3d8d63a56d6642e2ac23f0875c0bc2f3.ppt
- Количество слайдов: 28
 CLEAR III Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage Phase III Pharmacist Training Revised September 2010
CLEAR III Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage Phase III Pharmacist Training Revised September 2010
 AGENDA Overview Randomization Drug Supply Drug Shipment Drug Preparation Drug Accountability & Documentation Unblinding Procedure
AGENDA Overview Randomization Drug Supply Drug Shipment Drug Preparation Drug Accountability & Documentation Unblinding Procedure
 OVERVIEW Multicenter, international, double-blind, randomized study Approximately 500 patients will be enrolled across 75 study centers Primary objective- “define the long-term effects of lysing ventricular blood clots with rt-PA on functional outcomes
OVERVIEW Multicenter, international, double-blind, randomized study Approximately 500 patients will be enrolled across 75 study centers Primary objective- “define the long-term effects of lysing ventricular blood clots with rt-PA on functional outcomes
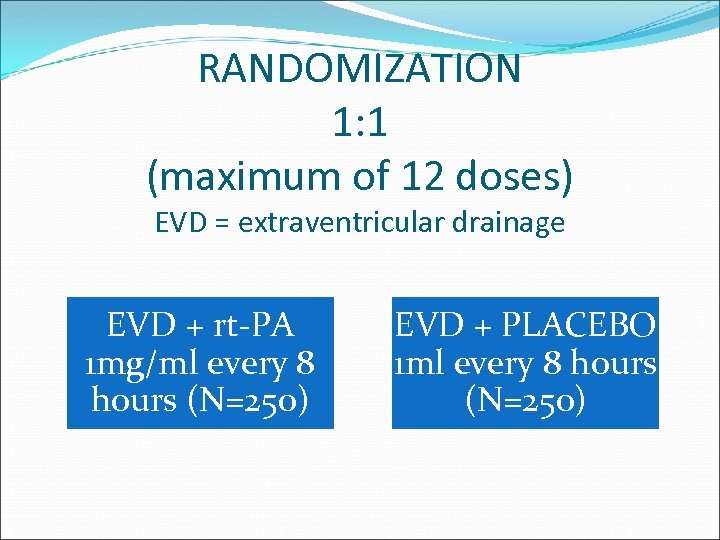 RANDOMIZATION 1: 1 (maximum of 12 doses) EVD = extraventricular drainage EVD + rt-PA 1 mg/ml every 8 hours (N=250) EVD + PLACEBO 1 ml every 8 hours (N=250)
RANDOMIZATION 1: 1 (maximum of 12 doses) EVD = extraventricular drainage EVD + rt-PA 1 mg/ml every 8 hours (N=250) EVD + PLACEBO 1 ml every 8 hours (N=250)
 DRUG SUPPLY Recombinant tissue plasminogen activator (rt-PA), Alteplase (Cathflo® Activase®) 2 mg vials for reconstitution Active drug Packaged in boxes containing 6 vials/box Manufactured by Genentech Storage – refrigerated 2 -8° C Provided for the study 0. 9% Sodium Chloride for Injection, USP Placebo and Flushes Use pharmacy supply- not provided
DRUG SUPPLY Recombinant tissue plasminogen activator (rt-PA), Alteplase (Cathflo® Activase®) 2 mg vials for reconstitution Active drug Packaged in boxes containing 6 vials/box Manufactured by Genentech Storage – refrigerated 2 -8° C Provided for the study 0. 9% Sodium Chloride for Injection, USP Placebo and Flushes Use pharmacy supply- not provided
 Alteplase (Cathflo® Activase®)
Alteplase (Cathflo® Activase®)
 Drug Shipments – USA Sites Distributed to sites by the Investigational Drug Service (IDS), Johns Hopkins Hospital (JHH) Fed. Ex utilized as the courier Shipped in multi-purpose insulated shippers with refrigerant packs Upon arrival, drug should be unpacked immediately, examined, verified and placed in a refrigerator A signed copy of the invoice must be faxed to the shipper Temperature monitoring devices are not used unless requested
Drug Shipments – USA Sites Distributed to sites by the Investigational Drug Service (IDS), Johns Hopkins Hospital (JHH) Fed. Ex utilized as the courier Shipped in multi-purpose insulated shippers with refrigerant packs Upon arrival, drug should be unpacked immediately, examined, verified and placed in a refrigerator A signed copy of the invoice must be faxed to the shipper Temperature monitoring devices are not used unless requested
 Drug Shipments - International Distributed to sites by the Investigational Drug Service (IDS), Johns Hopkins Hospital (JHH) or Wellspring Clinical Services Site will receive a “Notification of Shipment” e-mail message Upon arrival, drug should be unpacked immediately examined checked against the enclosed document (Shipping Manifest) quarantined in a refrigerator
Drug Shipments - International Distributed to sites by the Investigational Drug Service (IDS), Johns Hopkins Hospital (JHH) or Wellspring Clinical Services Site will receive a “Notification of Shipment” e-mail message Upon arrival, drug should be unpacked immediately examined checked against the enclosed document (Shipping Manifest) quarantined in a refrigerator
 Drug Shipment (continued) The temperature monitoring device will be secured and directions will be provided for the return of the device Complete the “Acknowledgement of Receipt” section on the shipping document (Shipping Manifest) and return to the shipper Temperature data will be reviewed by Qualified Pharmacist (QP)or Coordinating Center (CC)Pharmacist QP or CC Pharmacist will approve the release the drug from quarantine Site will receive written documentation (Final Order Release Certificate) regarding the release of the study drug
Drug Shipment (continued) The temperature monitoring device will be secured and directions will be provided for the return of the device Complete the “Acknowledgement of Receipt” section on the shipping document (Shipping Manifest) and return to the shipper Temperature data will be reviewed by Qualified Pharmacist (QP)or Coordinating Center (CC)Pharmacist QP or CC Pharmacist will approve the release the drug from quarantine Site will receive written documentation (Final Order Release Certificate) regarding the release of the study drug
 Drug Shipments (cont. ) DRUG CANNOT BE USED until you receive written documentation to release the drug Initial shipments will be sent when all regulatory documentation has been received from the site Any concerns about the drug supply should be reported to the shipper immediately To re-order drug Domestic -contact JHH IDS International- process will be communicated depending upon location
Drug Shipments (cont. ) DRUG CANNOT BE USED until you receive written documentation to release the drug Initial shipments will be sent when all regulatory documentation has been received from the site Any concerns about the drug supply should be reported to the shipper immediately To re-order drug Domestic -contact JHH IDS International- process will be communicated depending upon location
 DRUG PREPARATION (Active) Obtain study supply of Alteplase (Cathflo®Activase®) Utilizing aseptic technique, reconstitute* the vial with 2. 2 ml Sterile Water for Inj. , USP Slight foaming is not unusual. Let the vial stand undisturbed for several minutes to allow dissipation of any large bubbles. DO NOT shake the vial. *Based on product package insert for Cathflo® Activase®. Each vial contains 2. 2 mg of Alteplase, which includes a 10% overfill
DRUG PREPARATION (Active) Obtain study supply of Alteplase (Cathflo®Activase®) Utilizing aseptic technique, reconstitute* the vial with 2. 2 ml Sterile Water for Inj. , USP Slight foaming is not unusual. Let the vial stand undisturbed for several minutes to allow dissipation of any large bubbles. DO NOT shake the vial. *Based on product package insert for Cathflo® Activase®. Each vial contains 2. 2 mg of Alteplase, which includes a 10% overfill
 DRUG PREPARATION Final concentration is 1 mg/ml Draw up syringe(s) of 1 mg/ml Alteplase (Cathflo® Activase®) into 3 ml sterile syringes Cap the syringe with a sterile tip cap Label the syringe in a blinded fashion Expiration date for the syringe is 8 hours after reconstitution if stored at 2 -30°C
DRUG PREPARATION Final concentration is 1 mg/ml Draw up syringe(s) of 1 mg/ml Alteplase (Cathflo® Activase®) into 3 ml sterile syringes Cap the syringe with a sterile tip cap Label the syringe in a blinded fashion Expiration date for the syringe is 8 hours after reconstitution if stored at 2 -30°C
 DRUG PREPARATION (Placebo) Obtain a vial of 0. 9% Sodium Chloride for Injection, USP (preservative-free) Utilizing aseptic technique, draw up 1 ml into a 3 ml sterile syringe Cap the syringe with a sterile rubber tip cap Label the syringe in a blinded fashion Expiration date for the syringe is 8 hours after preparation (to match active drug syringe)
DRUG PREPARATION (Placebo) Obtain a vial of 0. 9% Sodium Chloride for Injection, USP (preservative-free) Utilizing aseptic technique, draw up 1 ml into a 3 ml sterile syringe Cap the syringe with a sterile rubber tip cap Label the syringe in a blinded fashion Expiration date for the syringe is 8 hours after preparation (to match active drug syringe)
 DRUG PREPARATION (cont. ) Complete the drug accountability log Used Alteplase (Cathflo® Activase®) vials can be discarded Expiration date for the syringe is 8 hours after reconstitution/preparation if stored at 2 -30°C
DRUG PREPARATION (cont. ) Complete the drug accountability log Used Alteplase (Cathflo® Activase®) vials can be discarded Expiration date for the syringe is 8 hours after reconstitution/preparation if stored at 2 -30°C
 PREPARATION of FLUSHES Obtain a 10 ml vial of 0. 9% Sodium Chloride Injection, preservative-free. Utilizing aseptic technique draw 4 ml of 0. 9% Sodium Chloride into a sterile 5 ml syringe Cap the syringe with a sterile tip cap Label the syringe Expiration of syringe is 24 hours
PREPARATION of FLUSHES Obtain a 10 ml vial of 0. 9% Sodium Chloride Injection, preservative-free. Utilizing aseptic technique draw 4 ml of 0. 9% Sodium Chloride into a sterile 5 ml syringe Cap the syringe with a sterile tip cap Label the syringe Expiration of syringe is 24 hours
 Can I Freeze TPA? At time of preparation of subject’s dose, draw up the appropriate dose into a syringe Cap syringe with a sterile tip cap Label syringe Store at -25° to -10°C Discard frozen syringe(s) after 45 days* Thaw syringe prior to dispensing Re-label the drug for enrolled subject in a blinded fashion *Based on USP 797 Beyond Use Dating
Can I Freeze TPA? At time of preparation of subject’s dose, draw up the appropriate dose into a syringe Cap syringe with a sterile tip cap Label syringe Store at -25° to -10°C Discard frozen syringe(s) after 45 days* Thaw syringe prior to dispensing Re-label the drug for enrolled subject in a blinded fashion *Based on USP 797 Beyond Use Dating
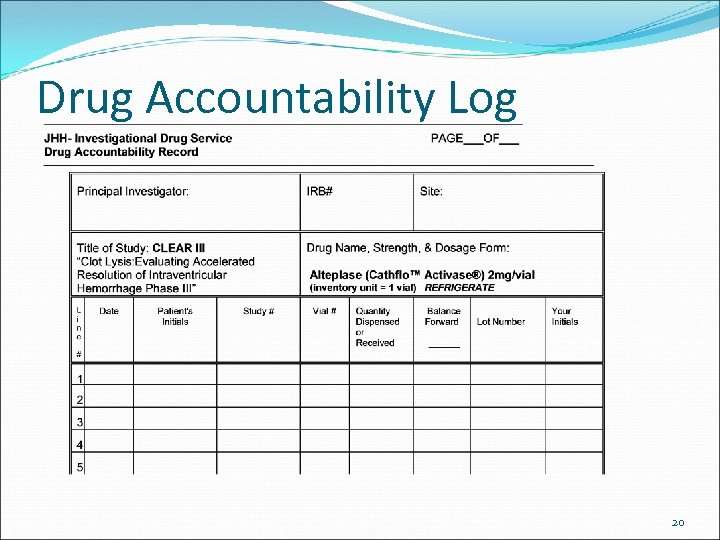
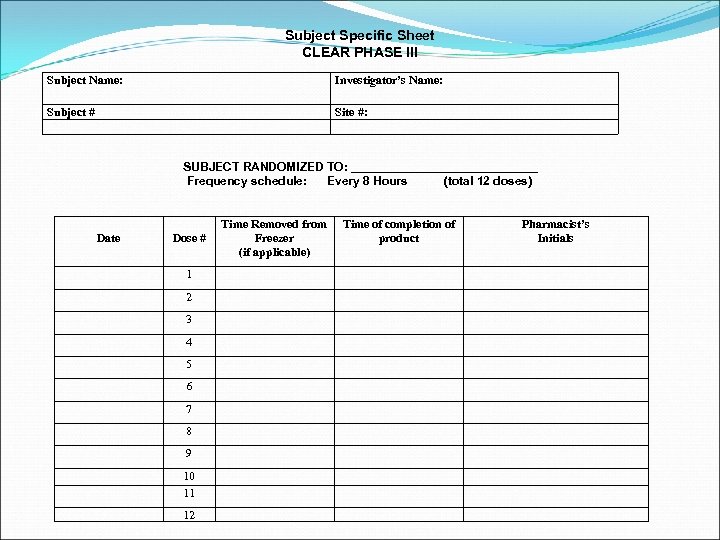
 DRUG ACCOUNTABILITY & DOCUMENTATION Drug Accountability log Document receipt and disposition of drug Complete a log for Alteplase and placebo Running balance must be maintained Subject Specific log Document preparation for an individual subject
DRUG ACCOUNTABILITY & DOCUMENTATION Drug Accountability log Document receipt and disposition of drug Complete a log for Alteplase and placebo Running balance must be maintained Subject Specific log Document preparation for an individual subject
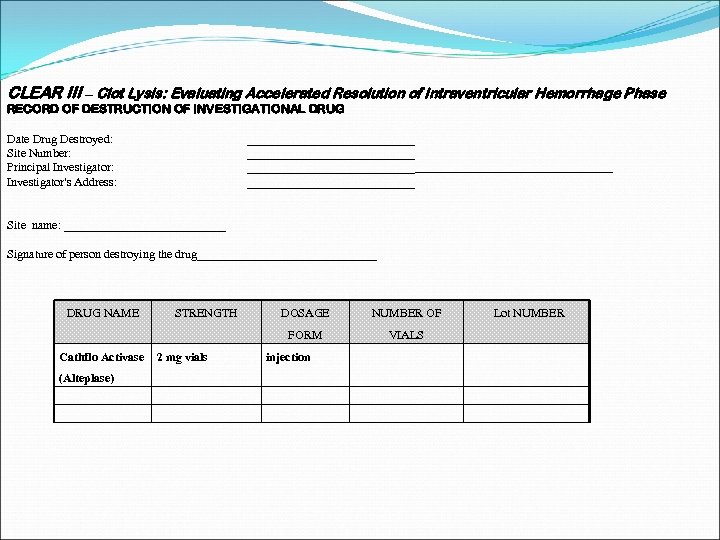
 DRUG ACCOUNTABILITY Receipt and disposition of study drug is documented on the drug accountability log Expired vials can be destroyed on site according to your site specific policy for drug destruction Prior to destroying the study drug on site a copy of your site’s policy for drug disposal/destruction must to sent to the Coordinating Center (CC) Pharmacist a drug destruction form must be completed
DRUG ACCOUNTABILITY Receipt and disposition of study drug is documented on the drug accountability log Expired vials can be destroyed on site according to your site specific policy for drug destruction Prior to destroying the study drug on site a copy of your site’s policy for drug disposal/destruction must to sent to the Coordinating Center (CC) Pharmacist a drug destruction form must be completed
 Drug Accountability Document the destruction of the drug on the drug accountability log Send a copy of the completed drug destruction form to the CC Pharmacist Send a copy of the drug accountability log to the CC Pharmacist
Drug Accountability Document the destruction of the drug on the drug accountability log Send a copy of the completed drug destruction form to the CC Pharmacist Send a copy of the drug accountability log to the CC Pharmacist
 Drug Accountability Log 20
Drug Accountability Log 20
 Subject Specific Sheet CLEAR PHASE III Subject Name: Investigator’s Name: Subject # Site #: SUBJECT RANDOMIZED TO: ______________ Frequency schedule: Every 8 Hours (total 12 doses) Date Dose # 1 2 3 4 5 6 7 8 9 10 11 12 Time Removed from Freezer (if applicable) Time of completion of product Pharmacist’s Initials
Subject Specific Sheet CLEAR PHASE III Subject Name: Investigator’s Name: Subject # Site #: SUBJECT RANDOMIZED TO: ______________ Frequency schedule: Every 8 Hours (total 12 doses) Date Dose # 1 2 3 4 5 6 7 8 9 10 11 12 Time Removed from Freezer (if applicable) Time of completion of product Pharmacist’s Initials
 CLEAR III – Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage Phase RECORD OF DESTRUCTION OF INVESTIGATIONAL DRUG Date Drug Destroyed: Site Number: Principal Investigator: Investigator's Address: ____________________________ Site name: ______________ Signature of person destroying the drug_______________ DRUG NAME (Alteplase) 2 mg vials DOSAGE NUMBER OF FORM Cathflo Activase STRENGTH VIALS injection Lot NUMBER
CLEAR III – Clot Lysis: Evaluating Accelerated Resolution of Intraventricular Hemorrhage Phase RECORD OF DESTRUCTION OF INVESTIGATIONAL DRUG Date Drug Destroyed: Site Number: Principal Investigator: Investigator's Address: ____________________________ Site name: ______________ Signature of person destroying the drug_______________ DRUG NAME (Alteplase) 2 mg vials DOSAGE NUMBER OF FORM Cathflo Activase STRENGTH VIALS injection Lot NUMBER
 Unblinding Procedure Site investigator must contact the Study Chairman The Study Chairman will consult with the Medical Safety Monitor Coordinating Center pharmacist or site pharmacist will be contacted if unblinding is necessary Written request for unblinding will be required
Unblinding Procedure Site investigator must contact the Study Chairman The Study Chairman will consult with the Medical Safety Monitor Coordinating Center pharmacist or site pharmacist will be contacted if unblinding is necessary Written request for unblinding will be required
 PHARMACIST RESPONSIBILITIES Training requirement Adherence to study protocol All staff preparing compounded sterile products are trained and competent (USP 797 Guidelines) All staff preparing study drug have been trained on protocol procedures and drug preparation Appropriate documentation is maintained Storage and security of investigational drug Temperature monitoring
PHARMACIST RESPONSIBILITIES Training requirement Adherence to study protocol All staff preparing compounded sterile products are trained and competent (USP 797 Guidelines) All staff preparing study drug have been trained on protocol procedures and drug preparation Appropriate documentation is maintained Storage and security of investigational drug Temperature monitoring
 Communication Contact the Coordinating Center Pharmacist (unblinded pharmacist) to communicate: Any drug related concerns or occurrences Storage deviations Refrigerator malfunctions Out of range temperatures Changes in pharmacy personnel listed in the randomization system Contact information (shipping address, phone #, fax#, e -mail addresses)
Communication Contact the Coordinating Center Pharmacist (unblinded pharmacist) to communicate: Any drug related concerns or occurrences Storage deviations Refrigerator malfunctions Out of range temperatures Changes in pharmacy personnel listed in the randomization system Contact information (shipping address, phone #, fax#, e -mail addresses)
 CLEAR III Website www. cleariii. com Pharmacy Manual Study Documents Current protocol version Pharmacist training presentation
CLEAR III Website www. cleariii. com Pharmacy Manual Study Documents Current protocol version Pharmacist training presentation
 REFERENCES CLEAR III website – www. cleariii. com Genentech- www. gene. com Alteplase (Cathflo® Activase®) product information - www. gene. com/gene/products/information/cardiovascular/cathfloactivase/index USP* 797 Guidelines - www. usp. org Coordinating Center (phone) 410. 614. 6996 JHH- Investigational Drug Service (phone) 410. 955. 6337 (fax) 410. 614. 8074 Pager 410. 283. 2936 *USP – United States Pharmacopeia
REFERENCES CLEAR III website – www. cleariii. com Genentech- www. gene. com Alteplase (Cathflo® Activase®) product information - www. gene. com/gene/products/information/cardiovascular/cathfloactivase/index USP* 797 Guidelines - www. usp. org Coordinating Center (phone) 410. 614. 6996 JHH- Investigational Drug Service (phone) 410. 955. 6337 (fax) 410. 614. 8074 Pager 410. 283. 2936 *USP – United States Pharmacopeia
 CONTACT INFORMATION Janet Mighty, R. Ph. MBA (Coordinating Center Pharmacist) Investigational Drug Service The Johns Hopkins Hospital 600 N. Wolfe Street, Carnegie 180 Baltimore, MD 21287 Phone 410. 955. 6337, Fax 410. 614. 8074 E-mail: jmighty@jhmi. edu
CONTACT INFORMATION Janet Mighty, R. Ph. MBA (Coordinating Center Pharmacist) Investigational Drug Service The Johns Hopkins Hospital 600 N. Wolfe Street, Carnegie 180 Baltimore, MD 21287 Phone 410. 955. 6337, Fax 410. 614. 8074 E-mail: jmighty@jhmi. edu


