e3a139d641d1e9bb8602b1995a6f8b4b.ppt
- Количество слайдов: 46

CLIA Challenges in Laboratory Consolidation 10/26/16 Kandice Kottke-Marchant, MD, Ph. D Robert J. Tomsich Pathology & Laboratory Medicine Institute (RT-PLMI)

Outline 1. 2. 3. Laboratory Integration Cost Reduction (Care Affordability) CLIA Challenges

1. Laboratory Integration (2007 -2012)
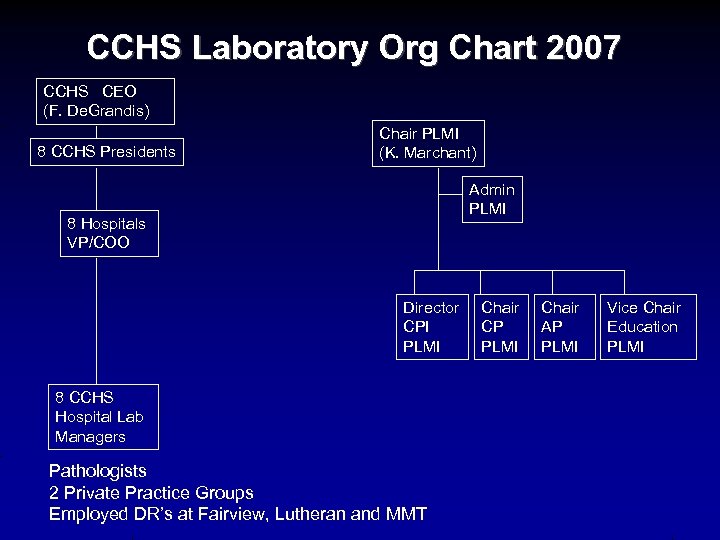
CCHS Laboratory Org Chart 2007 CCHS CEO (F. De. Grandis) 8 CCHS Presidents Chair PLMI (K. Marchant) Admin PLMI 8 Hospitals VP/COO Director CPI PLMI 8 CCHS Hospital Lab Managers Pathologists 2 Private Practice Groups Employed DR’s at Fairview, Lutheran and MMT Chair CP PLMI Chair AP PLMI Vice Chair Education PLMI
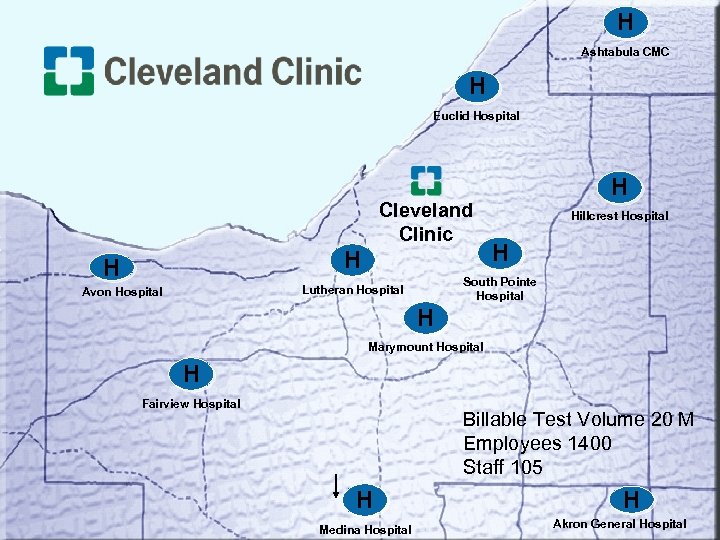
H Ashtabula CMC H Euclid Hospital Cleveland Clinic H H Hillcrest Hospital H South Pointe Hospital Lutheran Hospital Avon Hospital H H Marymount Hospital H Fairview Hospital Billable Test Volume 20 M Employees 1400 Staff 105 H Medina Hospital H Akron General Hospital

Strategic Aims for Laboratory Integration • Standardize - Test ordering - Testing platforms and methods - Quality - Policies and Procedures - Supply Chain • Reduce unit cost • Emphasize quality and consultation • Consolidate where possible - Core Laboratory - Centralize non-lab functions - Integrate services • Eliminate duplication

Issues for Laboratory Integration • • • 9 separate laboratories No common reporting structure 9 separate medical directors & managers Separate financial accounting Separate capital equipment process Separate IT systems (East, west, main) Different testing instruments Different reference ranges Different critical values Other Challenges: - Integration to be accomplished by consensus - No central leadership authority - No consolidated administrative structure
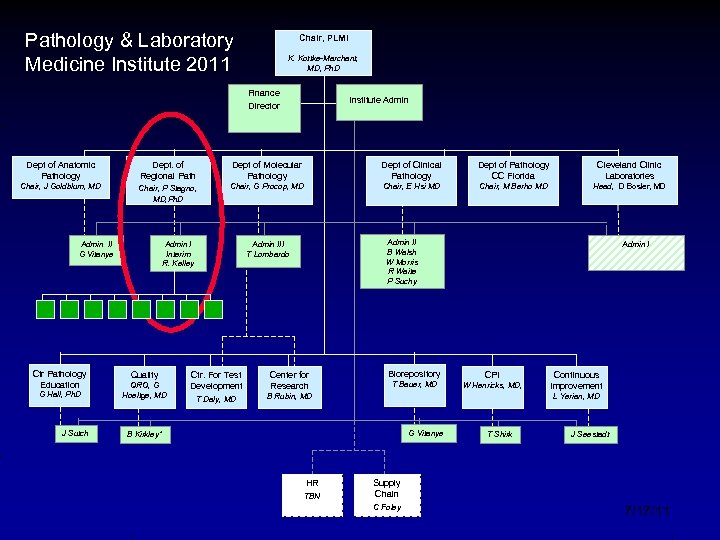
Pathology & Laboratory Medicine Institute 2011 Chair, PLMI K. Kottke-Marchant, MD, Ph. D Finance Director Institute Admin Dept of Anatomic Pathology Dept. of Regional Path Dept of Molecular Pathology Dept of Clinical Pathology Dept of Pathology CC Florida Cleveland Clinic Laboratories Chair, J Goldblum, MD Chair, P Stagno, MD, Ph. D Chair, G Procop, MD Chair, E Hsi MD Chair, M Berho MD Head, D Bosler, MD Admin II G Vitanye Ctr Pathology Education G Hall, Ph. D J Sutch Admin I Interim R. Kelley Quality QRO, G Hoeltge, MD Admin II B Walsh W Morris R Waite P Suchy Admin III T Lombardo Ctr. For Test Development Center for Research T Daly, MD Biorepository Admin I B Rubin, MD T Bauer, MD G Vitanye HR Continuous Improvement L Yerian, MD B Kirkley* TBN CPI W Henricks, MD, T Shirk J Seestadt Supply Chain C Foley 7/17/11

Accomplishments • Single IT platforms (Co. Path, Sun. Quest) • Standard Chemistry and hematology platforms • Std. reference ranges/critical values • Centralized molecular, flow cytometry, autopsy • Integrated pathologists to regional department • Consolidated regional microbiology, histology, cytology to one E & W hospital

Remaining Challenges • • Standardize policies and procedures Standardize quality management system Implement system-wide quality infrastructure Complete standardizing test menu Cross-train and use workforce regionally Streamline courier networks Define regional core labs

2. Cost Reduction: Care Affordability and Reimbursement Challenges (2014 -2015)
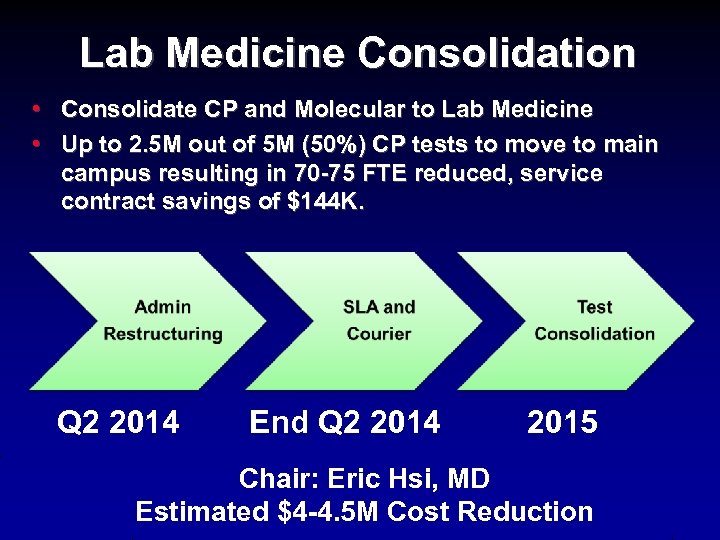
Lab Medicine Consolidation • Consolidate CP and Molecular to Lab Medicine • Up to 2. 5 M out of 5 M (50%) CP tests to move to main campus resulting in 70 -75 FTE reduced, service contract savings of $144 K. Q 2 2014 End Q 2 2014 2015 Chair: Eric Hsi, MD Estimated $4 -4. 5 M Cost Reduction
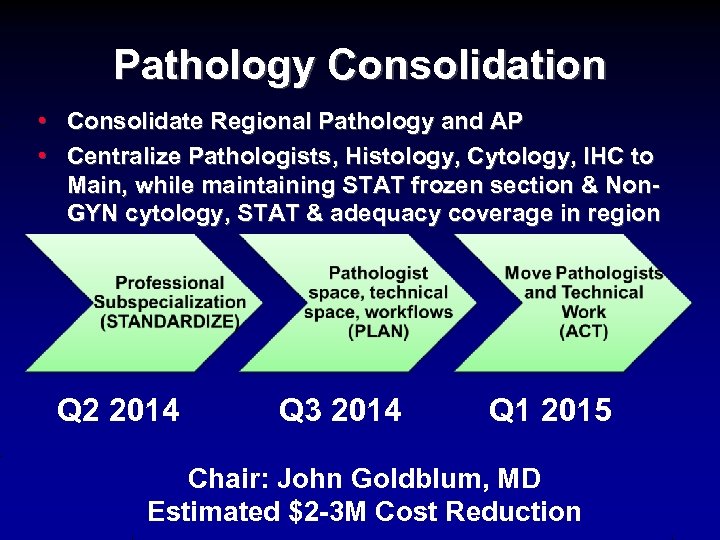
Pathology Consolidation • Consolidate Regional Pathology and AP • Centralize Pathologists, Histology, Cytology, IHC to Main, while maintaining STAT frozen section & Non. GYN cytology, STAT & adequacy coverage in region Q 2 2014 Q 3 2014 Q 1 2015 Chair: John Goldblum, MD Estimated $2 -3 M Cost Reduction

Challenges and Issues • Challenges - Setting service level expectations -Long-term, multi-year transformation - Change throughout organization - Organizational engagement - Communication to all stakeholders • Issues - Timeline for hiring new leaders -Onboarding new leaders -Elimination of Regional Pathology Department -Subspecialty leadership in regional laboratories -Employee fear and discontent

3. CLIA Challenges in Laboratory Integration (2015 -2016)

Complexity of Lab Quality • • >1000 CLIA Regulations >1500 unique Lab Tests 1400 employees 45 CLIA Lab Directors 16, 000 policies and procedures 3500 CAP requirements Competency assessment; QC; Proficiency Testing; equipment maintenance; SOP review - training

CMS Validation Survey • Occurs after about 5% of CAP inspections • To validate the accreditation agency’s process • Holds lab to CLIA standards • Focuses on documentation, personnel qualifications, competency, proficiency testing, QC
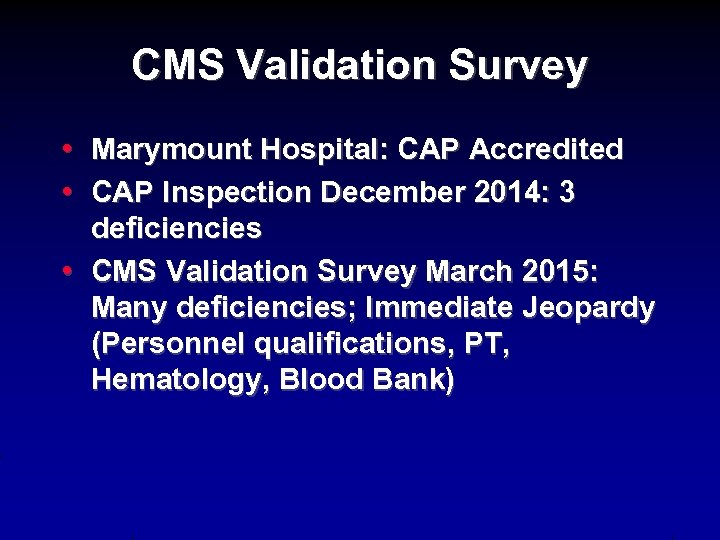
CMS Validation Survey • Marymount Hospital: CAP Accredited • CAP Inspection December 2014: 3 deficiencies • CMS Validation Survey March 2015: Many deficiencies; Immediate Jeopardy (Personnel qualifications, PT, Hematology, Blood Bank)
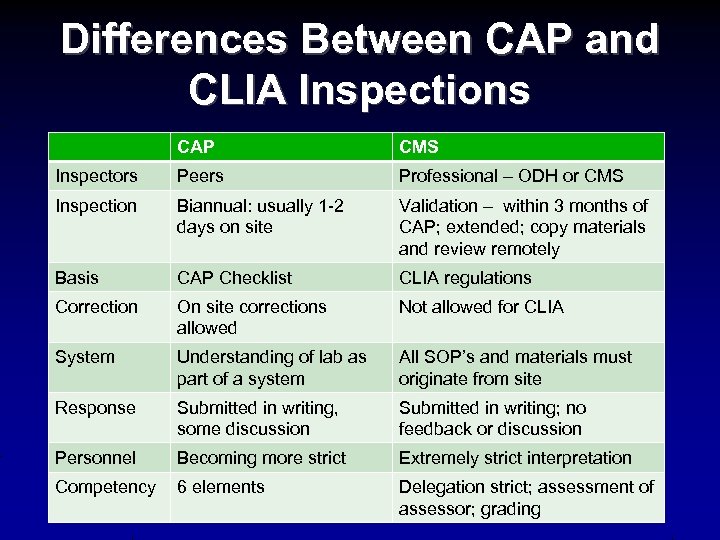
Differences Between CAP and CLIA Inspections CAP CMS Inspectors Peers Professional – ODH or CMS Inspection Biannual: usually 1 -2 days on site Validation – within 3 months of CAP; extended; copy materials and review remotely Basis CAP Checklist CLIA regulations Correction On site corrections allowed Not allowed for CLIA System Understanding of lab as part of a system All SOP’s and materials must originate from site Response Submitted in writing, some discussion Submitted in writing; no feedback or discussion Personnel Becoming more strict Extremely strict interpretation Competency 6 elements Delegation strict; assessment of assessor; grading
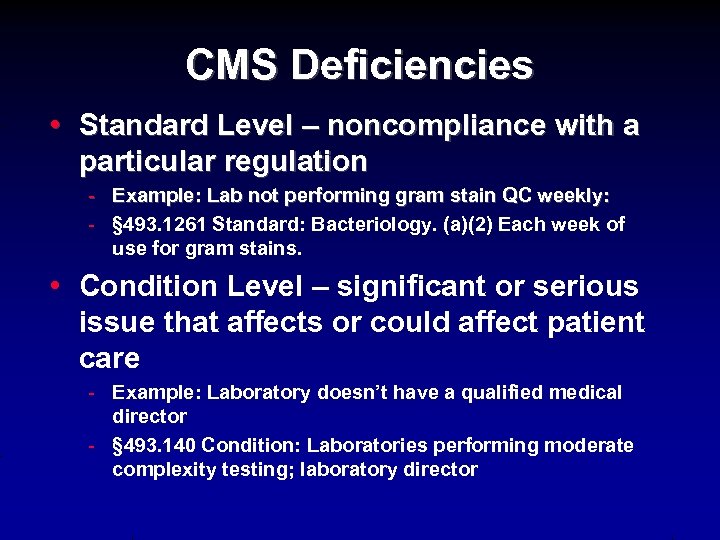
CMS Deficiencies • Standard Level – noncompliance with a particular regulation - Example: Lab not performing gram stain QC weekly: - § 493. 1261 Standard: Bacteriology. (a)(2) Each week of use for gram stains. • Condition Level – significant or serious issue that affects or could affect patient care - Example: Laboratory doesn’t have a qualified medical director - § 493. 140 Condition: Laboratories performing moderate complexity testing; laboratory director

CMS Deficiencies • Immediate Jeopardy (IJ) - 2 day notice to CMS Regional Office and provider - 23 day termination track - Submit “credible allegation of compliance” - Revisit to ensure IJ removed • If IJ cleared, 90 day termination track (67 extra days) • Submit “Plan of Correction” (Po. C) for remaining findings • Second revisit

Possible Sanctions - Suspend, limit, or revoke CLIA certificate - Directed Po. C • Specific corrective action • Notice to clients/patients - Suspension of all or part of lab Medicare payments - State onsite monitoring - Civil money penalty - Public notice

Plan of Correction • Submit in 10 days: - Description of how the deficient practice(s) will be or have been corrected - Impact on patient care - The method(s) to maintain and monitor compliance - A realistic date of correction (month, date and year)

Examples and Lessons from Deficiencies • • Proficiency Testing Point-of-Care Testing Analytics; LDT Competency Assessment Maintenance and Function Checks Personnel Qualifications; 209 Form Informatics
Proficiency Testing • CFR 493. 801 Enrollment and Testing of Samples - Not treating PT in same manner as patient; duplicate testing - Not tested with regular workload by persons who normally do testing • ACT, activity logs - TP consulted pathologist during PT of microscopic samples

Point of Care (ACT) • 493. 1269 QC not done with 2 levels of controls every 8 hours • Only “expert” users performed QC • Didn’t retain instrument printouts • Results not reported in EHR
Analytics; LDT • Moderate Complexity Testing - Exactly match IFU (instructions for use) - ANY changes elevate to high complexity LDT (tube, stability, centrifugation speed, rejection criteria) - Require full LDT validation - High complexity personnel requirements

Competency Assessment • Assessor had not been assessed • Individual observing competency not certified by LD for role as TS or GS • No direct observation • Didn’t include problem solving – or quizzes not graded • Pathologists as TP: Competency

Maintenance and Function Checks • Pipettes should be calibrated on site (under conditions of use) • Equipment verification must be performed by TP • 493. 1255 Calibration verification every 6 months • QC – Westgard rules

Personnel Qualifications and Delegations • • • Accuracy of CMS 209 form Degree in biological science Bachelor’s degree not stating field - Requires transcript and calculation of credit hours for chemistry/biology • Foreign degree - Requires translation and equivalency

Informatics • CFR 493. 1215 Hematology - Written procedures not available (document system went down during inspection – central IT)
Hospital vs. System Focus • • • SOP header – PLMI CMS wanted all SOP’s to be MMH only PT policy included PT catalog numbers for main campus, not just MMH • Validation survey – CMS will only speak with individuals listed on 209 form

Site Corrective Action • • POC Submitted Testing discontinuation CLIA consultant re-wrote SOP’s Rehired staff, Retrained IJ Removed November 2015 Testing reintroduced over next 8 months

Effect of Lab Integration • • • Central oversight – challenge Subject matter experts - central Local management – new, covering several sites Uncovered pre-existing practices Audits without local accountability for correction CLIA Lab Directors – focused on AP

System-wide Risk Mitigation • • • Audits Training Standardization Quality Structure ISO 15189

System-wide Risk Mitigation AUDITS • • • Engaged external consultant Performed mock CLIA audits Prioritized and enforced corrective action • Expanded and empowered internal audit group

Mock CMS Audit of CCHS Hospitals • Documentation practices • Non-standardized quality management system • Personnel qualifications (per CLIA) • Non-standard competency assessment • Variability in transfusion medicine processes • Variability in test validation • No centralized document governance • CLIA holders not engaged

System-wide Risk Mitigation TRAINING • CLIA holder accountability – fewer, more focused CLIA holders • Retrained all CLIA Lab Directors • Developed CLIA holder council • Required site specific CLIA holder monthly meetings • CLIA training for testing personnel

System-wide Risk Mitigation STANDARDIZATION • Adopt standardized quality management system • Metrics – central reporting/oversight • Develop systemic document control process and oversight • Standardized competency process • POSI – PLMI Optimization and Standardization Initiative (accountability for implementation)

System-wide risk Mitigation • Established new QA structure • Target ISO 15189 accreditation
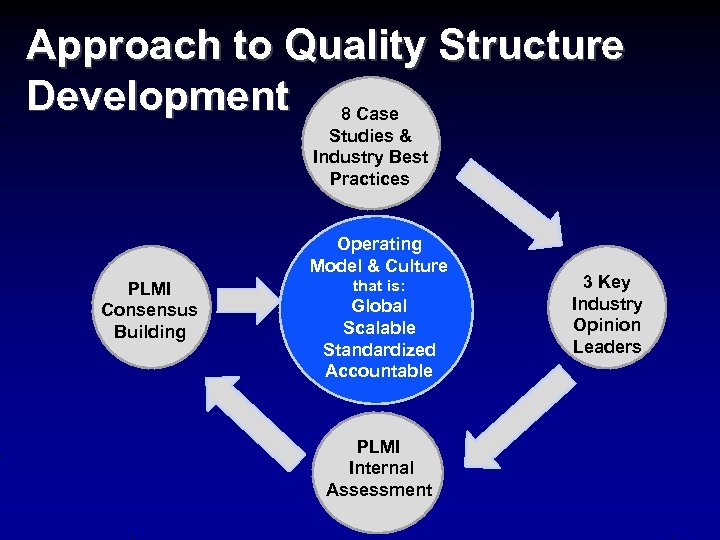
Approach to Quality Structure Development 8 Case Studies & Industry Best Practices Operating Model & Culture PLMI Consensus Building that is: Global Scalable Standardized Accountable PLMI Internal Assessment 3 Key Industry Opinion Leaders
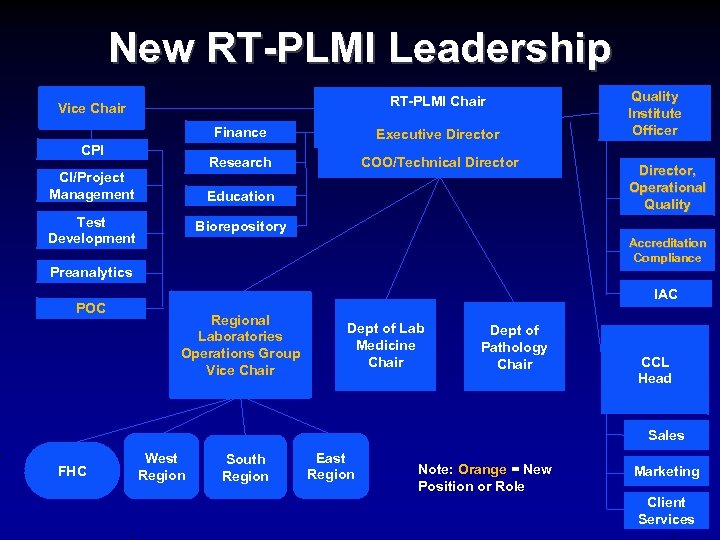
New RT-PLMI Leadership RT-PLMI Chair Vice Chair Finance Research CPI CI/Project Management Executive Director COO/Technical Director Education Test Development Director, Operational Quality Biorepository Accreditation Compliance Preanalytics POC Quality Institute Officer IAC Regional Laboratories Operations Group Vice Chair Dept of Lab Medicine Chair Dept of Pathology Chair CCL Head Sales FHC West Region South Region East Region Note: Orange = New Position or Role Marketing Client Services
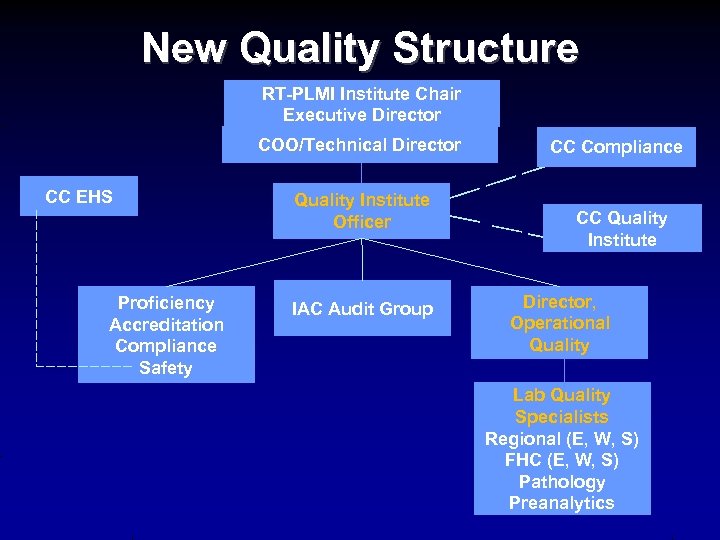
New Quality Structure RT-PLMI Institute Chair Executive Director COO/Technical Director CC EHS Proficiency Accreditation Compliance Safety Quality Institute Officer IAC Audit Group CC Compliance CC Quality Institute Director, Operational Quality Lab Quality Specialists Regional (E, W, S) FHC (E, W, S) Pathology Preanalytics
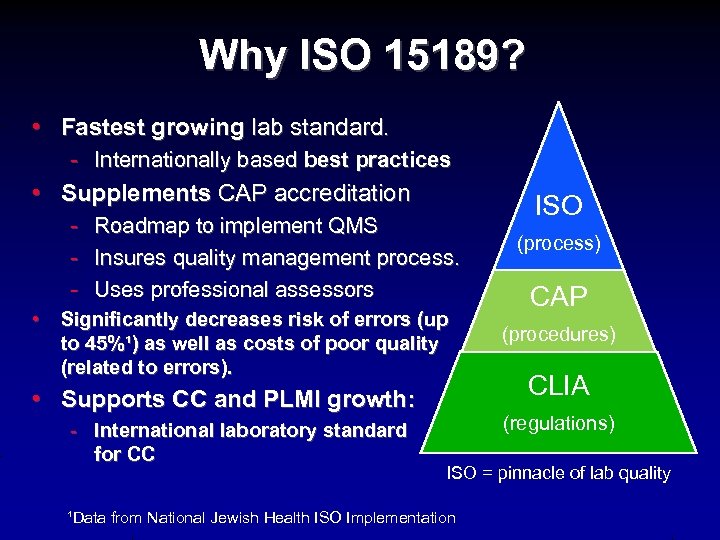
Why ISO 15189? • Fastest growing lab standard. - Internationally based best practices • Supplements CAP accreditation - Roadmap to implement QMS - Insures quality management process. - Uses professional assessors • Significantly decreases risk of errors (up to 45%¹) as well as costs of poor quality (related to errors). • Supports CC and PLMI growth: - International laboratory standard for CC ISO (process) CAP (procedures) CLIA (regulations) ISO = pinnacle of lab quality ¹Data from National Jewish Health ISO Implementation
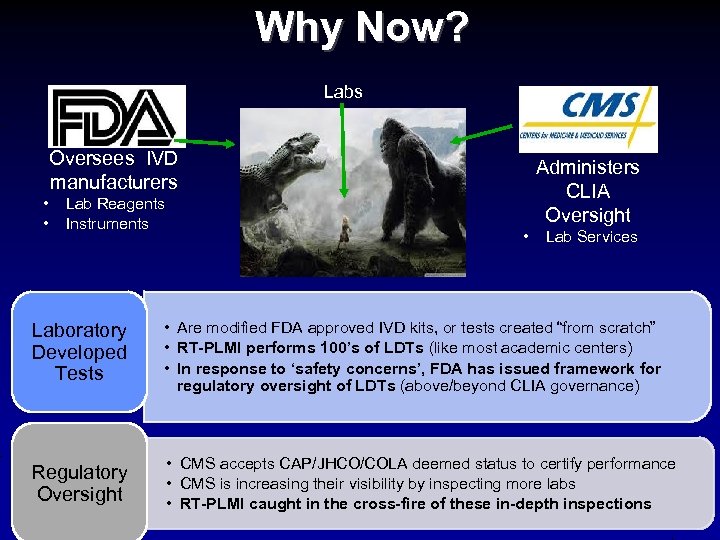
Why Now? Labs Oversees IVD manufacturers • • Lab Reagents Instruments Administers CLIA Oversight • Lab Services Laboratory Developed Tests • Are modified FDA approved IVD kits, or tests created “from scratch” • RT-PLMI performs 100’s of LDTs (like most academic centers) • In response to ‘safety concerns’, FDA has issued framework for regulatory oversight of LDTs (above/beyond CLIA governance) Regulatory Oversight • CMS accepts CAP/JHCO/COLA deemed status to certify performance • CMS is increasing their visibility by inspecting more labs • RT-PLMI caught in the cross-fire of these in-depth inspections

Summary: Lessons • Integration: - Maintain local accountability over laboratory - strong CLIA holder and manager • Cost Reduction: - leadership support for service levels • Quality: - Strengthen system-wide oversight - benefits of audit group
e3a139d641d1e9bb8602b1995a6f8b4b.ppt