fa1a841cf20b0b84fc0b345fa99d4a60.ppt
- Количество слайдов: 34
 CLIA: Yesterday, Today & Tomorrow! Judith Yost, M. A. , M. T. Director Division of Laboratory Services CLIA
CLIA: Yesterday, Today & Tomorrow! Judith Yost, M. A. , M. T. Director Division of Laboratory Services CLIA
 Topics for Discussion – Where did we begin? – Where are we today? • Technology • Personnel • Oversight/Quality – Where will we be? • Technology • Personnel • Oversight/Quality CLIA
Topics for Discussion – Where did we begin? – Where are we today? • Technology • Personnel • Oversight/Quality – Where will we be? • Technology • Personnel • Oversight/Quality CLIA
 232, 548 LABS Enrolled in CLIA in 2011! • • Compliance -----19, 319 Waiver ---- 146, 071 PPM ------ 37, 767 Accredited ------ 15, 787 Exempt (NY/WA) ----- 6, 802 Note: There were 150, 000 labs in 1994! Continued growth expected! • CLIA Source: CLIA Database June 2011
232, 548 LABS Enrolled in CLIA in 2011! • • Compliance -----19, 319 Waiver ---- 146, 071 PPM ------ 37, 767 Accredited ------ 15, 787 Exempt (NY/WA) ----- 6, 802 Note: There were 150, 000 labs in 1994! Continued growth expected! • CLIA Source: CLIA Database June 2011
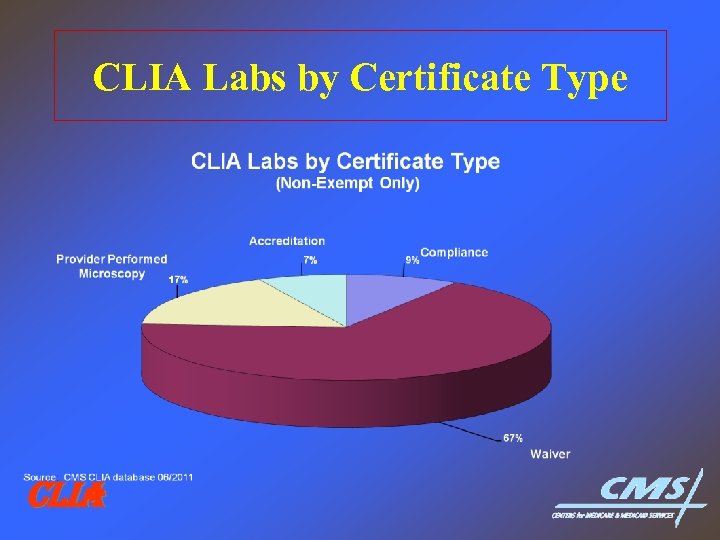 CLIA Labs by Certificate Type CLIA
CLIA Labs by Certificate Type CLIA
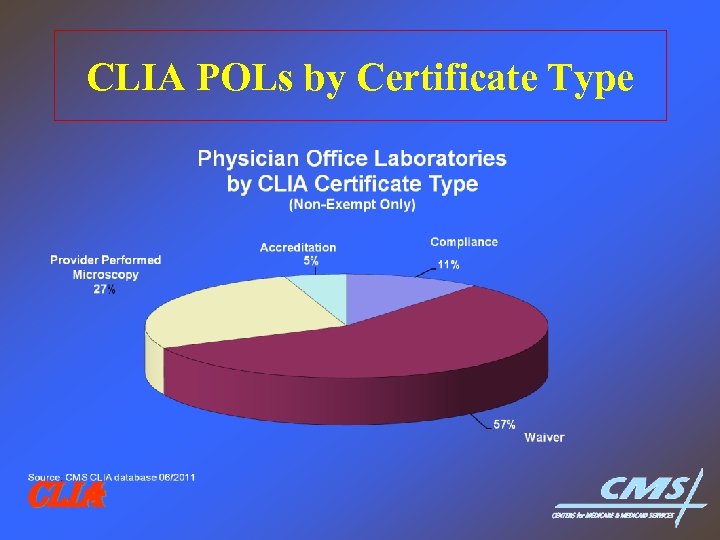 CLIA POLs by Certificate Type CLIA
CLIA POLs by Certificate Type CLIA
 CLIA Labs by Certificate Type 1995 vs 2011 1995 – Compliance---37, 578 vs – Waiver-----56, 031 vs – PPM------23, 089 vs – Accredited-----19, 426 vs CLIA 2011 19, 319 146, 071 37, 767 15, 767
CLIA Labs by Certificate Type 1995 vs 2011 1995 – Compliance---37, 578 vs – Waiver-----56, 031 vs – PPM------23, 089 vs – Accredited-----19, 426 vs CLIA 2011 19, 319 146, 071 37, 767 15, 767
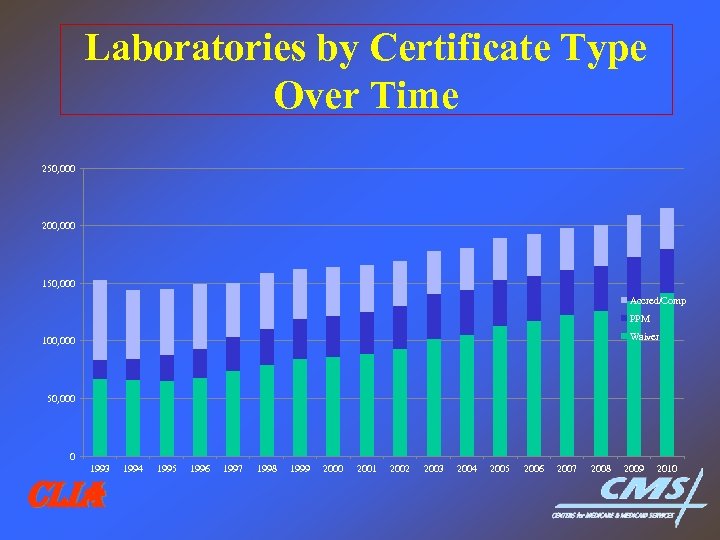 Laboratories by Certificate Type Over Time 250, 000 200, 000 150, 000 Accred/Comp PPM Waiver 100, 000 50, 000 0 1993 CLIA 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010
Laboratories by Certificate Type Over Time 250, 000 200, 000 150, 000 Accred/Comp PPM Waiver 100, 000 50, 000 0 1993 CLIA 1994 1995 1996 1997 1998 1999 2000 2001 2002 2003 2004 2005 2006 2007 2008 2009 2010
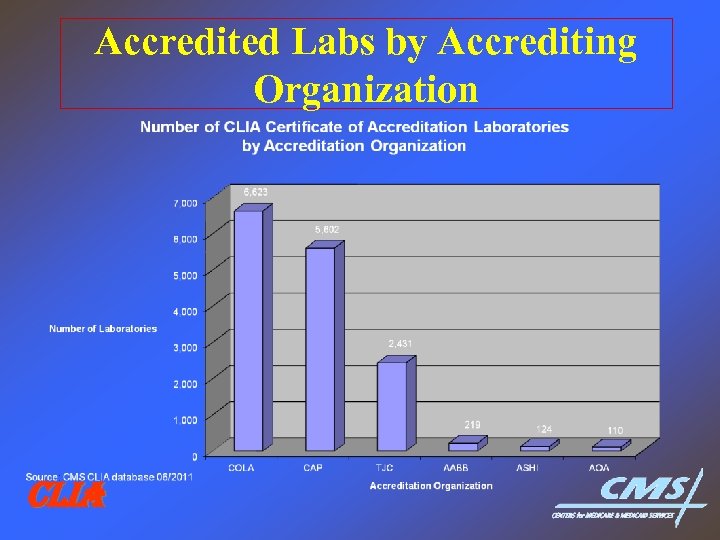 Accredited Labs by Accrediting Organization CLIA
Accredited Labs by Accrediting Organization CLIA
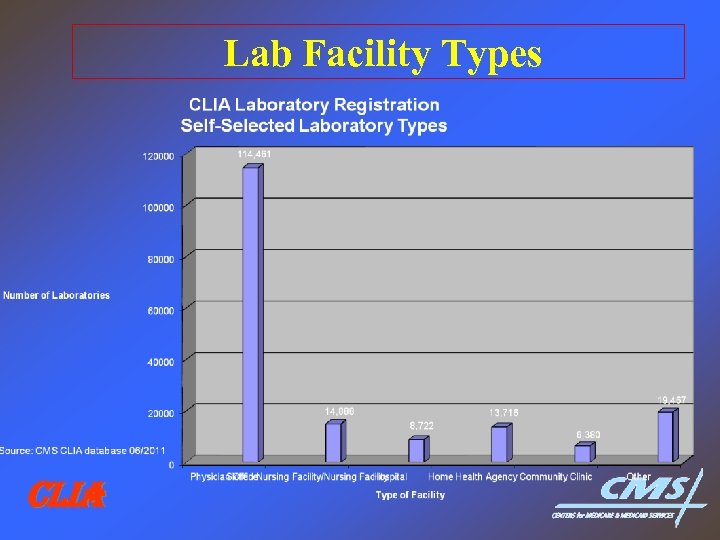 Lab Facility Types CLIA
Lab Facility Types CLIA
 Lab Oversight • Past Oversight – CLIA ’ 67, AO’s & States – Not all-inclusive • Current Oversight – Mostly regulation via: • CMS • AO &/or States • Rigid, difficult to change, outdated standards • Future Oversight – Same, but use regs as an umbrella w/ guidance: • Public/private partnerships • Professional & International standards • Performance Measures CLIA • Flexible, easier to adjust, current standards
Lab Oversight • Past Oversight – CLIA ’ 67, AO’s & States – Not all-inclusive • Current Oversight – Mostly regulation via: • CMS • AO &/or States • Rigid, difficult to change, outdated standards • Future Oversight – Same, but use regs as an umbrella w/ guidance: • Public/private partnerships • Professional & International standards • Performance Measures CLIA • Flexible, easier to adjust, current standards
 Pathology • Current Pathology – Review & interpretation of microscopic slides • Using a standard microscope • In a clinical laboratory • Future Pathology – Digital pathology—images transmitted to unlimited no. of locations via the Web • Telepathology; Virtual pathology • Excellent educational tool • Brings new compliance challenges • Now includes other specialties CLIA
Pathology • Current Pathology – Review & interpretation of microscopic slides • Using a standard microscope • In a clinical laboratory • Future Pathology – Digital pathology—images transmitted to unlimited no. of locations via the Web • Telepathology; Virtual pathology • Excellent educational tool • Brings new compliance challenges • Now includes other specialties CLIA
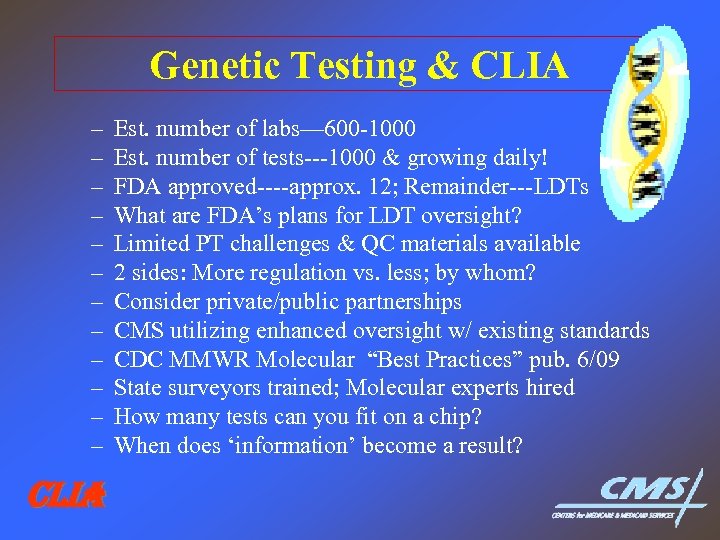 Genetic Testing & CLIA – – – CLIA Est. number of labs— 600 -1000 Est. number of tests---1000 & growing daily! FDA approved----approx. 12; Remainder---LDTs What are FDA’s plans for LDT oversight? Limited PT challenges & QC materials available 2 sides: More regulation vs. less; by whom? Consider private/public partnerships CMS utilizing enhanced oversight w/ existing standards CDC MMWR Molecular “Best Practices” pub. 6/09 State surveyors trained; Molecular experts hired How many tests can you fit on a chip? When does ‘information’ become a result?
Genetic Testing & CLIA – – – CLIA Est. number of labs— 600 -1000 Est. number of tests---1000 & growing daily! FDA approved----approx. 12; Remainder---LDTs What are FDA’s plans for LDT oversight? Limited PT challenges & QC materials available 2 sides: More regulation vs. less; by whom? Consider private/public partnerships CMS utilizing enhanced oversight w/ existing standards CDC MMWR Molecular “Best Practices” pub. 6/09 State surveyors trained; Molecular experts hired How many tests can you fit on a chip? When does ‘information’ become a result?
 Personnel Shortage – Growth of clinical labs is >27% (BLS) – 155, 822 MTs now; will need 187, 818 by 2014 – 146, 626 MLTs now; will need 183, 347 by 2014 – Median age is 48 yrs. – Skill set is mobile – Current shortfall 10, 000/yr. – National recruitment & retention strategies underway NOTE: Information courtesy Elissa Passiment, Exec. VP, ASCLS CLIA
Personnel Shortage – Growth of clinical labs is >27% (BLS) – 155, 822 MTs now; will need 187, 818 by 2014 – 146, 626 MLTs now; will need 183, 347 by 2014 – Median age is 48 yrs. – Skill set is mobile – Current shortfall 10, 000/yr. – National recruitment & retention strategies underway NOTE: Information courtesy Elissa Passiment, Exec. VP, ASCLS CLIA
 Personnel Shortage – Issues • Recognition • Power • Work environment – No opportunity for advancement – Risk of infectious disease – Work hours – Stress • Wages CLIA
Personnel Shortage – Issues • Recognition • Power • Work environment – No opportunity for advancement – Risk of infectious disease – Work hours – Stress • Wages CLIA
 Personnel Shortage – Multi-faceted Resolutions Underway • • • CLIA Send young MTs to recruit very young folks Develop HS science lesson plans Update MT curricula w/ today’s skills/needs Establish re-training pgm. former techs Obtain Congressional funds Brand labs as good workplace; offer creative opportunities Employers emphasize value & recognize techs Improve salaries & safety Create career ladders, innovative schedules
Personnel Shortage – Multi-faceted Resolutions Underway • • • CLIA Send young MTs to recruit very young folks Develop HS science lesson plans Update MT curricula w/ today’s skills/needs Establish re-training pgm. former techs Obtain Congressional funds Brand labs as good workplace; offer creative opportunities Employers emphasize value & recognize techs Improve salaries & safety Create career ladders, innovative schedules
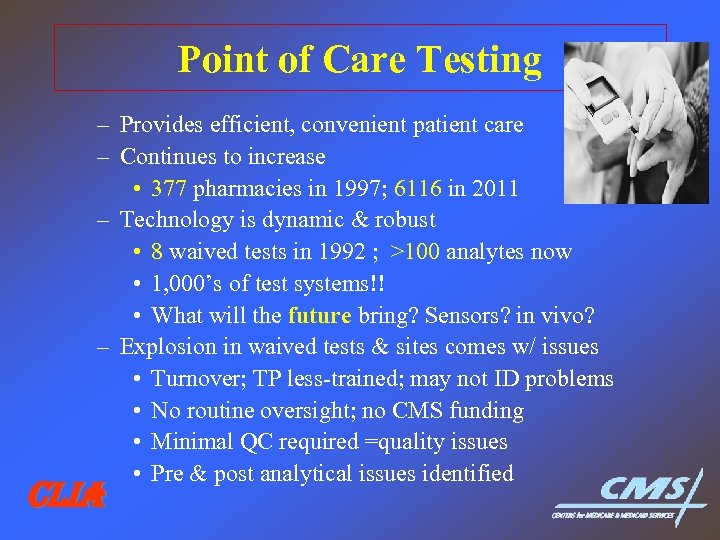 Point of Care Testing – Provides efficient, convenient patient care – Continues to increase • 377 pharmacies in 1997; 6116 in 2011 – Technology is dynamic & robust • 8 waived tests in 1992 ; >100 analytes now • 1, 000’s of test systems!! • What will the future bring? Sensors? in vivo? – Explosion in waived tests & sites comes w/ issues • Turnover; TP less-trained; may not ID problems • No routine oversight; no CMS funding • Minimal QC required =quality issues • Pre & post analytical issues identified CLIA
Point of Care Testing – Provides efficient, convenient patient care – Continues to increase • 377 pharmacies in 1997; 6116 in 2011 – Technology is dynamic & robust • 8 waived tests in 1992 ; >100 analytes now • 1, 000’s of test systems!! • What will the future bring? Sensors? in vivo? – Explosion in waived tests & sites comes w/ issues • Turnover; TP less-trained; may not ID problems • No routine oversight; no CMS funding • Minimal QC required =quality issues • Pre & post analytical issues identified CLIA
 CMS Certificate of Waiver (CW) Project – CMS suspected & found problems in CW labs – Conducted pilot studies: • Approx. 50% didn’t follow mfgr’s. instr. – Received support from CLIAC & CMS • Initiated 2% annual on site educational visits – Congress never anticipated 67% labs w/ o oversight! – 2 -4% CW labs/yr. have immediate jeopardy to patients’ health & safety! – Turnover, lack of training, etc. – Intervention yields significant improvement CLIA
CMS Certificate of Waiver (CW) Project – CMS suspected & found problems in CW labs – Conducted pilot studies: • Approx. 50% didn’t follow mfgr’s. instr. – Received support from CLIAC & CMS • Initiated 2% annual on site educational visits – Congress never anticipated 67% labs w/ o oversight! – 2 -4% CW labs/yr. have immediate jeopardy to patients’ health & safety! – Turnover, lack of training, etc. – Intervention yields significant improvement CLIA
 CMS Certificate of Waiver (CW) Project • 2004 – 39% not following instructions—initial visit • 2006 & Beyond – 34% not following instructions—initial visit • 70% improved conformance after educational intervention!! • 2011 - Address quality concerns w/ multifaceted plan in coordination w/ Partners; including A-19 CLIA
CMS Certificate of Waiver (CW) Project • 2004 – 39% not following instructions—initial visit • 2006 & Beyond – 34% not following instructions—initial visit • 70% improved conformance after educational intervention!! • 2011 - Address quality concerns w/ multifaceted plan in coordination w/ Partners; including A-19 CLIA
 CLIA & EHRs – CLIA requires results to authorized person • Or individual who will use them • Or to lab that ordered the test (referral) • Authorized person can designate ‘agent’(EHR) on their behalf – CLIA requires certain data elements on lab repts. – CLIA requires accurate, reliable, timely & confidential result transmission regardless of mechanism – New CMS guidance is available Mar. 2010! • Clarifies standard interpretations re EHRs. – Patient access reg pub. 9/12/11; Comments due 11/17/11 – More efforts underway w/ ONC CLIA
CLIA & EHRs – CLIA requires results to authorized person • Or individual who will use them • Or to lab that ordered the test (referral) • Authorized person can designate ‘agent’(EHR) on their behalf – CLIA requires certain data elements on lab repts. – CLIA requires accurate, reliable, timely & confidential result transmission regardless of mechanism – New CMS guidance is available Mar. 2010! • Clarifies standard interpretations re EHRs. – Patient access reg pub. 9/12/11; Comments due 11/17/11 – More efforts underway w/ ONC CLIA
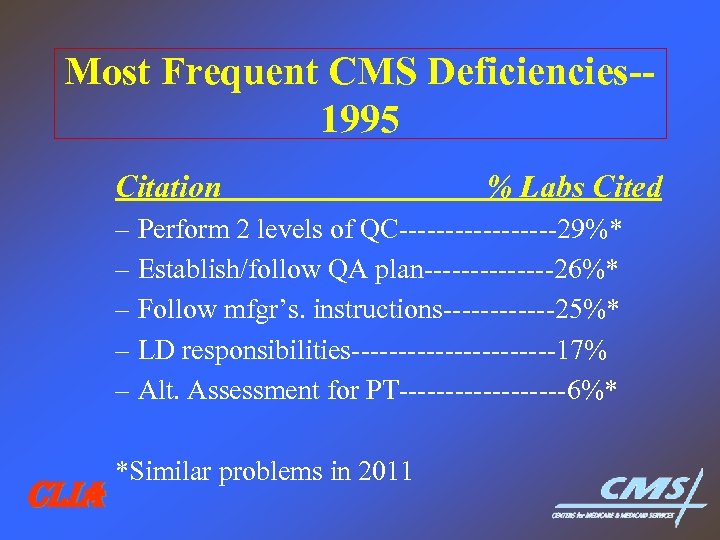 Most Frequent CMS Deficiencies-1995 Citation % Labs Cited – Perform 2 levels of QC---------29%* – Establish/follow QA plan-------26%* – Follow mfgr’s. instructions------25%* – LD responsibilities-----------17% – Alt. Assessment for PT---------6%* CLIA *Similar problems in 2011
Most Frequent CMS Deficiencies-1995 Citation % Labs Cited – Perform 2 levels of QC---------29%* – Establish/follow QA plan-------26%* – Follow mfgr’s. instructions------25%* – LD responsibilities-----------17% – Alt. Assessment for PT---------6%* CLIA *Similar problems in 2011
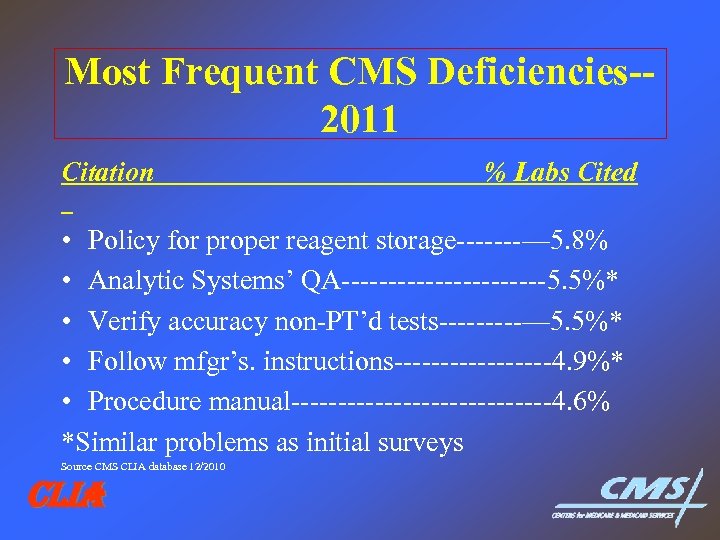 Most Frequent CMS Deficiencies-2011 Citation % Labs Cited • Policy for proper reagent storage-------— 5. 8% • Analytic Systems’ QA-----------5. 5%* • Verify accuracy non-PT’d tests-----— 5. 5%* • Follow mfgr’s. instructions---------4. 9%* • Procedure manual--------------4. 6% *Similar problems as initial surveys Source CMS CLIA database 12/2010 CLIA
Most Frequent CMS Deficiencies-2011 Citation % Labs Cited • Policy for proper reagent storage-------— 5. 8% • Analytic Systems’ QA-----------5. 5%* • Verify accuracy non-PT’d tests-----— 5. 5%* • Follow mfgr’s. instructions---------4. 9%* • Procedure manual--------------4. 6% *Similar problems as initial surveys Source CMS CLIA database 12/2010 CLIA
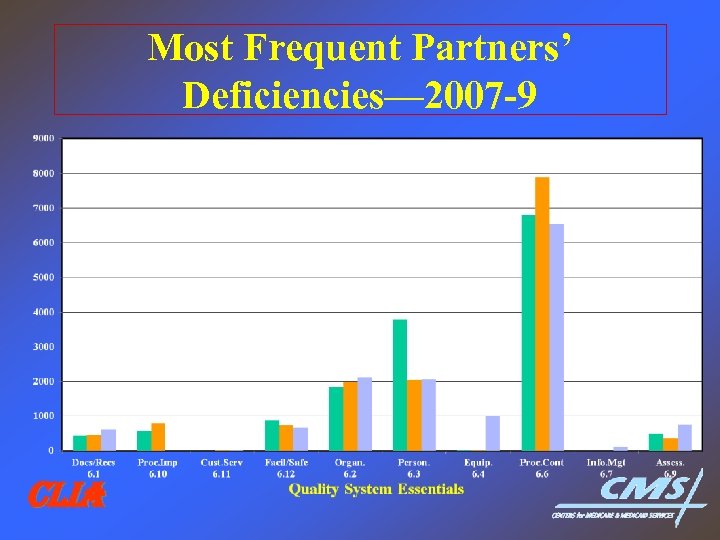 Most Frequent Partners’ Deficiencies— 2007 -9 CLIA
Most Frequent Partners’ Deficiencies— 2007 -9 CLIA
 Partners Performance Measures • Performance measures serve as an adjunct to validation protocol to identify what AO’s (and CMS) actually do! • Have identified weaknesses & facilitated improved & standardized oversight practices for all • Most frequent deficiencies for AO’s & CMS are similar • Provides focus for training, lab education; better requirements • Enhanced level of oversight achieved by Partners’ improved communication, coordination & collaboration CLIA
Partners Performance Measures • Performance measures serve as an adjunct to validation protocol to identify what AO’s (and CMS) actually do! • Have identified weaknesses & facilitated improved & standardized oversight practices for all • Most frequent deficiencies for AO’s & CMS are similar • Provides focus for training, lab education; better requirements • Enhanced level of oversight achieved by Partners’ improved communication, coordination & collaboration CLIA
 2003 QC Regulation Each laboratory that performs nonwaived testing must meet the applicable analytic systems requirements, unless HHS approves a procedure, specified in the Interpretive Guidelines, that provides equivalent quality. CLIA
2003 QC Regulation Each laboratory that performs nonwaived testing must meet the applicable analytic systems requirements, unless HHS approves a procedure, specified in the Interpretive Guidelines, that provides equivalent quality. CLIA
 CMS Interpretive Guidelines (IG)Published 2004 – Provides regulatory interpretations, policies for labs & surveyors • Located on CMS/CLIA web site – Introduces “Equivalent QC” (EQC) – CMS will make adjustments w/ experience &/or data or new policies CLIA
CMS Interpretive Guidelines (IG)Published 2004 – Provides regulatory interpretations, policies for labs & surveyors • Located on CMS/CLIA web site – Introduces “Equivalent QC” (EQC) – CMS will make adjustments w/ experience &/or data or new policies CLIA
 “QC for the Future” • ‘ 05 CLSI meeting w/ lab prof. orgs. , gov’t, industry & AOs: – Labs need more info from manufacturers. – One-size-fits-all QC won’t work for all test systems, different patient pop. & lab types. • CLSI Evaluation Protocol (EP) doc completed – Alternative QC for labs using ISO risk management • Interpretive Guidelines will be revised accordingly CLIA
“QC for the Future” • ‘ 05 CLSI meeting w/ lab prof. orgs. , gov’t, industry & AOs: – Labs need more info from manufacturers. – One-size-fits-all QC won’t work for all test systems, different patient pop. & lab types. • CLSI Evaluation Protocol (EP) doc completed – Alternative QC for labs using ISO risk management • Interpretive Guidelines will be revised accordingly CLIA
 EP-23: Laboratory QC Based on Risk Management • CMS working w/ CLSI to develop new guidance “Evaluation Protocol” documents – Includes experts from labs, industry & gov’t. – Utilizing a consensus process – Exciting, groundbreaking efforts – Utilizes much of what the lab already does • Laboratories’ guidance to design custom alternative QC w/ manufacturer’s information—EP-23 – Chaired by James Nichols, Baystate Health CLIA
EP-23: Laboratory QC Based on Risk Management • CMS working w/ CLSI to develop new guidance “Evaluation Protocol” documents – Includes experts from labs, industry & gov’t. – Utilizing a consensus process – Exciting, groundbreaking efforts – Utilizes much of what the lab already does • Laboratories’ guidance to design custom alternative QC w/ manufacturer’s information—EP-23 – Chaired by James Nichols, Baystate Health CLIA
 EP-23: Laboratory QC Based on Risk Management – – – CLIA Intended for laboratory & POC users Uses mfgrs. ’ risk mgmt. info about test limitations Reduces negative impact of test limits on QC Enables lab to develop cost-efficient, effective QC protocols While ensuring compliance with applicable regulations Considers labs’ unique environmental factors: • Technology, personnel competency, temperature, • Storage conditions • Clinical use of test results (if known)
EP-23: Laboratory QC Based on Risk Management – – – CLIA Intended for laboratory & POC users Uses mfgrs. ’ risk mgmt. info about test limitations Reduces negative impact of test limits on QC Enables lab to develop cost-efficient, effective QC protocols While ensuring compliance with applicable regulations Considers labs’ unique environmental factors: • Technology, personnel competency, temperature, • Storage conditions • Clinical use of test results (if known)
 PT Regulation Update • Plan w/ milestones developed; No firm ETA – Includes target values, grading system, PT pgms. , labs, PT referral, alt. assessment; analyte selection – Requires a proposed rule w/ comment & final • PT providers’ meeting held Nov. ’ 08 • Utilize CLIAC process w/ SME WG • Medicare & other data reviewed for test frequency • Evaluating methods for analyte selection & grading • WG mtg. March 2010; rept. to CLIAC Sept. ‘ 10 & Mar. ‘ 11 • Ongoing mtgs. & data analysis w/ CDC • Stay tuned…. CLIA
PT Regulation Update • Plan w/ milestones developed; No firm ETA – Includes target values, grading system, PT pgms. , labs, PT referral, alt. assessment; analyte selection – Requires a proposed rule w/ comment & final • PT providers’ meeting held Nov. ’ 08 • Utilize CLIAC process w/ SME WG • Medicare & other data reviewed for test frequency • Evaluating methods for analyte selection & grading • WG mtg. March 2010; rept. to CLIAC Sept. ‘ 10 & Mar. ‘ 11 • Ongoing mtgs. & data analysis w/ CDC • Stay tuned…. CLIA
 Goals for the Future – Publish standards/performance measures reflective of current technology & state of lab medicine – Improve consistency among AOs, CMS regions, State surveyors, where possible – Use professional standards in lieu of regulations for flexibility, where appropriate – Engage in public/private partnerships to garner expertise – Collaborate w/ FDA on LDT oversight – Ensure quality in CW labs via education & other approaches – Assume waived lab oversight if A-19 passes: develop quality standards based on data from CW visits CLIA
Goals for the Future – Publish standards/performance measures reflective of current technology & state of lab medicine – Improve consistency among AOs, CMS regions, State surveyors, where possible – Use professional standards in lieu of regulations for flexibility, where appropriate – Engage in public/private partnerships to garner expertise – Collaborate w/ FDA on LDT oversight – Ensure quality in CW labs via education & other approaches – Assume waived lab oversight if A-19 passes: develop quality standards based on data from CW visits CLIA
 Goals for the Future – Become more data driven to enhance program management & lab oversight – Increase CMS involvement in personnel shortages – Maintain solvency of CLIA program – Finalize strategy for oversight of international labs – Address digitized/virtual testing in multiple sites – Update personnel, certificate, etc. regulations – Determine oversight of non-invasive tests – Help to enhance patient-centric care – Augment relationships betw. providers & labs CLIA
Goals for the Future – Become more data driven to enhance program management & lab oversight – Increase CMS involvement in personnel shortages – Maintain solvency of CLIA program – Finalize strategy for oversight of international labs – Address digitized/virtual testing in multiple sites – Update personnel, certificate, etc. regulations – Determine oversight of non-invasive tests – Help to enhance patient-centric care – Augment relationships betw. providers & labs CLIA
 CLIA Retrospective Thoughts…. . – Balance of extremes to craft quality standards • Assure access; minimize cost/burden • Maintain integrity of quality oversight – Work w/ AOs, exempt States to improve the level & consistency of oversight-Partner’s – Use an educational, not punitive, QA focus to survey labs & monitor outcomes (test results) CLIA
CLIA Retrospective Thoughts…. . – Balance of extremes to craft quality standards • Assure access; minimize cost/burden • Maintain integrity of quality oversight – Work w/ AOs, exempt States to improve the level & consistency of oversight-Partner’s – Use an educational, not punitive, QA focus to survey labs & monitor outcomes (test results) CLIA
 Where to find more info – CMS CLIA web site • www. cms. hhs. gov/clia • Brochures, lab look-up; guidelines; data, etc. – CMS Central office in Baltimore • 410 -786 -3531 – Judy Yost’s email • Judith. yost@cms. hhs. gov CLIA
Where to find more info – CMS CLIA web site • www. cms. hhs. gov/clia • Brochures, lab look-up; guidelines; data, etc. – CMS Central office in Baltimore • 410 -786 -3531 – Judy Yost’s email • Judith. yost@cms. hhs. gov CLIA
 THE END! The future is now! Thank you!! CLIA
THE END! The future is now! Thank you!! CLIA


