53d308c8ad0bb4f88e70378793f52e0c.ppt
- Количество слайдов: 23

Clinical Activity Observed in a Phase 1 Dose-Escalation Trial of an Oral MET and ALK Inhibitor, PF-02341066 EL Kwak 1, DR Camidge 2, J Clark 1, GI Shapiro 3, RG Maki 4, MJ Ratain 5, B Solomon 6, Y-J Bang 7, S-H Ou 8, R Salgia 5 1. Massachusetts General Hospital 5. University of Chicago Cancer Center 2. University of Colorado Cancer Center 6. Peter Mac. Callum Cancer Centre 3. Dana-Farber Cancer Institute 7. Seoul National University 4. Memorial Sloan-Kettering Cancer Center 8. University of California at Irvine
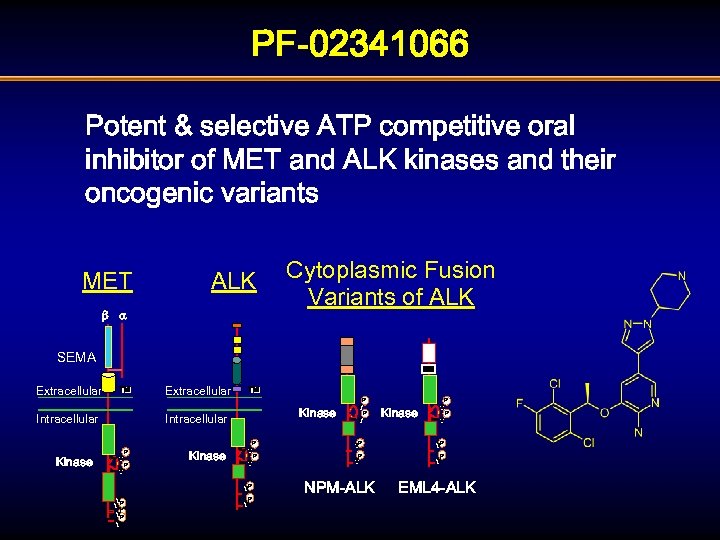
PF-02341066 Potent & selective ATP competitive oral inhibitor of MET and ALK kinases and their oncogenic variants MET ALK b a Cytoplasmic Fusion Variants of ALK SEMA Extracellular TM TM P Kinase Intracellular Y Y P Kinase Y Y P P Kinase P Y Y YP P Y YP P P Y Y NPM-ALK P Kinase Y Y P Y YP P Y Y EML 4 -ALK
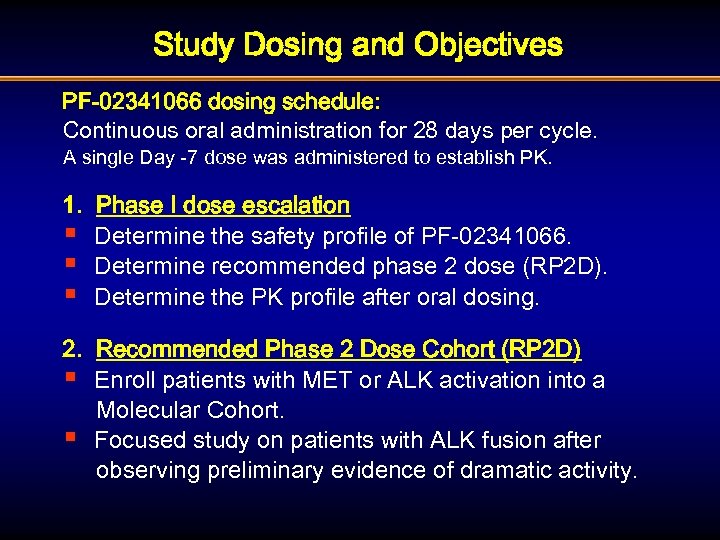
Study Dosing and Objectives PF-02341066 dosing schedule: Continuous oral administration for 28 days per cycle. A single Day -7 dose was administered to establish PK. 1. Phase I dose escalation § Determine the safety profile of PF-02341066. § Determine recommended phase 2 dose (RP 2 D). § Determine the PK profile after oral dosing. 2. Recommended Phase 2 Dose Cohort (RP 2 D) § Enroll patients with MET or ALK activation into a Molecular Cohort. § Focused study on patients with ALK fusion after observing preliminary evidence of dramatic activity.
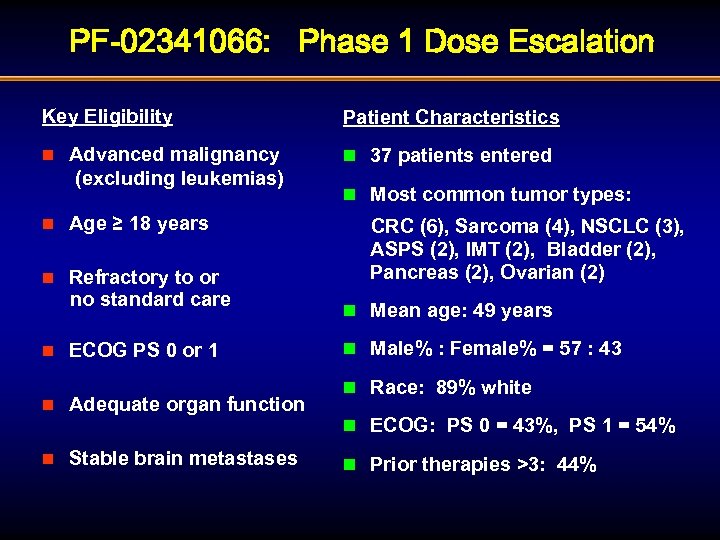
PF-02341066: Phase 1 Dose Escalation Key Eligibility Patient Characteristics Advanced malignancy (excluding leukemias) n 37 patients entered n n Age ≥ 18 years Refractory to or no standard care n n ECOG PS 0 or 1 n Adequate organ function n Stable brain metastases n Most common tumor types: CRC (6), Sarcoma (4), NSCLC (3), ASPS (2), IMT (2), Bladder (2), Pancreas (2), Ovarian (2) n Mean age: 49 years n Male% : Female% = 57 : 43 n Race: 89% white n ECOG: PS 0 = 43%, PS 1 = 54% n Prior therapies >3: 44%
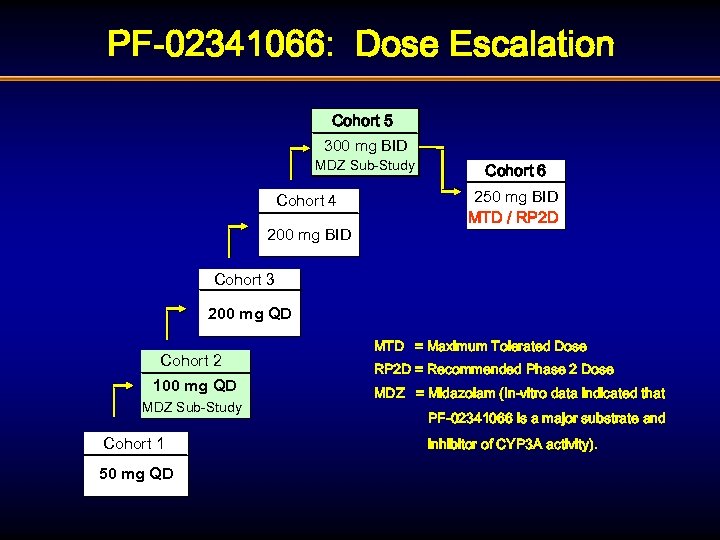
PF-02341066: Dose Escalation Cohort 5 300 mg BID MDZ Sub-Study Cohort 4 200 mg BID Cohort 6 250 mg BID MTD / RP 2 D Cohort 3 200 mg QD Cohort 2 100 mg QD MDZ Sub-Study Cohort 1 50 mg QD MTD = Maximum Tolerated Dose RP 2 D = Recommended Phase 2 Dose MDZ = Midazolam (In-vitro data indicated that PF-02341066 is a major substrate and inhibitor of CYP 3 A activity).
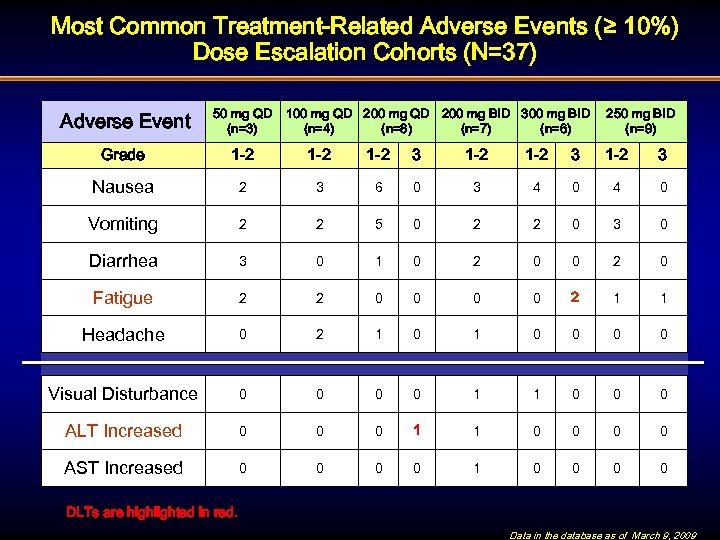
Most Common Treatment-Related Adverse Events (≥ 10%) Dose Escalation Cohorts (N=37) Adverse Event 50 mg QD (n=3) 100 mg QD 200 mg BID 300 mg BID (n=4) (n=8) (n=7) (n=6) 250 mg BID (n=9) Grade 1 -2 1 -2 3 Nausea 2 3 6 0 3 4 0 Vomiting 2 2 5 0 2 2 0 3 0 Diarrhea 3 0 1 0 2 0 Fatigue 2 2 0 0 2 1 1 Headache 0 2 1 0 0 0 0 Visual Disturbance 0 0 1 1 0 0 0 ALT Increased 0 0 0 1 1 0 0 AST Increased 0 0 1 0 0 DLTs are highlighted in red. Data in the database as of March 9, 2009

PF-02341066: Overview of Pharmacokinetics § Peak plasma concentration occurred at 4 hr after single doses § Plasma elimination half life ~53 hr (at 250 mg BID) § No evidence of non-linearity in PK at doses between 100 mg QD - 300 mg BID § Moderate inter-subject variability (CV 30 -69% for AUC and Cmax) § Moderate CYP 3 A 4 inhibitor (mean 3. 6 -fold increase in oral MDZ AUC, 90%CI: 2. 7 -4. 9)
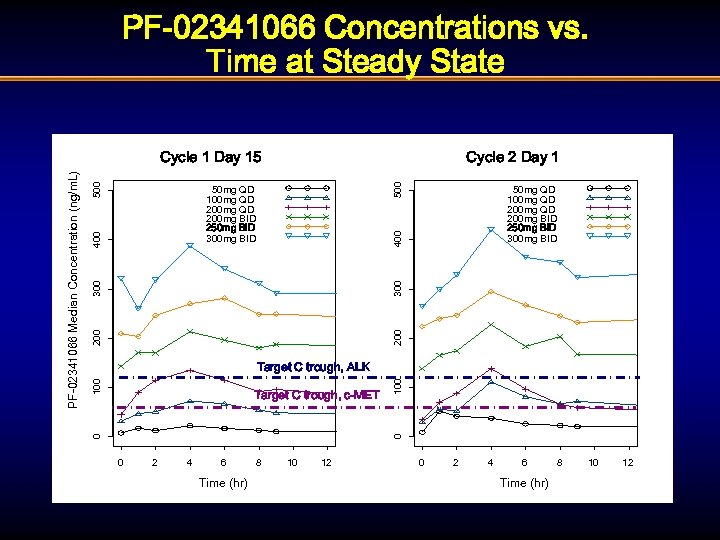
PF-02341066 Concentrations vs. Time at Steady State 500 Cycle 2 Day 1 400 50 mg QD 100 mg QD 200 mg BID 250 mg BID 300 mg BID 200 300 400 50 mg QD 100 mg QD 200 mg BID 250 mg BID 300 mg BID 0 Target C trough, c-MET 100 Target C trough, ALK 0 PF-02341066 Median Concentration (ng/m. L) Cycle 1 Day 15 0 2 4 6 Time (hr) 8 10 12
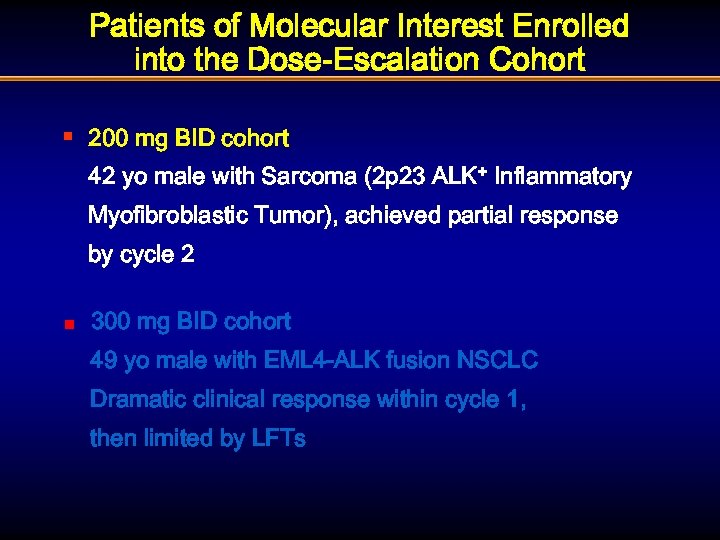
Patients of Molecular Interest Enrolled into the Dose-Escalation Cohort § 200 mg BID cohort 42 yo male with Sarcoma (2 p 23 ALK+ Inflammatory Myofibroblastic Tumor), achieved partial response by cycle 2 n 300 mg BID cohort 49 yo male with EML 4 -ALK fusion NSCLC Dramatic clinical response within cycle 1, then limited by LFTs
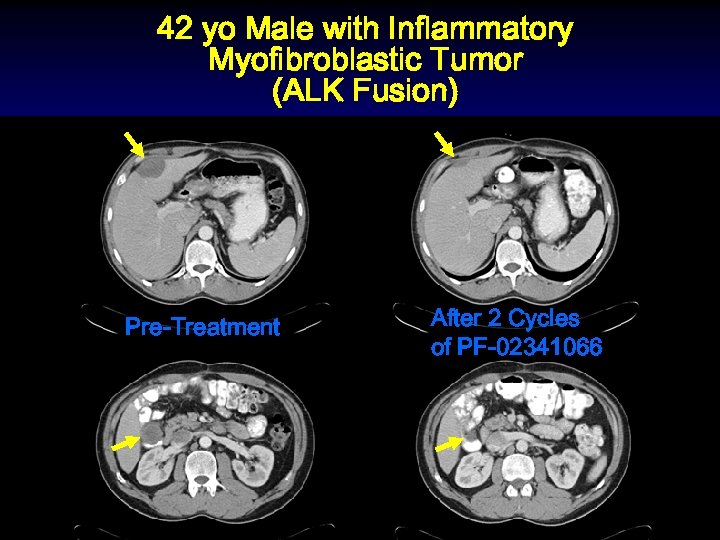
42 yo Male with Inflammatory Myofibroblastic Tumor (ALK Fusion) Pre-Treatment After 2 Cycles of PF-02341066
Patients of Molecular Interest Enrolled into the Dose-Escalation Cohort § 200 mg BID cohort 42 yo male with Sarcoma (2 p 23 Inflammatory Myofibroblastic Tumor), achieved partial response by cycle 2 n 300 mg BID cohort 49 yo male with EML 4 -ALK fusion NSCLC. Dramatic clinical response within cycle 1, then limited by LFTs
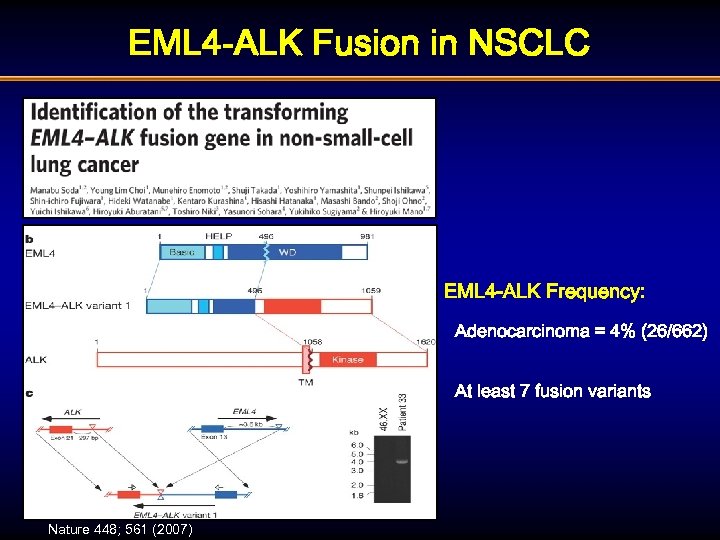
EML 4 -ALK Fusion in NSCLC EML 4 -ALK Frequency: Adenocarcinoma = 4% (26/662) At least 7 fusion variants Nature 448; 561 (2007)
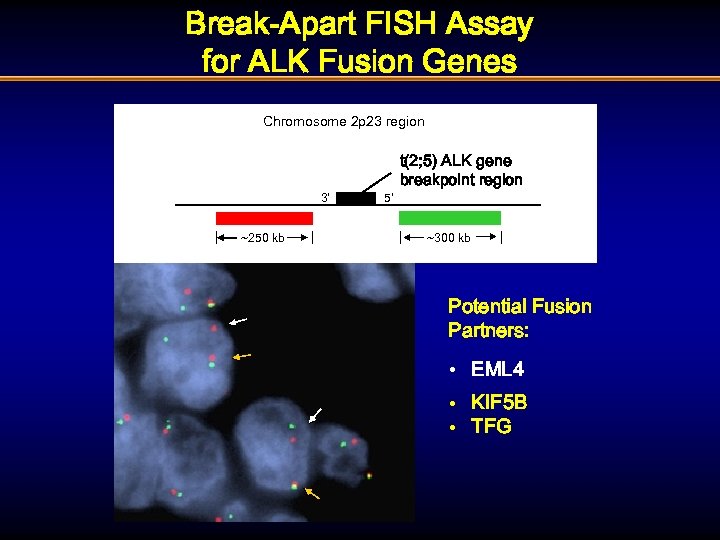
Break-Apart FISH Assay for ALK Fusion Genes Chromosome 2 p 23 region 3’ ~250 kb 5’ t(2; 5) ALK gene breakpoint region ~300 kb Potential Fusion Partners: • EML 4 • KIF 5 B • TFG
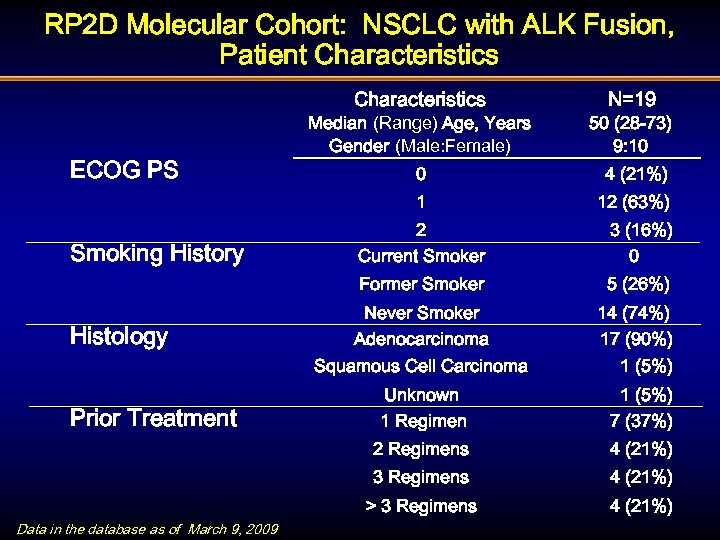
RP 2 D Molecular Cohort: NSCLC with ALK Fusion, Patient Characteristics N=19 Smoking History 50 (28 -73) 9: 10 0 4 (21%) 1 ECOG PS Median (Range) Age, Years Gender (Male: Female) 12 (63%) 3 (16%) 0 Former Smoker Histology 2 Current Smoker 5 (26%) Never Smoker Adenocarcinoma 14 (74%) 17 (90%) Squamous Cell Carcinoma Data in the database as of March 9, 2009 1 (5%) 7 (37%) 4 (21%) 3 Regimens Unknown 1 Regimen 2 Regimens Prior Treatment 1 (5%) 4 (21%) > 3 Regimens 4 (21%)

Study Status – NSCLC ALK Patients § 27 Patients Dosed: Data Collection Ongoing § 18 Patients Entered into Safety Database § 19 Patients Evaluable for Response
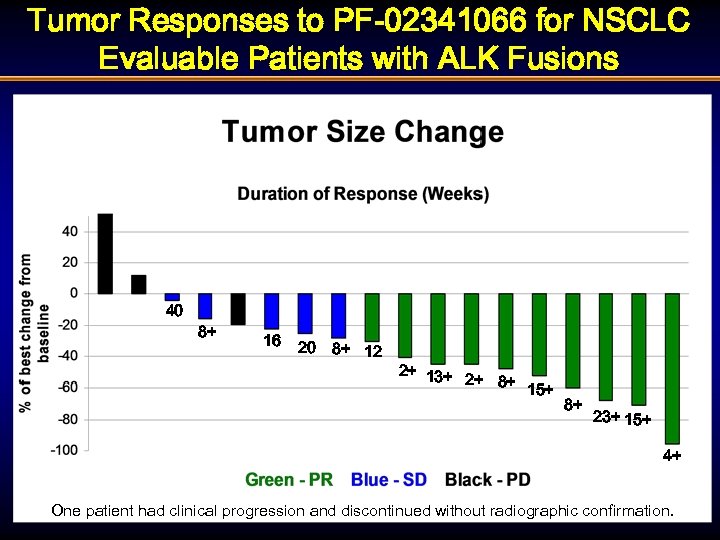
Tumor Responses to PF-02341066 for NSCLC Evaluable Patients with ALK Fusions 40 8+ 16 20 8+ 12 2+ 13+ 2+ 8+ 15+ 8+ 23+ 15+ 4+ One patient had clinical progression and discontinued without radiographic confirmation.
Molecular Cohort: NSCLC ALK Fusion § Overall Response Rate = 53% (10/19 pts) § Disease Control Rate at 8 weeks = 79% (15/19 pts) § 4 patients had progression at first evaluation
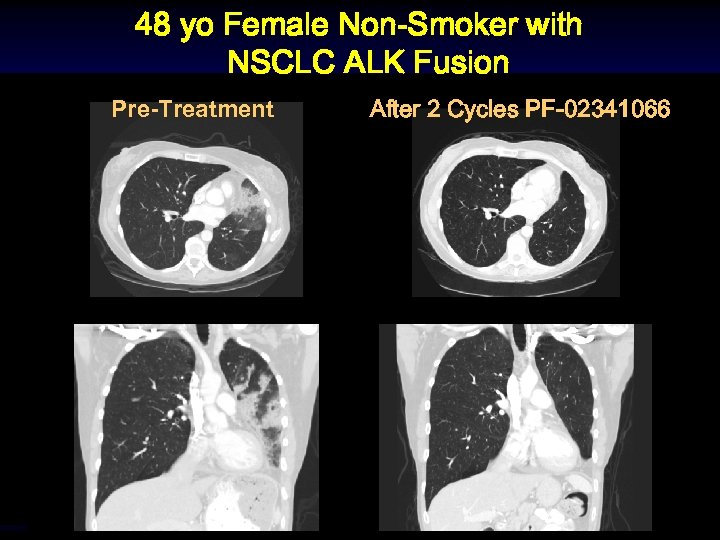
48 yo Female Non-Smoker with NSCLC ALK Fusion Pre-Treatment After 2 Cycles PF-02341066
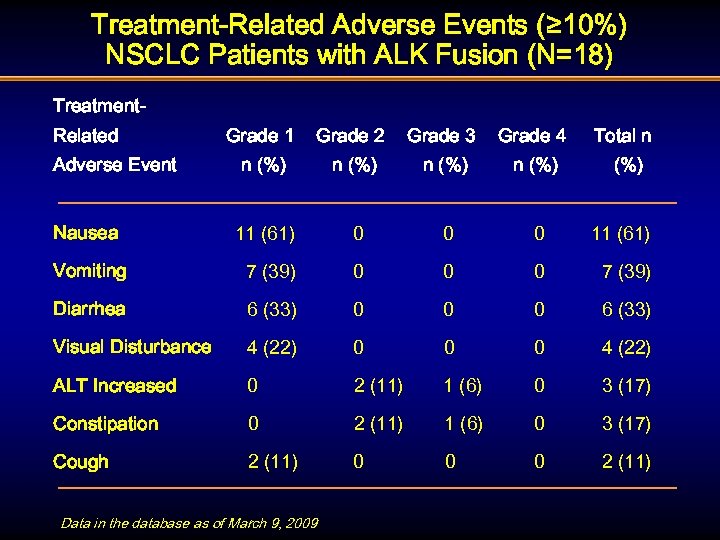
Treatment-Related Adverse Events (≥ 10%) NSCLC Patients with ALK Fusion (N=18) Treatment. Related Grade 1 Grade 2 Grade 3 Grade 4 n (%) 11 (61) 0 0 0 11 (61) Vomiting 7 (39) 0 0 0 7 (39) Diarrhea 6 (33) 0 0 0 6 (33) Visual Disturbance 4 (22) 0 0 0 4 (22) ALT Increased 0 2 (11) 1 (6) 0 3 (17) Constipation 0 2 (11) 1 (6) 0 3 (17) Cough 2 (11) 0 0 0 2 (11) Adverse Event Nausea Data in the database as of March 9, 2009 Total n (%)

Conclusions § The MTD and RP 2 D of oral PF-02341066 is 250 mg BID. § The most frequent AEs were mild and moderate GI-related and fatigue. All AEs were manageable and reversible. § Treatment with PF-02341066 resulted in dramatic clinical activity against tumors carrying activating ALK gene fusions. § Results of this trial support the importance of incorporating prospective molecular profiling into early-phase clinical trials for targeted therapies.
PF-02341066: Future Directions § For the Molecular Cohort Focus efforts on identifying patients with MET amplification or mutations § Conduct genetic characterization of ALK fusion partners and EML 4 -ALK variants in responders and non-responders § Conduct molecular analyses of other determinants of response § § Clinical Development of PF-02341066 § Conduct a Phase 3 clinical trial in NSCLC patients harboring ALK fusions

Acknowledgments Massachusetts General Hospital John Iafrate*, Jeffrey Clark, Eunice Kwak Thomas Lynch, Alice Shaw, Panos Fidias Jeffrey Engelman, Marguerite Parkman Dana-Farber Cancer Institute Geoffrey Shapiro, Pasi Janne*, James Butrynski, Leena Gandhi, Andrew Wolanski Suzanne Hitchcock-Bryan, Charles Lee Beth Israel Deaconess Medical Center Bruce Dezube, Daniel Costa, Myles Clancy Memorial Sloan Kettering Robert Maki, Suresh C. Jhanwar* Linda Ahn, Cory Ornelas All The Research Staff All The Patients * Molecular Profiling Contributor Seoul National University Yung-Jue Bang, Woo-Ho Kim*, Dong-Wan Kim Se-Hoon Lee, Do Youn Oh, Sae-Won Han Peter Mac. Callum Cancer Centre Benjamin Solomon, Alex Dobrovic*, Stephen Fox*, Hongdo Do*, Toni-Maree Rogers* University of Colorado Ross Camidge, Marileila Garcia*, S. Gail Eckhardt, Wells Messersmith University of California - Irvine Sai Hong Ou University of Chicago Ravi Salgia, Mark Ratain, David Geary Leonardo Faoro, Rajani Kanteti Pfizer Isan Chen, James Christensen, Victoria Cohan, Gina Emory, Ray Lu, Sophia Randolph, Weiwei Tan, Greg Wei, Keith Wilner Funding provided by Pfizer
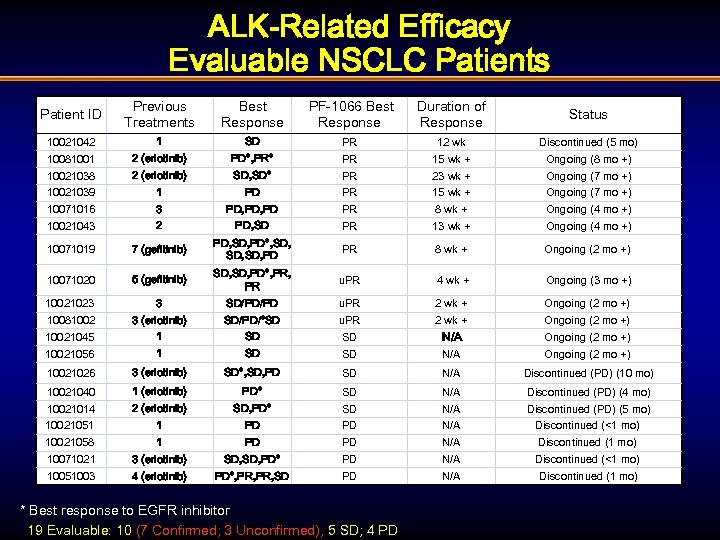
ALK-Related Efficacy Evaluable NSCLC Patients Patient ID Previous Treatments Best Response PF-1066 Best Response Duration of Response Status 10021042 1 SD PR 12 wk Discontinued (5 mo) 10081001 2 (erlotinib) PD*, PR* PR 15 wk + Ongoing (8 mo +) 10021038 2 (erlotinib) SD, SD* PR 23 wk + Ongoing (7 mo +) 10021039 1 PD PR 15 wk + Ongoing (7 mo +) 10071016 3 PD, PD PR 8 wk + Ongoing (4 mo +) 10021043 2 PD, SD PR 13 wk + Ongoing (4 mo +) 10071019 7 (gefitinib) PD, SD, PD*, SD, SD, PD PR 8 wk + Ongoing (2 mo +) 10071020 5 (gefitinib) SD, PD*, PR u. PR 4 wk + Ongoing (3 mo +) 10021023 3 SD/PD/PD u. PR 2 wk + Ongoing (2 mo +) 10081002 3 (erlotinib) SD/PD/*SD u. PR 2 wk + Ongoing (2 mo +) 10021045 1 SD SD N/A Ongoing (2 mo +) 10021056 1 SD SD N/A Ongoing (2 mo +) 10021026 3 (erlotinib) SD*, SD, PD SD N/A Discontinued (PD) (10 mo) 10021040 1 (erlotinib) PD* SD N/A Discontinued (PD) (4 mo) 10021014 2 (erlotinib) SD, PD* SD N/A Discontinued (PD) (5 mo) 10021051 1 PD PD N/A Discontinued (<1 mo) 10021058 1 PD PD N/A Discontinued (1 mo) 10071021 3 (erlotinib) SD, PD* PD N/A Discontinued (<1 mo) 10051003 4 (erlotinib) PD*, PR, SD PD N/A Discontinued (1 mo) * Best response to EGFR inhibitor 19 Evaluable: 10 (7 Confirmed; 3 Unconfirmed), 5 SD; 4 PD
53d308c8ad0bb4f88e70378793f52e0c.ppt