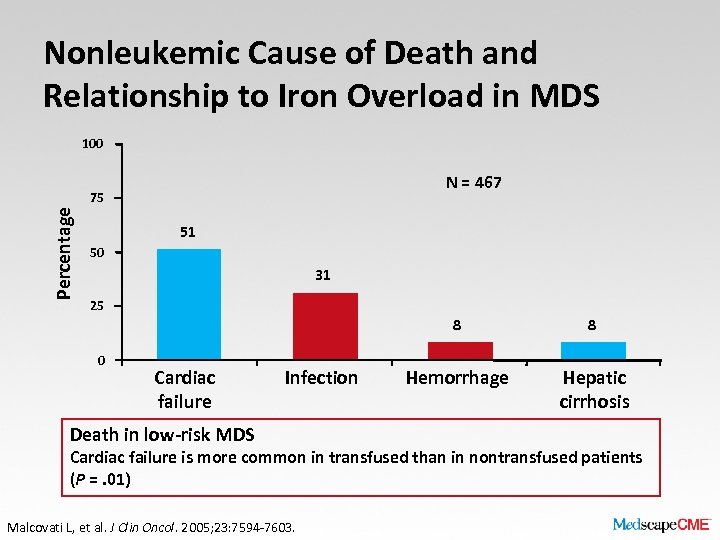 Nonleukemic Cause of Death and Relationship to Iron Overload in MDS 100 N = 467 Percentage 75 51 50 31 25 8 0 Cardiac failure Infection Death in low-risk MDS 8 Hemorrhage Hepatic cirrhosis Cardiac failure is more common in transfused than in nontransfused patients (P =. 01) Malcovati L, et al. J Clin Oncol. 2005; 23: 7594 -7603.
Nonleukemic Cause of Death and Relationship to Iron Overload in MDS 100 N = 467 Percentage 75 51 50 31 25 8 0 Cardiac failure Infection Death in low-risk MDS 8 Hemorrhage Hepatic cirrhosis Cardiac failure is more common in transfused than in nontransfused patients (P =. 01) Malcovati L, et al. J Clin Oncol. 2005; 23: 7594 -7603.
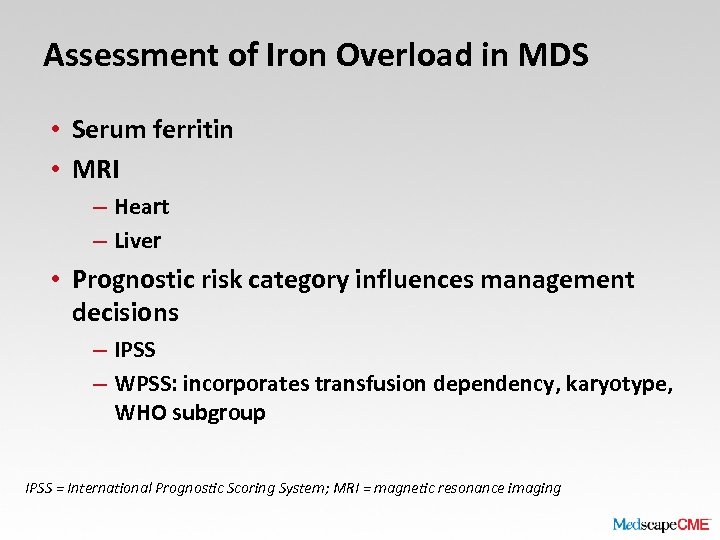 Assessment of Iron Overload in MDS • Serum ferritin • MRI – Heart – Liver • Prognostic risk category influences management decisions – IPSS – WPSS: incorporates transfusion dependency, karyotype, WHO subgroup IPSS = International Prognostic Scoring System; MRI = magnetic resonance imaging
Assessment of Iron Overload in MDS • Serum ferritin • MRI – Heart – Liver • Prognostic risk category influences management decisions – IPSS – WPSS: incorporates transfusion dependency, karyotype, WHO subgroup IPSS = International Prognostic Scoring System; MRI = magnetic resonance imaging
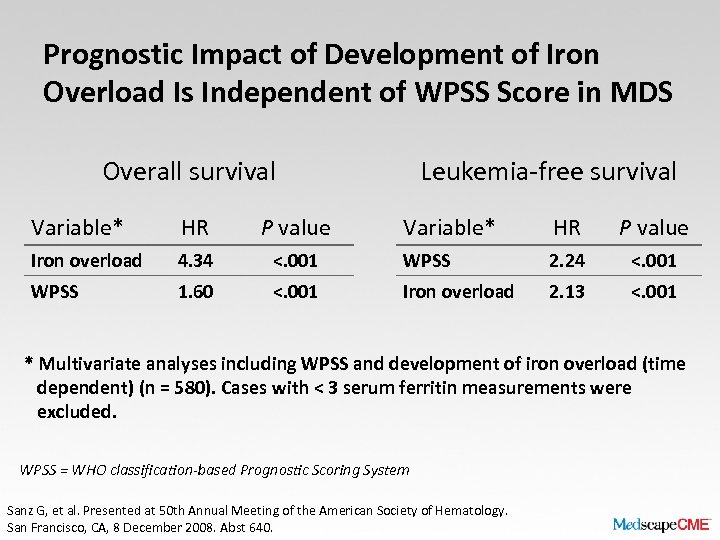 Prognostic Impact of Development of Iron Overload Is Independent of WPSS Score in MDS Overall survival Variable* HR P value Iron overload 4. 34 WPSS 1. 60 Leukemia-free survival Variable* HR P value <. 001 WPSS 2. 24 <. 001 Iron overload 2. 13 <. 001 * Multivariate analyses including WPSS and development of iron overload (time dependent) (n = 580). Cases with < 3 serum ferritin measurements were excluded. WPSS = WHO classification-based Prognostic Scoring System Sanz G, et al. Presented at 50 th Annual Meeting of the American Society of Hematology. San Francisco, CA, 8 December 2008. Abst 640.
Prognostic Impact of Development of Iron Overload Is Independent of WPSS Score in MDS Overall survival Variable* HR P value Iron overload 4. 34 WPSS 1. 60 Leukemia-free survival Variable* HR P value <. 001 WPSS 2. 24 <. 001 Iron overload 2. 13 <. 001 * Multivariate analyses including WPSS and development of iron overload (time dependent) (n = 580). Cases with < 3 serum ferritin measurements were excluded. WPSS = WHO classification-based Prognostic Scoring System Sanz G, et al. Presented at 50 th Annual Meeting of the American Society of Hematology. San Francisco, CA, 8 December 2008. Abst 640.
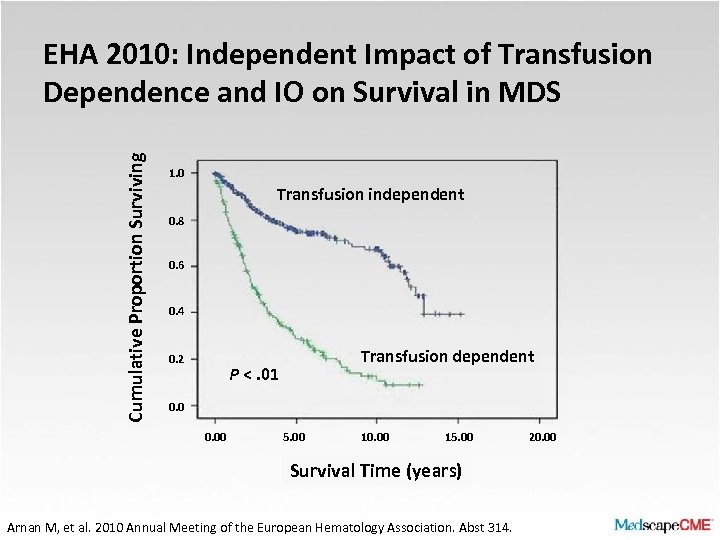 Cumulative Proportion Surviving EHA 2010: Independent Impact of Transfusion Dependence and IO on Survival in MDS 1. 0 Transfusion independent 0. 8 0. 6 0. 4 0. 2 Transfusion dependent P <. 01 0. 00 5. 00 10. 00 15. 00 Survival Time (years) Arnan M, et al. 2010 Annual Meeting of the European Hematology Association. Abst 314. 20. 00
Cumulative Proportion Surviving EHA 2010: Independent Impact of Transfusion Dependence and IO on Survival in MDS 1. 0 Transfusion independent 0. 8 0. 6 0. 4 0. 2 Transfusion dependent P <. 01 0. 00 5. 00 10. 00 15. 00 Survival Time (years) Arnan M, et al. 2010 Annual Meeting of the European Hematology Association. Abst 314. 20. 00
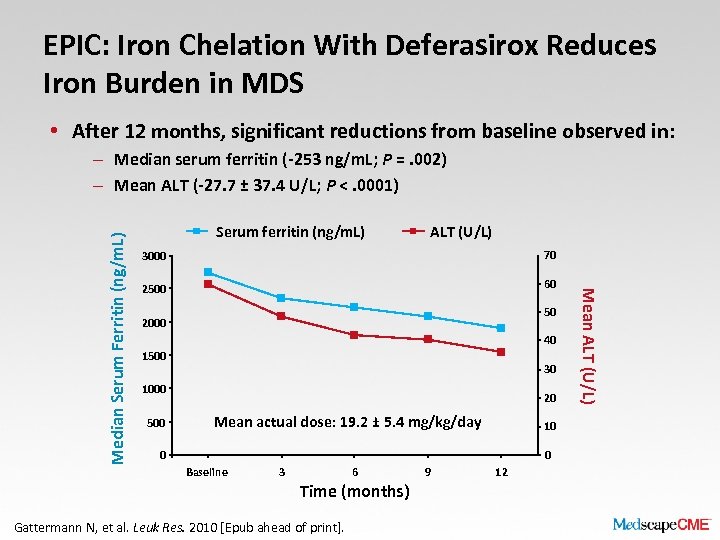 EPIC: Iron Chelation With Deferasirox Reduces Iron Burden in MDS • After 12 months, significant reductions from baseline observed in: Serum ferritin (ng/m. L) ALT (U/L) 3000 70 2500 60 50 2000 40 1500 30 1000 500 20 Mean actual dose: 19. 2 ± 5. 4 mg/kg/day 10 0 0 Baseline 3 6 Time (months) Gattermann N, et al. Leuk Res. 2010 [Epub ahead of print]. 9 12 Mean ALT (U/L) Median Serum Ferritin (ng/m. L) – Median serum ferritin (-253 ng/m. L; P =. 002) – Mean ALT (-27. 7 ± 37. 4 U/L; P <. 0001)
EPIC: Iron Chelation With Deferasirox Reduces Iron Burden in MDS • After 12 months, significant reductions from baseline observed in: Serum ferritin (ng/m. L) ALT (U/L) 3000 70 2500 60 50 2000 40 1500 30 1000 500 20 Mean actual dose: 19. 2 ± 5. 4 mg/kg/day 10 0 0 Baseline 3 6 Time (months) Gattermann N, et al. Leuk Res. 2010 [Epub ahead of print]. 9 12 Mean ALT (U/L) Median Serum Ferritin (ng/m. L) – Median serum ferritin (-253 ng/m. L; P =. 002) – Mean ALT (-27. 7 ± 37. 4 U/L; P <. 0001)
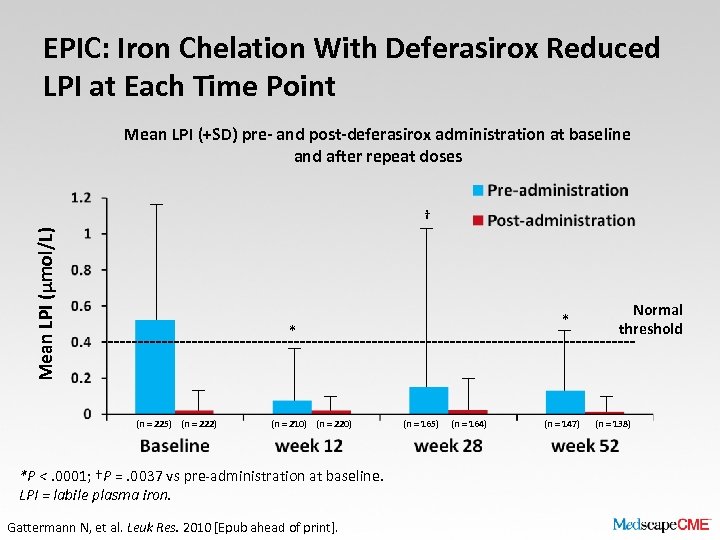 EPIC: Iron Chelation With Deferasirox Reduced LPI at Each Time Point Mean LPI (+SD) pre- and post-deferasirox administration at baseline and after repeat doses Mean LPI (μmol/L) † * * (n = 225) (n = 222) (n = 210) (n = 220) *P <. 0001; †P =. 0037 vs pre-administration at baseline. LPI = labile plasma iron. Gattermann N, et al. Leuk Res. 2010 [Epub ahead of print]. (n = 165) (n = 164) (n = 147) Normal threshold (n = 138)
EPIC: Iron Chelation With Deferasirox Reduced LPI at Each Time Point Mean LPI (+SD) pre- and post-deferasirox administration at baseline and after repeat doses Mean LPI (μmol/L) † * * (n = 225) (n = 222) (n = 210) (n = 220) *P <. 0001; †P =. 0037 vs pre-administration at baseline. LPI = labile plasma iron. Gattermann N, et al. Leuk Res. 2010 [Epub ahead of print]. (n = 165) (n = 164) (n = 147) Normal threshold (n = 138)
 Matched-Pair Analysis: Iron Chelation Therapy vs Transfusion Therapy Only in MDS Aim: To evaluate survival in patients with MDS following chelation therapy by matched-pair analysis • Retrospective matched-pair analysis: – 94 MDS patients undergoing long-term chelation therapy – 93 patients in Düsseldorf MDS Registry receiving supportive care only • Pairs matched according to age at diagnosis, gender, MDS type (WHO classification), and IPSS score • All patients had iron overload (serum ferritin > 500 ng/m. L) • Patients were followed until death or June 30, 2009 Fox F, et al. Blood. 2009; 114(11): abst 1747.
Matched-Pair Analysis: Iron Chelation Therapy vs Transfusion Therapy Only in MDS Aim: To evaluate survival in patients with MDS following chelation therapy by matched-pair analysis • Retrospective matched-pair analysis: – 94 MDS patients undergoing long-term chelation therapy – 93 patients in Düsseldorf MDS Registry receiving supportive care only • Pairs matched according to age at diagnosis, gender, MDS type (WHO classification), and IPSS score • All patients had iron overload (serum ferritin > 500 ng/m. L) • Patients were followed until death or June 30, 2009 Fox F, et al. Blood. 2009; 114(11): abst 1747.
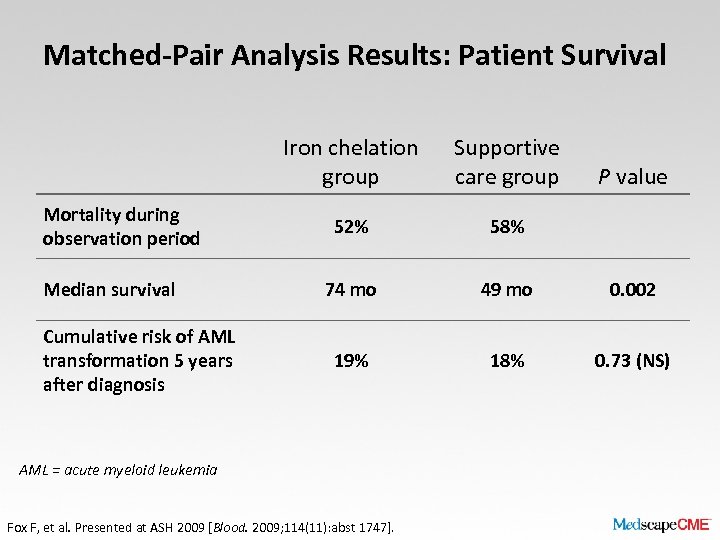 Matched-Pair Analysis Results: Patient Survival Iron chelation group Mortality during observation period Median survival Cumulative risk of AML transformation 5 years after diagnosis Supportive care group 52% 58% 74 mo 49 mo 0. 002 19% 18% 0. 73 (NS) AML = acute myeloid leukemia Fox F, et al. Presented at ASH 2009 [Blood. 2009; 114(11): abst 1747]. P value
Matched-Pair Analysis Results: Patient Survival Iron chelation group Mortality during observation period Median survival Cumulative risk of AML transformation 5 years after diagnosis Supportive care group 52% 58% 74 mo 49 mo 0. 002 19% 18% 0. 73 (NS) AML = acute myeloid leukemia Fox F, et al. Presented at ASH 2009 [Blood. 2009; 114(11): abst 1747]. P value
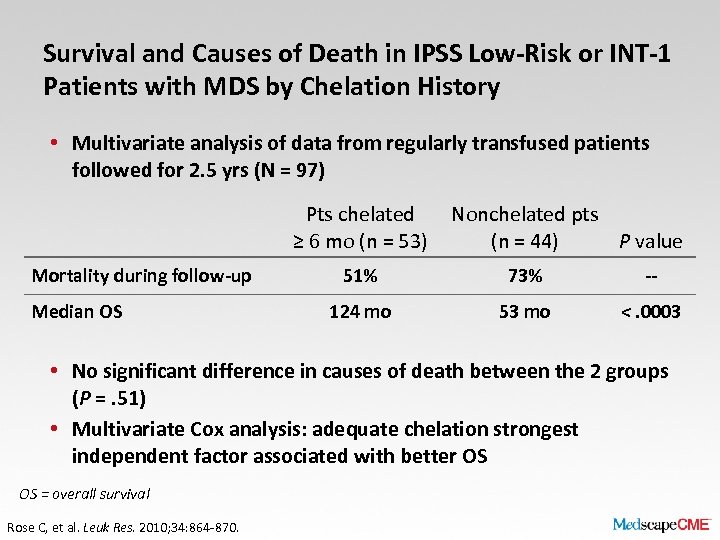 Survival and Causes of Death in IPSS Low-Risk or INT-1 Patients with MDS by Chelation History • Multivariate analysis of data from regularly transfused patients followed for 2. 5 yrs (N = 97) Pts chelated ≥ 6 mo (n = 53) Mortality during follow-up Median OS Nonchelated pts (n = 44) P value 51% 73% -- 124 mo 53 mo <. 0003 • No significant difference in causes of death between the 2 groups (P =. 51) • Multivariate Cox analysis: adequate chelation strongest independent factor associated with better OS OS = overall survival Rose C, et al. Leuk Res. 2010; 34: 864 -870.
Survival and Causes of Death in IPSS Low-Risk or INT-1 Patients with MDS by Chelation History • Multivariate analysis of data from regularly transfused patients followed for 2. 5 yrs (N = 97) Pts chelated ≥ 6 mo (n = 53) Mortality during follow-up Median OS Nonchelated pts (n = 44) P value 51% 73% -- 124 mo 53 mo <. 0003 • No significant difference in causes of death between the 2 groups (P =. 51) • Multivariate Cox analysis: adequate chelation strongest independent factor associated with better OS OS = overall survival Rose C, et al. Leuk Res. 2010; 34: 864 -870.
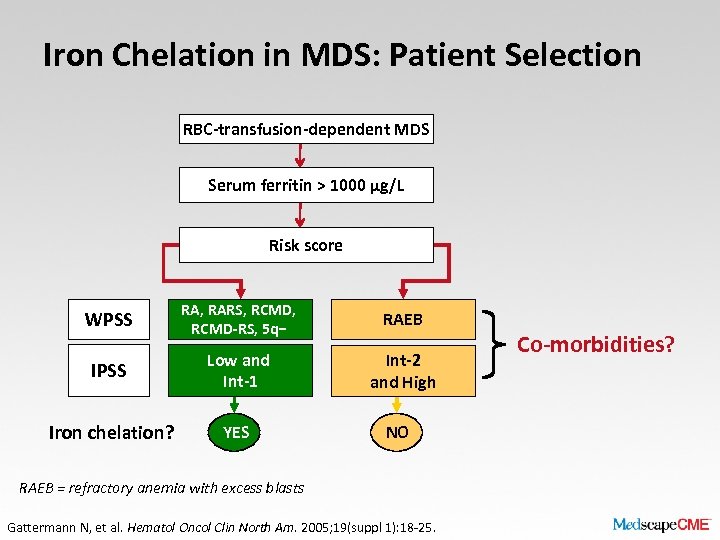 Iron Chelation in MDS: Patient Selection RBC-transfusion-dependent MDS Serum ferritin > 1000 µg/L Risk score WPSS RA, RARS, RCMD-RS, 5 q− RAEB IPSS Low and Int-1 Int-2 and High Iron chelation? YES NO RAEB = refractory anemia with excess blasts Gattermann N, et al. Hematol Oncol Clin North Am. 2005; 19(suppl 1): 18 -25. Co-morbidities?
Iron Chelation in MDS: Patient Selection RBC-transfusion-dependent MDS Serum ferritin > 1000 µg/L Risk score WPSS RA, RARS, RCMD-RS, 5 q− RAEB IPSS Low and Int-1 Int-2 and High Iron chelation? YES NO RAEB = refractory anemia with excess blasts Gattermann N, et al. Hematol Oncol Clin North Am. 2005; 19(suppl 1): 18 -25. Co-morbidities?
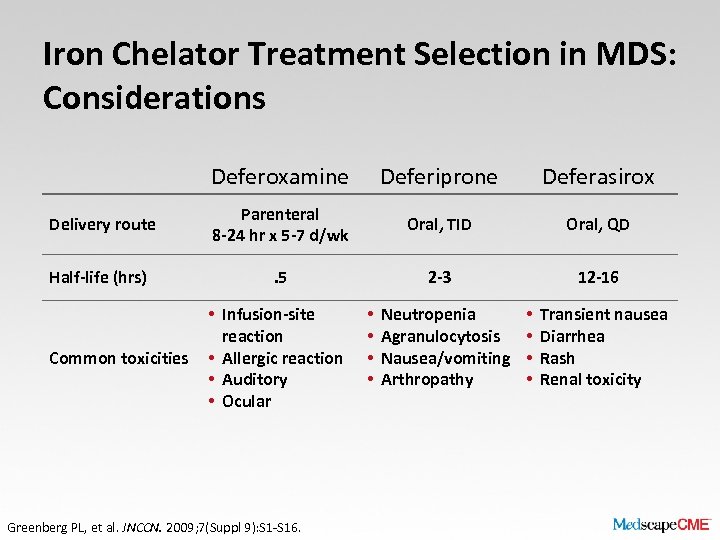 Iron Chelator Treatment Selection in MDS: Considerations Deferoxamine Deferiprone Deferasirox Delivery route Parenteral 8 -24 hr x 5 -7 d/wk Oral, TID Oral, QD Half-life (hrs) . 5 2 -3 12 -16 Common toxicities • Infusion-site reaction • Allergic reaction • Auditory • Ocular Greenberg PL, et al. JNCCN. 2009; 7(Suppl 9): S 1 -S 16. • • Neutropenia Agranulocytosis Nausea/vomiting Arthropathy • • Transient nausea Diarrhea Rash Renal toxicity
Iron Chelator Treatment Selection in MDS: Considerations Deferoxamine Deferiprone Deferasirox Delivery route Parenteral 8 -24 hr x 5 -7 d/wk Oral, TID Oral, QD Half-life (hrs) . 5 2 -3 12 -16 Common toxicities • Infusion-site reaction • Allergic reaction • Auditory • Ocular Greenberg PL, et al. JNCCN. 2009; 7(Suppl 9): S 1 -S 16. • • Neutropenia Agranulocytosis Nausea/vomiting Arthropathy • • Transient nausea Diarrhea Rash Renal toxicity
 Ongoing Studies • TELESTO: Myelodysplastic Syndromes (MDS) Event. Free Survival With Iron Chelation Therapy Study – Phase 3, multicenter, randomized, double-blind, placebo -controlled trial of deferasirox in patients with Low/Int 1–risk MDS and transfusional iron overload – Primary endpoint: Event-free survival (composite endpoint including death and nonfatal events related to cardiac and liver function) – Secondary endpoints: overall survival, TSH, glucosetolerance testing, IPSS score, change in hematologic function expressed in total number of blood transfusions
Ongoing Studies • TELESTO: Myelodysplastic Syndromes (MDS) Event. Free Survival With Iron Chelation Therapy Study – Phase 3, multicenter, randomized, double-blind, placebo -controlled trial of deferasirox in patients with Low/Int 1–risk MDS and transfusional iron overload – Primary endpoint: Event-free survival (composite endpoint including death and nonfatal events related to cardiac and liver function) – Secondary endpoints: overall survival, TSH, glucosetolerance testing, IPSS score, change in hematologic function expressed in total number of blood transfusions
 Conclusions • Transfusion dependency and iron overload: adverse effects on morbidity and mortality of patients with MDS – Particular issue in low-risk MDS due to longer-term transfusion therapy • Assessment: serum ferritin, liver/heart MRI, IPSS/WPSS prognostic scoring • Iron chelation shown to reduce iron burden and LPI, improve survival in patients with MDS and iron overload • Treatment selection considerations with iron chelators: – Efficiency, administration route/treatment compliance, tolerability in primarily elderly patients
Conclusions • Transfusion dependency and iron overload: adverse effects on morbidity and mortality of patients with MDS – Particular issue in low-risk MDS due to longer-term transfusion therapy • Assessment: serum ferritin, liver/heart MRI, IPSS/WPSS prognostic scoring • Iron chelation shown to reduce iron burden and LPI, improve survival in patients with MDS and iron overload • Treatment selection considerations with iron chelators: – Efficiency, administration route/treatment compliance, tolerability in primarily elderly patients


