5-CrystalForms(5) - copie.ppt
- Количество слайдов: 130
 Classes of symmetry and forms Recall : 1) Depending upon what elements of symmetry are present, all crystals may be divided into 32 distinct groups called CLASSES OF SYMMETRY (crystal classes). 2) Crystal classes belong to 6 or (7) crystal systems based on orientations and lenghts of crystallograpic axes There are 48 possible crystal forms or forms that can be developed as the result of the 32 combinations of symmetry.
Classes of symmetry and forms Recall : 1) Depending upon what elements of symmetry are present, all crystals may be divided into 32 distinct groups called CLASSES OF SYMMETRY (crystal classes). 2) Crystal classes belong to 6 or (7) crystal systems based on orientations and lenghts of crystallograpic axes There are 48 possible crystal forms or forms that can be developed as the result of the 32 combinations of symmetry.
 What is a crystal form ?
What is a crystal form ?
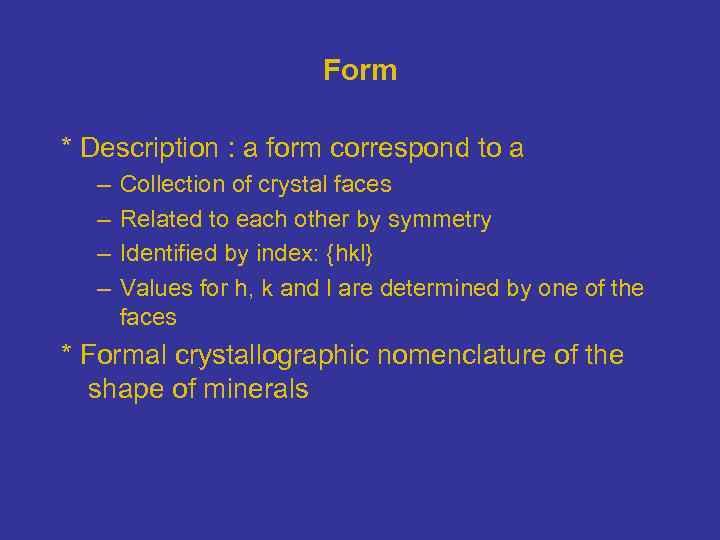 Form * Description : a form correspond to a – – Collection of crystal faces Related to each other by symmetry Identified by index: {hkl} Values for h, k and l are determined by one of the faces * Formal crystallographic nomenclature of the shape of minerals
Form * Description : a form correspond to a – – Collection of crystal faces Related to each other by symmetry Identified by index: {hkl} Values for h, k and l are determined by one of the faces * Formal crystallographic nomenclature of the shape of minerals
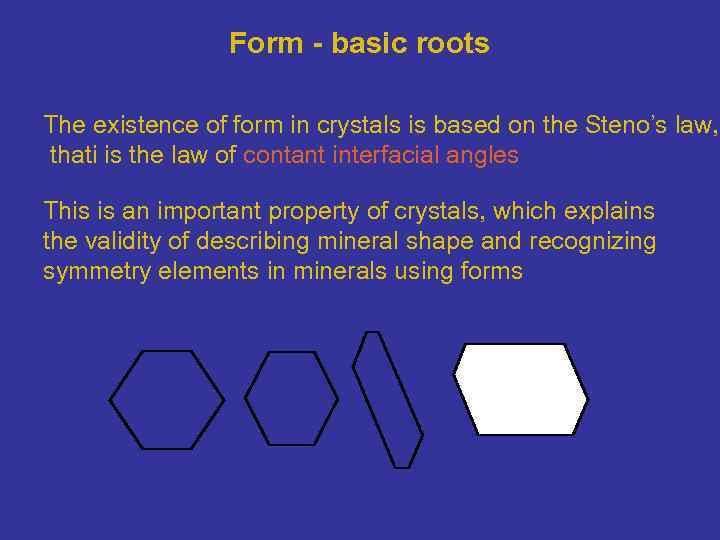 Form - basic roots The existence of form in crystals is based on the Steno’s law, thati is the law of contant interfacial angles This is an important property of crystals, which explains the validity of describing mineral shape and recognizing symmetry elements in minerals using forms
Form - basic roots The existence of form in crystals is based on the Steno’s law, thati is the law of contant interfacial angles This is an important property of crystals, which explains the validity of describing mineral shape and recognizing symmetry elements in minerals using forms
 Determination of a form • The shape of a form is determined with: 1) Miller index of one face in form (its location) 2) Point symmetry of the crystal class (Hermann-Mauguin notation) • The form is created by operating point symmetry on the initial face • Number of faces in a form depends on crystal class
Determination of a form • The shape of a form is determined with: 1) Miller index of one face in form (its location) 2) Point symmetry of the crystal class (Hermann-Mauguin notation) • The form is created by operating point symmetry on the initial face • Number of faces in a form depends on crystal class
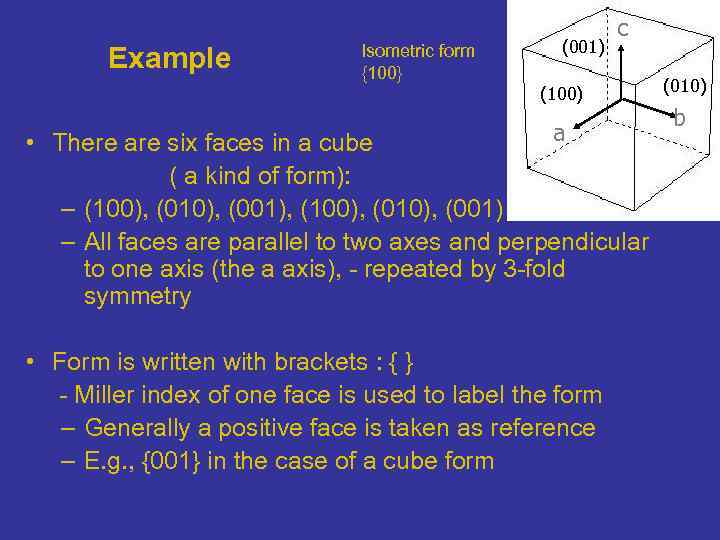 Example Isometric form {100} (001) c (100) a • There are six faces in a cube ( a kind of form): – (100), (010), (001), (100), (010), (001) – All faces are parallel to two axes and perpendicular to one axis (the a axis), - repeated by 3 -fold symmetry • Form is written with brackets : { } - Miller index of one face is used to label the form – Generally a positive face is taken as reference – E. g. , {001} in the case of a cube form (010) b
Example Isometric form {100} (001) c (100) a • There are six faces in a cube ( a kind of form): – (100), (010), (001), (100), (010), (001) – All faces are parallel to two axes and perpendicular to one axis (the a axis), - repeated by 3 -fold symmetry • Form is written with brackets : { } - Miller index of one face is used to label the form – Generally a positive face is taken as reference – E. g. , {001} in the case of a cube form (010) b
 Plane, zone axes, zone symbol (100) - Miller index; denote a plane of the crystal structure, and regular repetition of that plane with a particular spacing. In the cubic system, the normal to plane (hkl) is the direction [hkl]. In lower symmetry cases, the normal to (hkl) is not parallel to [hkl]. [100] - Zone symbol : direction of a line at the intersection of planes Coordinate in square brackets denote a direction vector {100} - zone form - Indices in curly brackets denote a family of directions (often plane direction), related by symmetry operations. From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Plane, zone axes, zone symbol (100) - Miller index; denote a plane of the crystal structure, and regular repetition of that plane with a particular spacing. In the cubic system, the normal to plane (hkl) is the direction [hkl]. In lower symmetry cases, the normal to (hkl) is not parallel to [hkl]. [100] - Zone symbol : direction of a line at the intersection of planes Coordinate in square brackets denote a direction vector {100} - zone form - Indices in curly brackets denote a family of directions (often plane direction), related by symmetry operations. From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
 Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
 Height: 5. 5 to 7. 5 cm Form : St-Etienne School of Mines, France Collection of 400 wooden crystal models : 112 of the Dana serie from Krantz and 250 older models Collection of 100 terracotta models http: //www. mineralogy. eu/models/pyritohedron 01. html
Height: 5. 5 to 7. 5 cm Form : St-Etienne School of Mines, France Collection of 400 wooden crystal models : 112 of the Dana serie from Krantz and 250 older models Collection of 100 terracotta models http: //www. mineralogy. eu/models/pyritohedron 01. html
 Height: 5. 5 to 7. 5 cm Wooden crystal models Collection of 135 wooden crystal models for teaching at the end of the 19 th in Germany and Austria (private collection) http: //www. mineralogy. eu/models/pyritohedron 01. html
Height: 5. 5 to 7. 5 cm Wooden crystal models Collection of 135 wooden crystal models for teaching at the end of the 19 th in Germany and Austria (private collection) http: //www. mineralogy. eu/models/pyritohedron 01. html
 Height: 3. 5 to 5. 5 cm Groth & Frantz collection 1880 Wooden crystal models Top stephanite (392), krennerite (394), hessite (47) bottom row: sylvanite (533), proustite (215) freislebenite (539) 6 silver- and/or gold-containing minerals http: //www. mineralogy. eu/models/pyritohedron 01. html Haüy -. J. Annales de Chimie, 17, 225 -319 (1793)
Height: 3. 5 to 5. 5 cm Groth & Frantz collection 1880 Wooden crystal models Top stephanite (392), krennerite (394), hessite (47) bottom row: sylvanite (533), proustite (215) freislebenite (539) 6 silver- and/or gold-containing minerals http: //www. mineralogy. eu/models/pyritohedron 01. html Haüy -. J. Annales de Chimie, 17, 225 -319 (1793)
 Height: 5. 5 to 7. 5 cm top row: gypsum (564), lazulite (589) center: trydymite (711) bottom row: titanite (702), calcite (307) http: //www. mineralogy. eu/models/pyritohedron 01. html Groth & Frantz collection 1880 Wooden crystal models - Twins
Height: 5. 5 to 7. 5 cm top row: gypsum (564), lazulite (589) center: trydymite (711) bottom row: titanite (702), calcite (307) http: //www. mineralogy. eu/models/pyritohedron 01. html Groth & Frantz collection 1880 Wooden crystal models - Twins
 Size: 25/10 cm F. Krantz cardboard crystal model A tourmaline crystal from a series of 60. These models were offered varnished to prevent damage. http: //www. mineralogy. eu/models/pyritohedron 01. html
Size: 25/10 cm F. Krantz cardboard crystal model A tourmaline crystal from a series of 60. These models were offered varnished to prevent damage. http: //www. mineralogy. eu/models/pyritohedron 01. html
 Height: 5. 5 to 7. 5 cm Porcelain crystal models (ca. 1841) Unsigned [John Joseph Griffin], England, ca. 1841 From a collection of 24 biscuit porcelain crystal models http: //www. mineralogy. eu/models/pyritohedron 01. html
Height: 5. 5 to 7. 5 cm Porcelain crystal models (ca. 1841) Unsigned [John Joseph Griffin], England, ca. 1841 From a collection of 24 biscuit porcelain crystal models http: //www. mineralogy. eu/models/pyritohedron 01. html
 Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
 Open forms and closed forms The octahedral form is given as {111}. It’s a closed form The pinacoidal form is given by {0001}- rare in quartz. It’s an open form A closed form is a set of crystal faces that completely enclose space. Thus, in crystal classes that contain closed forms, a crystal can be made up of a single form. An open form is one or more crystal faces that do not completely enclose space. Two, or more forms are needed to define the crystal.
Open forms and closed forms The octahedral form is given as {111}. It’s a closed form The pinacoidal form is given by {0001}- rare in quartz. It’s an open form A closed form is a set of crystal faces that completely enclose space. Thus, in crystal classes that contain closed forms, a crystal can be made up of a single form. An open form is one or more crystal faces that do not completely enclose space. Two, or more forms are needed to define the crystal.
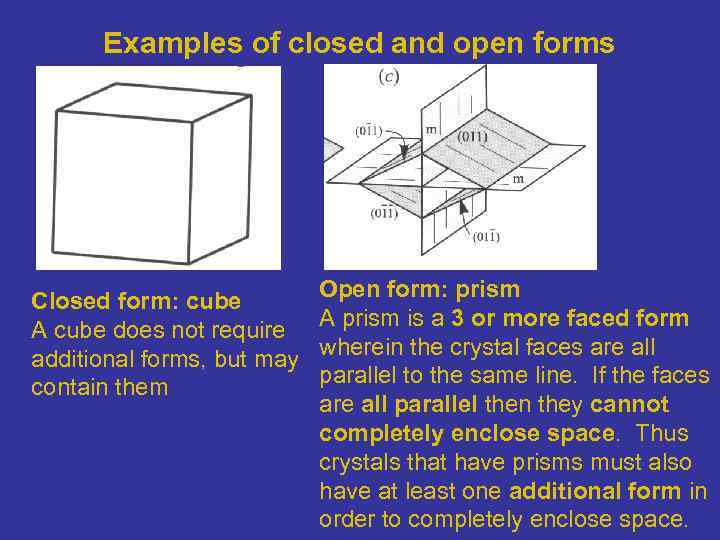 Examples of closed and open forms Closed form: cube A cube does not require additional forms, but may contain them Open form: prism A prism is a 3 or more faced form wherein the crystal faces are all parallel to the same line. If the faces are all parallel then they cannot completely enclose space. Thus crystals that have prisms must also have at least one additional form in order to completely enclose space.
Examples of closed and open forms Closed form: cube A cube does not require additional forms, but may contain them Open form: prism A prism is a 3 or more faced form wherein the crystal faces are all parallel to the same line. If the faces are all parallel then they cannot completely enclose space. Thus crystals that have prisms must also have at least one additional form in order to completely enclose space.
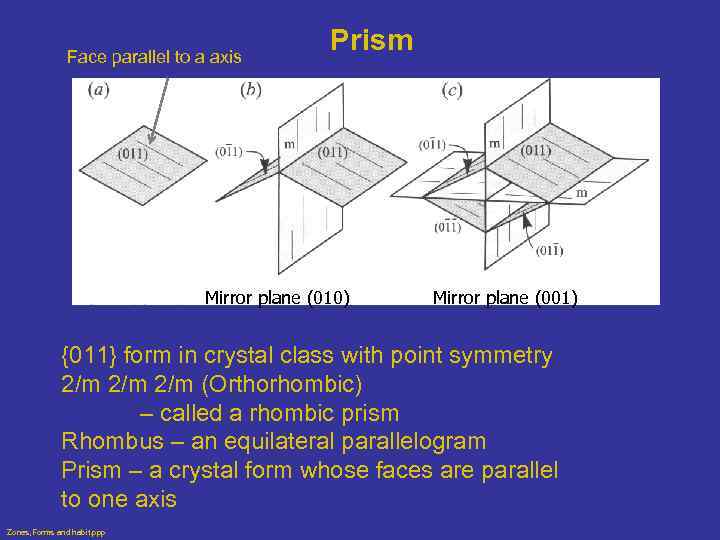 Face parallel to a axis Prism Mirror plane (010) Mirror plane (001) {011} form in crystal class with point symmetry 2/m 2/m (Orthorhombic) – called a rhombic prism Rhombus – an equilateral parallelogram Prism – a crystal form whose faces are parallel to one axis Zones, Forms and habit. ppp
Face parallel to a axis Prism Mirror plane (010) Mirror plane (001) {011} form in crystal class with point symmetry 2/m 2/m (Orthorhombic) – called a rhombic prism Rhombus – an equilateral parallelogram Prism – a crystal form whose faces are parallel to one axis Zones, Forms and habit. ppp
 Closed and open forms There are 17 or 18 open forms and 30 closed forms. - Minerals must have more than one form if they have an open form - Minerals may have only one closed form - Minerals could have more than 1 form, closed or open.
Closed and open forms There are 17 or 18 open forms and 30 closed forms. - Minerals must have more than one form if they have an open form - Minerals may have only one closed form - Minerals could have more than 1 form, closed or open.
 Examples of open forms Pedions are single faced forms. Since there is only one face in the form, a pedion cannot completely enclose space. Thus, a crystal that has only pedions, must have at least 3 different pedions to completely enclose space.
Examples of open forms Pedions are single faced forms. Since there is only one face in the form, a pedion cannot completely enclose space. Thus, a crystal that has only pedions, must have at least 3 different pedions to completely enclose space.
 Closed and open forms : Pyramid and dipyramid A Pyramid usually ends a prism. It is an open form as the crystal symmetry should be described by other forms. On the other hand, a dipyramid has at least 6 faces that meet in points at opposite ends of the crystal. These faces can completely enclose space, so a dipyramid is closed form. Although a crystal may be made up of a single dipyramid form, it may also have other forms present. Hexagonal Pyramid : 6 faces dihexagonal Pyramid: 12 faces Dihexagonal Dipyramid: 12 x 2 faces
Closed and open forms : Pyramid and dipyramid A Pyramid usually ends a prism. It is an open form as the crystal symmetry should be described by other forms. On the other hand, a dipyramid has at least 6 faces that meet in points at opposite ends of the crystal. These faces can completely enclose space, so a dipyramid is closed form. Although a crystal may be made up of a single dipyramid form, it may also have other forms present. Hexagonal Pyramid : 6 faces dihexagonal Pyramid: 12 faces Dihexagonal Dipyramid: 12 x 2 faces
 Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms : the different forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms : the different forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
 Non-isometric form Open forms Closed forms* 1 - Pedion 2 - Pinacoid 3 - Dihedron - dome - sphenoid 4 - Prism 5 - Pyramid 1 - Dipyramid 2 - Disphenoid 3 - Trapezohedron 4 - Scalenohedron 5 - Rhombohedron 6 - Tetrahedron * Other closed forms will be shown with the isometric system
Non-isometric form Open forms Closed forms* 1 - Pedion 2 - Pinacoid 3 - Dihedron - dome - sphenoid 4 - Prism 5 - Pyramid 1 - Dipyramid 2 - Disphenoid 3 - Trapezohedron 4 - Scalenohedron 5 - Rhombohedron 6 - Tetrahedron * Other closed forms will be shown with the isometric system
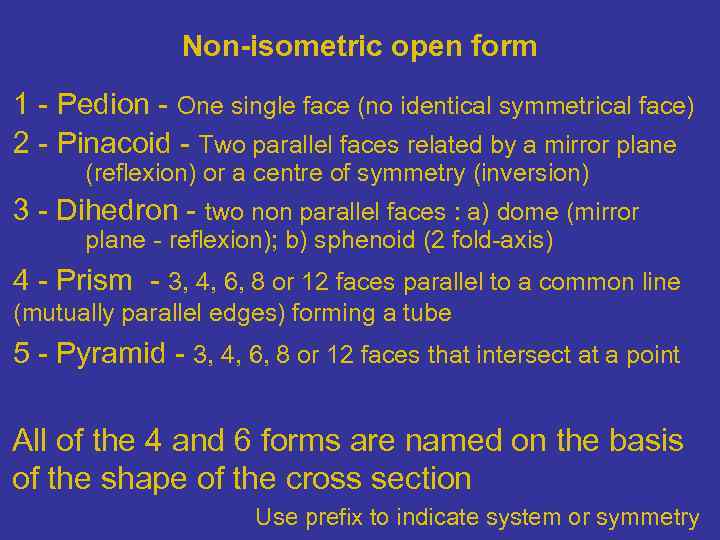 Non-isometric open form 1 - Pedion - One single face (no identical symmetrical face) 2 - Pinacoid - Two parallel faces related by a mirror plane (reflexion) or a centre of symmetry (inversion) 3 - Dihedron - two non parallel faces : a) dome (mirror plane - reflexion); b) sphenoid (2 fold-axis) 4 - Prism - 3, 4, 6, 8 or 12 faces parallel to a common line (mutually parallel edges) forming a tube 5 - Pyramid - 3, 4, 6, 8 or 12 faces that intersect at a point All of the 4 and 6 forms are named on the basis of the shape of the cross section Use prefix to indicate system or symmetry
Non-isometric open form 1 - Pedion - One single face (no identical symmetrical face) 2 - Pinacoid - Two parallel faces related by a mirror plane (reflexion) or a centre of symmetry (inversion) 3 - Dihedron - two non parallel faces : a) dome (mirror plane - reflexion); b) sphenoid (2 fold-axis) 4 - Prism - 3, 4, 6, 8 or 12 faces parallel to a common line (mutually parallel edges) forming a tube 5 - Pyramid - 3, 4, 6, 8 or 12 faces that intersect at a point All of the 4 and 6 forms are named on the basis of the shape of the cross section Use prefix to indicate system or symmetry
 Non-isometric closed form 1 - Dipyramid - Two 3 -, 4 -, 6 -, 8 - or 12 - sided pyramids (top and bottom) related by an horizontal mirror plane 2 - Disphenoid - 4 non-equilateral triangular faces 3 - Trapezohedron - 4 trapezium-shaped faces 4 - Scalenohedron - 8 or 12 scalene triangle-shaped faces 5 - Rhombohedron - 6 rhomb-shaped faces 6 - Tetrahedron - 4 equilateral triangular faces Use prefix to indicate system or symmetry
Non-isometric closed form 1 - Dipyramid - Two 3 -, 4 -, 6 -, 8 - or 12 - sided pyramids (top and bottom) related by an horizontal mirror plane 2 - Disphenoid - 4 non-equilateral triangular faces 3 - Trapezohedron - 4 trapezium-shaped faces 4 - Scalenohedron - 8 or 12 scalene triangle-shaped faces 5 - Rhombohedron - 6 rhomb-shaped faces 6 - Tetrahedron - 4 equilateral triangular faces Use prefix to indicate system or symmetry
 Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms : the different forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms : the different forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
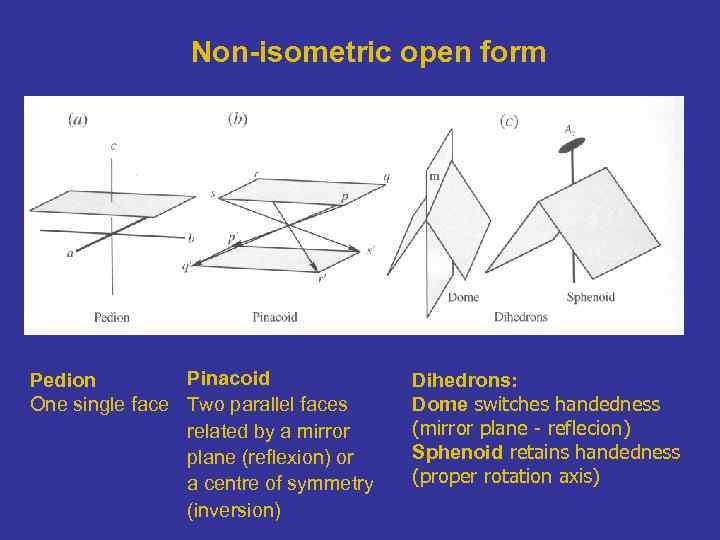 Non-isometric open form Pinacoid Pedion One single face Two parallel faces related by a mirror plane (reflexion) or a centre of symmetry (inversion) Dihedrons: Dome switches handedness (mirror plane - reflecion) Sphenoid retains handedness (proper rotation axis)
Non-isometric open form Pinacoid Pedion One single face Two parallel faces related by a mirror plane (reflexion) or a centre of symmetry (inversion) Dihedrons: Dome switches handedness (mirror plane - reflecion) Sphenoid retains handedness (proper rotation axis)
 Open form 1 -Pedion A single face unrelated to any other by symmetry. ------ > Open form • Triclinic system: – Point group (i. e. crystal class) = 1 –Symmetry content = (1 A 1) –{111} has only 2 faces Elbaite Ex: Elbaite : 3 m Na(Li, Al)3 Al 6 Si 6 O 18(BO 3)3 (OH)4, Sodium Lithium Aluminum Boro-Silicate Hydroxide Group: tourmaline (cyclosilicate)
Open form 1 -Pedion A single face unrelated to any other by symmetry. ------ > Open form • Triclinic system: – Point group (i. e. crystal class) = 1 –Symmetry content = (1 A 1) –{111} has only 2 faces Elbaite Ex: Elbaite : 3 m Na(Li, Al)3 Al 6 Si 6 O 18(BO 3)3 (OH)4, Sodium Lithium Aluminum Boro-Silicate Hydroxide Group: tourmaline (cyclosilicate)
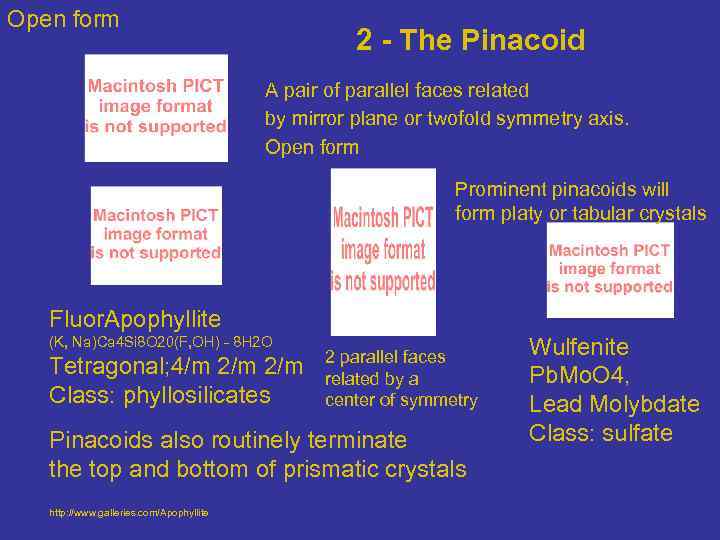 Open form 2 - The Pinacoid A pair of parallel faces related by mirror plane or twofold symmetry axis. Open form Prominent pinacoids will form platy or tabular crystals Fluor. Apophyllite (K, Na)Ca 4 Si 8 O 20(F, OH) - 8 H 2 O Tetragonal; 4/m 2/m Class: phyllosilicates 2 parallel faces related by a center of symmetry Pinacoids also routinely terminate the top and bottom of prismatic crystals http: //www. galleries. com/Apophyllite Wulfenite Pb. Mo. O 4, Lead Molybdate Class: sulfate
Open form 2 - The Pinacoid A pair of parallel faces related by mirror plane or twofold symmetry axis. Open form Prominent pinacoids will form platy or tabular crystals Fluor. Apophyllite (K, Na)Ca 4 Si 8 O 20(F, OH) - 8 H 2 O Tetragonal; 4/m 2/m Class: phyllosilicates 2 parallel faces related by a center of symmetry Pinacoids also routinely terminate the top and bottom of prismatic crystals http: //www. galleries. com/Apophyllite Wulfenite Pb. Mo. O 4, Lead Molybdate Class: sulfate
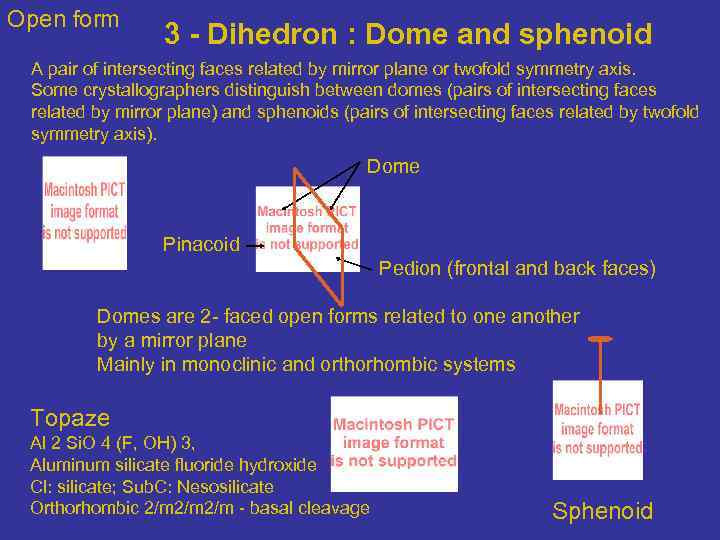 Open form 3 - Dihedron : Dome and sphenoid A pair of intersecting faces related by mirror plane or twofold symmetry axis. Some crystallographers distinguish between domes (pairs of intersecting faces related by mirror plane) and sphenoids (pairs of intersecting faces related by twofold symmetry axis). Dome Pinacoid Pedion (frontal and back faces) Domes are 2 - faced open forms related to one another by a mirror plane Mainly in monoclinic and orthorhombic systems Topaze Al 2 Si. O 4 (F, OH) 3, Aluminum silicate fluoride hydroxide Cl: silicate; Sub. C: Nesosilicate Orthorhombic 2/m 2/m - basal cleavage Sphenoid
Open form 3 - Dihedron : Dome and sphenoid A pair of intersecting faces related by mirror plane or twofold symmetry axis. Some crystallographers distinguish between domes (pairs of intersecting faces related by mirror plane) and sphenoids (pairs of intersecting faces related by twofold symmetry axis). Dome Pinacoid Pedion (frontal and back faces) Domes are 2 - faced open forms related to one another by a mirror plane Mainly in monoclinic and orthorhombic systems Topaze Al 2 Si. O 4 (F, OH) 3, Aluminum silicate fluoride hydroxide Cl: silicate; Sub. C: Nesosilicate Orthorhombic 2/m 2/m - basal cleavage Sphenoid
 Open form 4 - The different prisms A prism is an open form consisting of three or more parallel faces (set of faces) depending on the symmetry All the faces of one prism must be separated form each other by a specific amount of degrees: 1 - Rhombic prism 2 - Tetragonal prism 3 - Ditetragonal prism 4 - Trigonal prism 5 - Ditrigonal prism 6 - Hexagonal prism 7 - Dihexagonal prism !!! Prismatic habit does not mean that the mineral faces are prism faces for they could be two sets of pinacoids that are parallel to the same axes.
Open form 4 - The different prisms A prism is an open form consisting of three or more parallel faces (set of faces) depending on the symmetry All the faces of one prism must be separated form each other by a specific amount of degrees: 1 - Rhombic prism 2 - Tetragonal prism 3 - Ditetragonal prism 4 - Trigonal prism 5 - Ditrigonal prism 6 - Hexagonal prism 7 - Dihexagonal prism !!! Prismatic habit does not mean that the mineral faces are prism faces for they could be two sets of pinacoids that are parallel to the same axes.
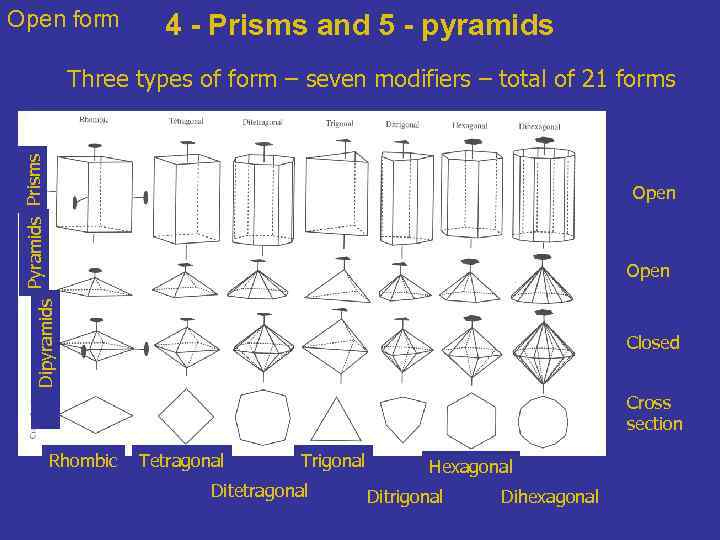 Open form 4 - Prisms and 5 - pyramids Prisms Three types of form – seven modifiers – total of 21 forms Open Dipyramids Open Closed Cross section Rhombic Tetragonal Trigonal Ditetragonal Hexagonal Ditrigonal Dihexagonal
Open form 4 - Prisms and 5 - pyramids Prisms Three types of form – seven modifiers – total of 21 forms Open Dipyramids Open Closed Cross section Rhombic Tetragonal Trigonal Ditetragonal Hexagonal Ditrigonal Dihexagonal
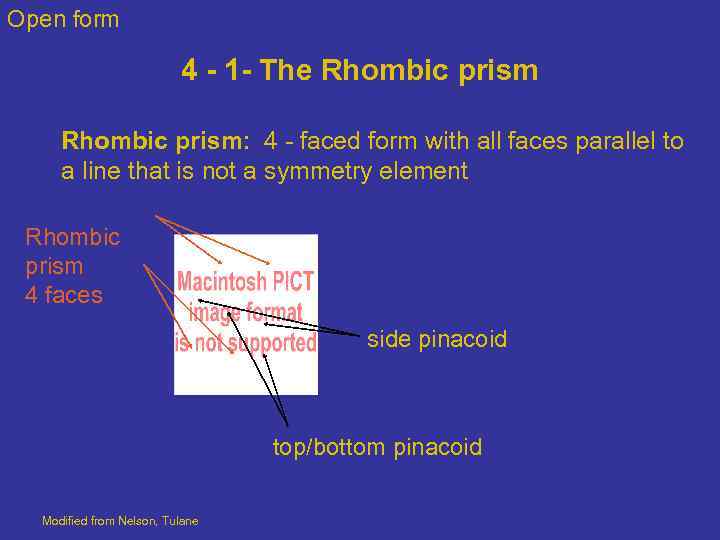 Open form 4 - 1 - The Rhombic prism: 4 - faced form with all faces parallel to a line that is not a symmetry element Rhombic prism 4 faces side pinacoid top/bottom pinacoid Modified from Nelson, Tulane
Open form 4 - 1 - The Rhombic prism: 4 - faced form with all faces parallel to a line that is not a symmetry element Rhombic prism 4 faces side pinacoid top/bottom pinacoid Modified from Nelson, Tulane
 Open form 4 - The 2 - Tetragonal prism and 3 - Ditetragonal prism Tetragonal prism Ditetragonal prism top/bottom pinacoid Tetragonal prism : 4 - faced open form with all faces parallel to a 4 -fold rotation axis. Ditetragonal prism : 8 - faced form with all faces parallel to a 4 -fold rotation axis Modified from Nelson, Tulane
Open form 4 - The 2 - Tetragonal prism and 3 - Ditetragonal prism Tetragonal prism Ditetragonal prism top/bottom pinacoid Tetragonal prism : 4 - faced open form with all faces parallel to a 4 -fold rotation axis. Ditetragonal prism : 8 - faced form with all faces parallel to a 4 -fold rotation axis Modified from Nelson, Tulane
 Open form 4 - The 4 - Trigonal prism and 5 - Ditrigonal prism Trigonal prism Cross section with no 6 -fold rotational Symmetry Distinct from an hexagonal form Trigonal prism : 3 - faced form with all faces parallel to a 3 -fold rotation axis Ditrigonal prism : 6 - faced form with all 6 faces parallel to a 3 -fold rotation axis. Modified from Nelson, Tulane
Open form 4 - The 4 - Trigonal prism and 5 - Ditrigonal prism Trigonal prism Cross section with no 6 -fold rotational Symmetry Distinct from an hexagonal form Trigonal prism : 3 - faced form with all faces parallel to a 3 -fold rotation axis Ditrigonal prism : 6 - faced form with all 6 faces parallel to a 3 -fold rotation axis. Modified from Nelson, Tulane
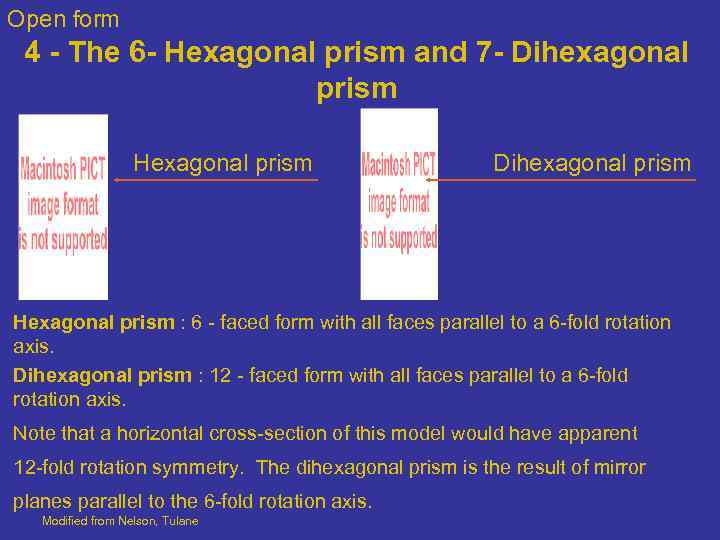 Open form 4 - The 6 - Hexagonal prism and 7 - Dihexagonal prism Hexagonal prism : 6 - faced form with all faces parallel to a 6 -fold rotation axis. Dihexagonal prism : 12 - faced form with all faces parallel to a 6 -fold rotation axis. Note that a horizontal cross-section of this model would have apparent 12 -fold rotation symmetry. The dihexagonal prism is the result of mirror planes parallel to the 6 -fold rotation axis. Modified from Nelson, Tulane
Open form 4 - The 6 - Hexagonal prism and 7 - Dihexagonal prism Hexagonal prism : 6 - faced form with all faces parallel to a 6 -fold rotation axis. Dihexagonal prism : 12 - faced form with all faces parallel to a 6 -fold rotation axis. Note that a horizontal cross-section of this model would have apparent 12 -fold rotation symmetry. The dihexagonal prism is the result of mirror planes parallel to the 6 -fold rotation axis. Modified from Nelson, Tulane
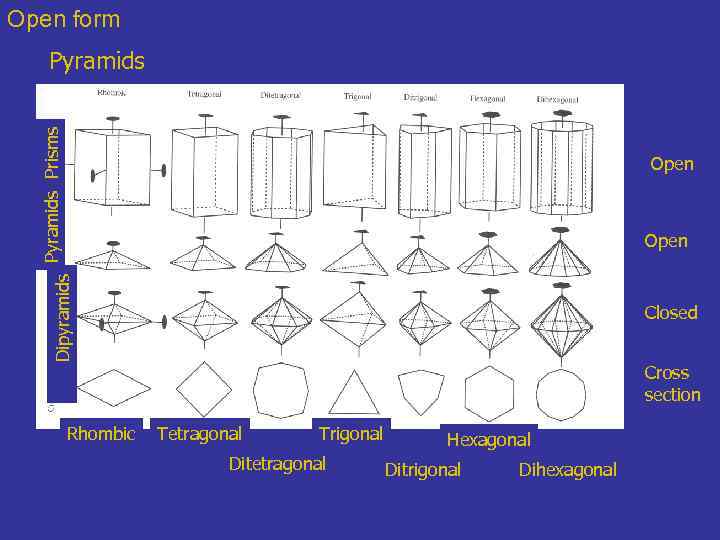 Open form Pyramids Prisms Pyramids Open Dipyramids Open Rhombic Closed Cross section Tetragonal Trigonal Ditetragonal Hexagonal Ditrigonal Dihexagonal
Open form Pyramids Prisms Pyramids Open Dipyramids Open Rhombic Closed Cross section Tetragonal Trigonal Ditetragonal Hexagonal Ditrigonal Dihexagonal
 Open form 5 - Crystal Forms : 3 -, 4 -, and 6 pyramids The basal face is a pedion. Pyramids, like prisms, are composed of either 3, 4, 6, 8, 12 faces. The faces are not parallel and in fact converge around a crystallographic axes forming a multi-sided tent (compare the dome and sphenoid) with a point unless capped by a pedion or pinacoid. The steepness of the faces of a single pyramid must remain constant. Terminaison of minerals can Be done by several pyramids. Variably inclined terminaisons would result. Rhombic pyramid: 4 -faced form where the faces are related by mirror planes. The faces labeled "p" are the four faces of the rhombic pyramid. If extend, these 4 faces would meet at a point. Modified from Nelson, Tulane
Open form 5 - Crystal Forms : 3 -, 4 -, and 6 pyramids The basal face is a pedion. Pyramids, like prisms, are composed of either 3, 4, 6, 8, 12 faces. The faces are not parallel and in fact converge around a crystallographic axes forming a multi-sided tent (compare the dome and sphenoid) with a point unless capped by a pedion or pinacoid. The steepness of the faces of a single pyramid must remain constant. Terminaison of minerals can Be done by several pyramids. Variably inclined terminaisons would result. Rhombic pyramid: 4 -faced form where the faces are related by mirror planes. The faces labeled "p" are the four faces of the rhombic pyramid. If extend, these 4 faces would meet at a point. Modified from Nelson, Tulane
 Open form 5 - Example: Tetragonal pyramid 4 -faced form where the faces are related by a 4 axis. In the drawing the small triangular faces that cut the corners represent the tetragonal pyramid. Note that if extended, these 4 faces would meet at a point. Modified from Nelson, Tulane
Open form 5 - Example: Tetragonal pyramid 4 -faced form where the faces are related by a 4 axis. In the drawing the small triangular faces that cut the corners represent the tetragonal pyramid. Note that if extended, these 4 faces would meet at a point. Modified from Nelson, Tulane
 Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
 Closed forms 1 - dipyramids : 3 -, 4 -, and 6 dipyramids Dipyramids are closed forms consisting of 6, 8, 12, 16, or 24 faces. Dipyramidsare pyramids that are reflected across a mirror plane. Thus, they occur in crystal classes that have a mirror plane perpendicular to a rotation or rotoinversion axis. Mirror plane
Closed forms 1 - dipyramids : 3 -, 4 -, and 6 dipyramids Dipyramids are closed forms consisting of 6, 8, 12, 16, or 24 faces. Dipyramidsare pyramids that are reflected across a mirror plane. Thus, they occur in crystal classes that have a mirror plane perpendicular to a rotation or rotoinversion axis. Mirror plane
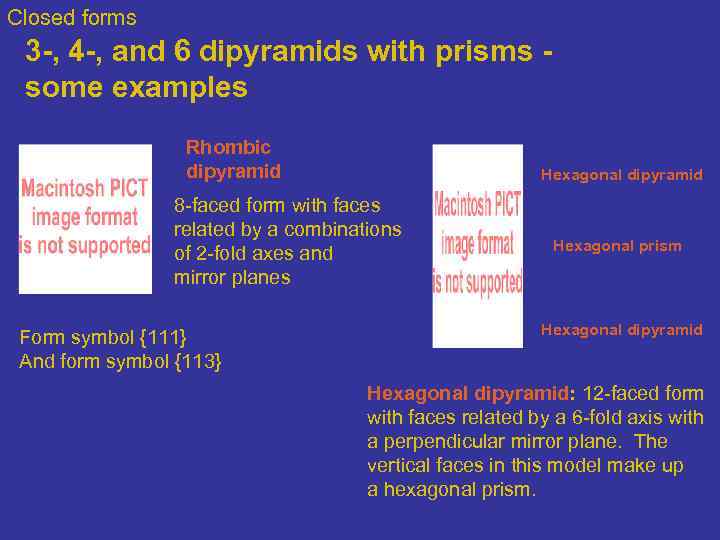 Closed forms 3 -, 4 -, and 6 dipyramids with prisms - some examples Rhombic dipyramid Hexagonal dipyramid 8 -faced form with faces related by a combinations of 2 -fold axes and mirror planes Form symbol {111} And form symbol {113} Hexagonal prism Hexagonal dipyramid: 12 -faced form with faces related by a 6 -fold axis with a perpendicular mirror plane. The vertical faces in this model make up a hexagonal prism.
Closed forms 3 -, 4 -, and 6 dipyramids with prisms - some examples Rhombic dipyramid Hexagonal dipyramid 8 -faced form with faces related by a combinations of 2 -fold axes and mirror planes Form symbol {111} And form symbol {113} Hexagonal prism Hexagonal dipyramid: 12 -faced form with faces related by a 6 -fold axis with a perpendicular mirror plane. The vertical faces in this model make up a hexagonal prism.
 Closed forms 3 -, 4 -, and 6 dipyramids with prisms - some examples Tetragonal dipyramid: 8 -faced form with faces related by a 4 -fold axis with a perpendicular mirror plane. The drawing shows the 8 -faced tetragonal dipyramid. Also shown is the 4 -faced tetragonal prism, and the 2 -faced top/bottom pinacoid. Ditetragonal dipyramid: 16 -faced form with faces related by a 4 -fold axis with a perpendicular mirror plane. The ditetragonal dipyramid is shown here. Note the vertical faces belong to a ditetragonal prism.
Closed forms 3 -, 4 -, and 6 dipyramids with prisms - some examples Tetragonal dipyramid: 8 -faced form with faces related by a 4 -fold axis with a perpendicular mirror plane. The drawing shows the 8 -faced tetragonal dipyramid. Also shown is the 4 -faced tetragonal prism, and the 2 -faced top/bottom pinacoid. Ditetragonal dipyramid: 16 -faced form with faces related by a 4 -fold axis with a perpendicular mirror plane. The ditetragonal dipyramid is shown here. Note the vertical faces belong to a ditetragonal prism.
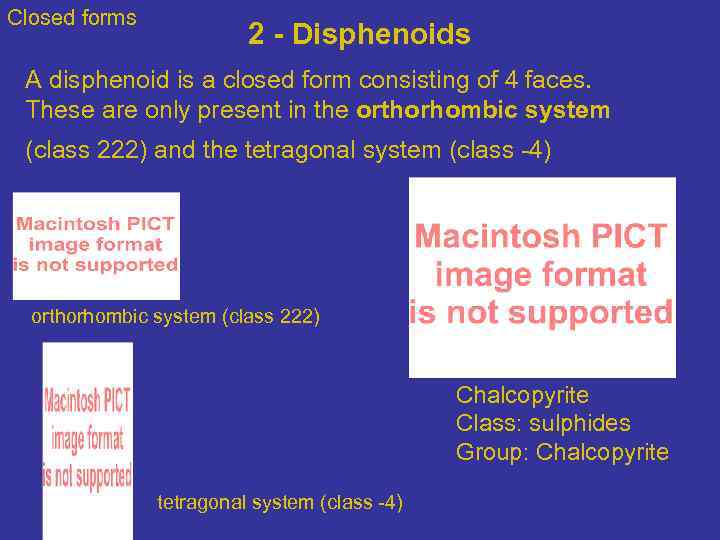 Closed forms 2 - Disphenoids A disphenoid is a closed form consisting of 4 faces. These are only present in the orthorhombic system (class 222) and the tetragonal system (class -4) orthorhombic system (class 222) Chalcopyrite Class: sulphides Group: Chalcopyrite tetragonal system (class -4)
Closed forms 2 - Disphenoids A disphenoid is a closed form consisting of 4 faces. These are only present in the orthorhombic system (class 222) and the tetragonal system (class -4) orthorhombic system (class 222) Chalcopyrite Class: sulphides Group: Chalcopyrite tetragonal system (class -4)
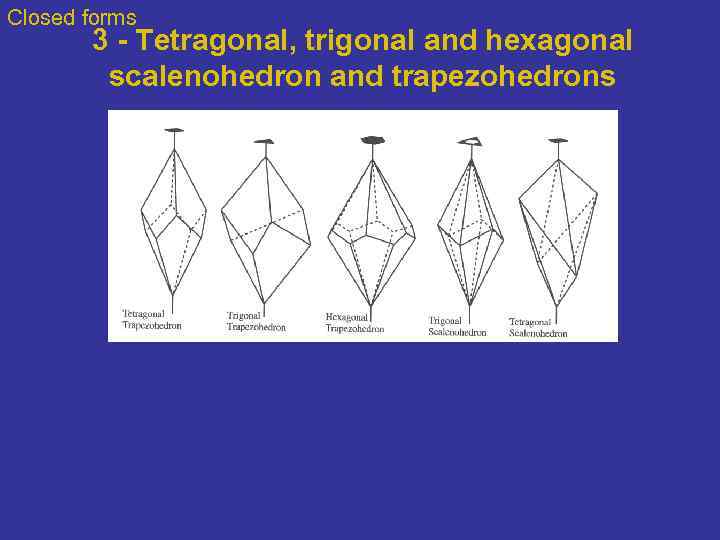 Closed forms 3 - Tetragonal, trigonal and hexagonal scalenohedron and trapezohedrons
Closed forms 3 - Tetragonal, trigonal and hexagonal scalenohedron and trapezohedrons
 Closed forms 3 - Trapezohedron: a solid made of trapezia (irregular quadrilaterals) Trapezohedron are closed 6, 8, or 12 faced forms, with 3, 4, or 6 upper faces offset from 3, 4, or 6 lower faces. The trapezohedron results from 3 -, 4 -, or 6 -fold axes combined with a perpendicular 2 -fold axis. Named according to the number of faces. The names of the three different forms are preceded by either trigonal, tetragonal or hexagonal respectively (ie. hexagonal trapezohedron) to avoid confusion. This group of forms should not be confused with the isometric trapezohedron which is not related but has trapezium shaped faces. Pb 2 CO 3 Cl 2, Lead Carbonate Chloride Carbonate Rare carbonate crystallizing in the tetragonal system Form from oxidation of lead-bearing minerals (former slags). http: //www. galleries. com/Leucite
Closed forms 3 - Trapezohedron: a solid made of trapezia (irregular quadrilaterals) Trapezohedron are closed 6, 8, or 12 faced forms, with 3, 4, or 6 upper faces offset from 3, 4, or 6 lower faces. The trapezohedron results from 3 -, 4 -, or 6 -fold axes combined with a perpendicular 2 -fold axis. Named according to the number of faces. The names of the three different forms are preceded by either trigonal, tetragonal or hexagonal respectively (ie. hexagonal trapezohedron) to avoid confusion. This group of forms should not be confused with the isometric trapezohedron which is not related but has trapezium shaped faces. Pb 2 CO 3 Cl 2, Lead Carbonate Chloride Carbonate Rare carbonate crystallizing in the tetragonal system Form from oxidation of lead-bearing minerals (former slags). http: //www. galleries. com/Leucite
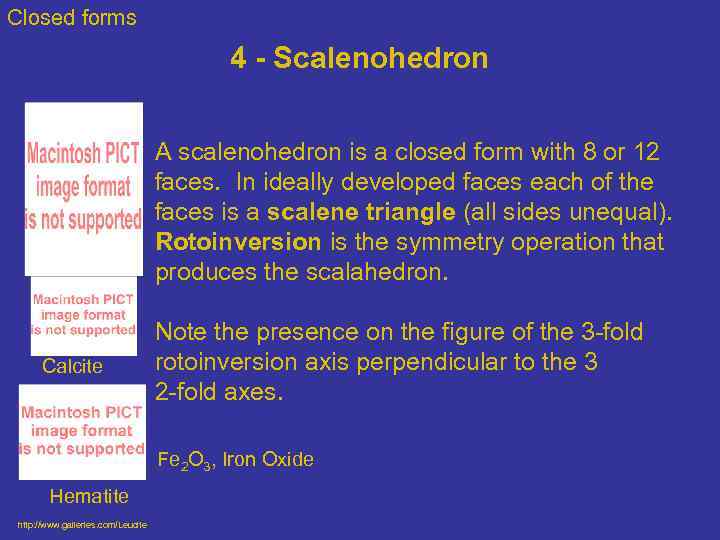 Closed forms 4 - Scalenohedron Calcite A scalenohedron is a closed form with 8 or 12 faces. In ideally developed faces each of the faces is a scalene triangle (all sides unequal). Rotoinversion is the symmetry operation that produces the scalahedron. Note the presence on the figure of the 3 -fold rotoinversion axis perpendicular to the 3 2 -fold axes. Fe 2 O 3, Iron Oxide Hematite http: //www. galleries. com/Leucite
Closed forms 4 - Scalenohedron Calcite A scalenohedron is a closed form with 8 or 12 faces. In ideally developed faces each of the faces is a scalene triangle (all sides unequal). Rotoinversion is the symmetry operation that produces the scalahedron. Note the presence on the figure of the 3 -fold rotoinversion axis perpendicular to the 3 2 -fold axes. Fe 2 O 3, Iron Oxide Hematite http: //www. galleries. com/Leucite
 Closed forms 5 - Rhombohedron A solid with six congruent parallelogram faces - A distorded cube along one of its diagonal three-fold symmetry axes. 3 faces on top are offset by 3 identical upside down faces on the bottom, as a result of a 3 -fold rotoinversion axis. Rhombohedrons can also result from a 3 -fold axis with perpendicular 2 -fold axes. Rhombohedrons only occur in the crystal classes 2/m , 32, and. The steepness of the rhombohedron is variable for different minerals. The diagonal of "deformation" becomes the prominent axes of symmetry and is trigonal with a three fold rotation about the axes. Six mirror planes protrude through the middle of the faces and edges that converge on the top and bottom points. There are six faces (like a cube), each rhomb shaped (4 equal sides, no 90 angles) If the "deformation" is not severe then the crystal can appear to be pseudo-cubic. With severe "deformation" the crystal can appear tabular. The rhombohedron is the only non-isometric closed form with parallel faces. Looks like a stretched or shortened cube
Closed forms 5 - Rhombohedron A solid with six congruent parallelogram faces - A distorded cube along one of its diagonal three-fold symmetry axes. 3 faces on top are offset by 3 identical upside down faces on the bottom, as a result of a 3 -fold rotoinversion axis. Rhombohedrons can also result from a 3 -fold axis with perpendicular 2 -fold axes. Rhombohedrons only occur in the crystal classes 2/m , 32, and. The steepness of the rhombohedron is variable for different minerals. The diagonal of "deformation" becomes the prominent axes of symmetry and is trigonal with a three fold rotation about the axes. Six mirror planes protrude through the middle of the faces and edges that converge on the top and bottom points. There are six faces (like a cube), each rhomb shaped (4 equal sides, no 90 angles) If the "deformation" is not severe then the crystal can appear to be pseudo-cubic. With severe "deformation" the crystal can appear tabular. The rhombohedron is the only non-isometric closed form with parallel faces. Looks like a stretched or shortened cube
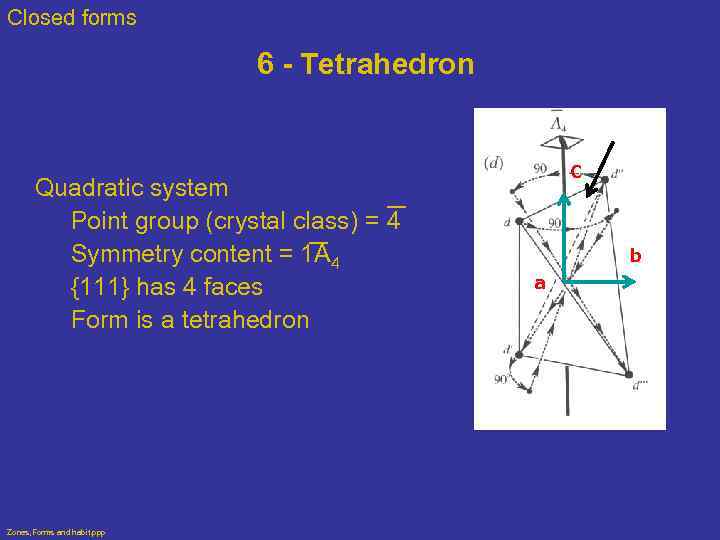 Closed forms 6 - Tetrahedron Quadratic system Point group (crystal class) = 4 Symmetry content = 1 A 4 {111} has 4 faces Form is a tetrahedron Zones, Forms and habit. ppp C b a
Closed forms 6 - Tetrahedron Quadratic system Point group (crystal class) = 4 Symmetry content = 1 A 4 {111} has 4 faces Form is a tetrahedron Zones, Forms and habit. ppp C b a
 Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
 Isometric forms • 15 possible forms • 4 common ones – Cube {001} – 4/m 3 2/m symmetry – Octahedron {111} – 4/m 3 2/m symmetry – Tetrahedron {111} – 4 symmetry – Dodecahedron {110} Zones, Forms and habit. ppp
Isometric forms • 15 possible forms • 4 common ones – Cube {001} – 4/m 3 2/m symmetry – Octahedron {111} – 4/m 3 2/m symmetry – Tetrahedron {111} – 4 symmetry – Dodecahedron {110} Zones, Forms and habit. ppp
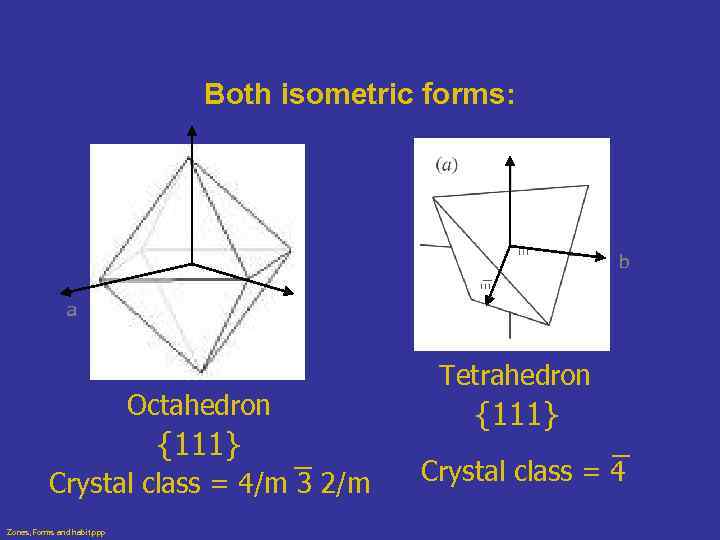 Both isometric forms: c c b a b Octahedron {111} Crystal class = 4/m 3 2/m Zones, Forms and habit. ppp a Tetrahedron {111} Crystal class = 4
Both isometric forms: c c b a b Octahedron {111} Crystal class = 4/m 3 2/m Zones, Forms and habit. ppp a Tetrahedron {111} Crystal class = 4
 Closed isometric Forms 1 - Hexahedron or cube Symmetrical 6 side-box. The cube can only be formed by isometric minerals. 4 -fold axes are perpendicular to the face of the cube, and four axes run through the corners of the cube. Note that the form symbol for a hexahedron is {100}, and it consists of the following 6 faces: (100), (010), (001), (100), (010), and (001). The face 100 generates all the other faces Galena is a mineral that forms cubes.
Closed isometric Forms 1 - Hexahedron or cube Symmetrical 6 side-box. The cube can only be formed by isometric minerals. 4 -fold axes are perpendicular to the face of the cube, and four axes run through the corners of the cube. Note that the form symbol for a hexahedron is {100}, and it consists of the following 6 faces: (100), (010), (001), (100), (010), and (001). The face 100 generates all the other faces Galena is a mineral that forms cubes.
 Closed isometric Forms 2 - Octahedron An octahedron is an 8 faced form that results form three 4 -fold axes with perpendicular mirror planes. The octahedron has the form symbol {111} and consists of the following 8 faces: (111), (111), and (111). Note that four 3 -fold axes are present that are perpendicular to the triangular faces of the octahedron. Diamond The face 111 generates all the other faces. The resulting set of faces is designated (111) and is called an octahedron. C: elemental carbon - Native element, non metallic
Closed isometric Forms 2 - Octahedron An octahedron is an 8 faced form that results form three 4 -fold axes with perpendicular mirror planes. The octahedron has the form symbol {111} and consists of the following 8 faces: (111), (111), and (111). Note that four 3 -fold axes are present that are perpendicular to the triangular faces of the octahedron. Diamond The face 111 generates all the other faces. The resulting set of faces is designated (111) and is called an octahedron. C: elemental carbon - Native element, non metallic
 Closed isometric Forms 2 - Octahedron - diamond Diamonds, C, South Africa. Point group: 4/m 3 bar 2/m. Average crystal size: 1 cm. Crystal shape: top row and first crystal in the bottom row: octahedron; middle crystal in bottom row: tetrahedron; third crystal bottom row: two intergrown octahedra. http: //webmineral. brgm. fr: 8003
Closed isometric Forms 2 - Octahedron - diamond Diamonds, C, South Africa. Point group: 4/m 3 bar 2/m. Average crystal size: 1 cm. Crystal shape: top row and first crystal in the bottom row: octahedron; middle crystal in bottom row: tetrahedron; third crystal bottom row: two intergrown octahedra. http: //webmineral. brgm. fr: 8003
 Closed isometric Forms 3 - Dodecahedron A dodecahedron is a closed 12 -faced form. Dodecahedrons can be formed by cutting off the edges of a cube. The form symbol for a dodecahedron is {110}. Grossular garnet Ca 3 Al 2 Si 3 O 12, Jeffrey Quarry, Asbestos, Quebec, Canada. Crystal size: 12 mm. Point group: 4/m 3 bar 2/m. The crystal shape is a dodecahedron. The faces show growth steps. http: //www. rockhounds. com/rockshop/xtal/part 2. html
Closed isometric Forms 3 - Dodecahedron A dodecahedron is a closed 12 -faced form. Dodecahedrons can be formed by cutting off the edges of a cube. The form symbol for a dodecahedron is {110}. Grossular garnet Ca 3 Al 2 Si 3 O 12, Jeffrey Quarry, Asbestos, Quebec, Canada. Crystal size: 12 mm. Point group: 4/m 3 bar 2/m. The crystal shape is a dodecahedron. The faces show growth steps. http: //www. rockhounds. com/rockshop/xtal/part 2. html
 Closed isometric Forms 4 - Tetrahexahedron The tetrahexahedron is a 24 -faced form with a general form symbol of {0 hl} This means that all faces are parallel to one of the a axes, and intersect the other 2 axes at different lengths.
Closed isometric Forms 4 - Tetrahexahedron The tetrahexahedron is a 24 -faced form with a general form symbol of {0 hl} This means that all faces are parallel to one of the a axes, and intersect the other 2 axes at different lengths.
 Closed isometric Forms 5 - Trapezohedron An isometric trapezohedron is a 12 -faced closed form with the general form symbol {hhl}. All the faces intersect two of the a axes at equal length and intersect the third a axis at a different length The mineral analcime is commonly seen in this form Tectosilicate - group : zeolites Na. Al. Si 2 O 6 -H 2 O, Hydrated Sodium Aluminum Silicate Leucite : KAl. Si 2 O 6, Potassium Aluminum Silicate Tectosilicates; Group: feldspathoïdes Spessartine garnet Mn 3 Al 2 Si 3 O 12. Haramosh Mountains Baltistan, Northern Areas, Pakistan.
Closed isometric Forms 5 - Trapezohedron An isometric trapezohedron is a 12 -faced closed form with the general form symbol {hhl}. All the faces intersect two of the a axes at equal length and intersect the third a axis at a different length The mineral analcime is commonly seen in this form Tectosilicate - group : zeolites Na. Al. Si 2 O 6 -H 2 O, Hydrated Sodium Aluminum Silicate Leucite : KAl. Si 2 O 6, Potassium Aluminum Silicate Tectosilicates; Group: feldspathoïdes Spessartine garnet Mn 3 Al 2 Si 3 O 12. Haramosh Mountains Baltistan, Northern Areas, Pakistan.
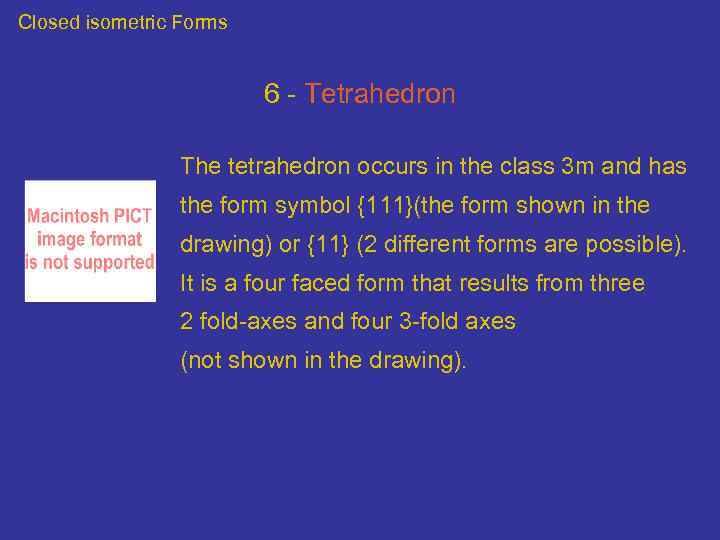 Closed isometric Forms 6 - Tetrahedron The tetrahedron occurs in the class 3 m and has the form symbol {111}(the form shown in the drawing) or {11} (2 different forms are possible). It is a four faced form that results from three 2 fold-axes and four 3 -fold axes (not shown in the drawing).
Closed isometric Forms 6 - Tetrahedron The tetrahedron occurs in the class 3 m and has the form symbol {111}(the form shown in the drawing) or {11} (2 different forms are possible). It is a four faced form that results from three 2 fold-axes and four 3 -fold axes (not shown in the drawing).
 Closed isometric Forms 7 - Gyroid A gyroid is a form in the class 432 24 congruent irregular pentagonal faces [note no mirror planes]
Closed isometric Forms 7 - Gyroid A gyroid is a form in the class 432 24 congruent irregular pentagonal faces [note no mirror planes]
 Closed isometric Forms 8 - Pyritohedron or pentagondodecahedron 102 The pyritohedron is a 12 -faced form (special form) that occurs in the crystal class 2/m. Note that there are no 4 -fold axes in this class. Faces are each perpendicular to a mirror plane, reducing the number of faces to 12 pentagonal faces. Although this superficially looks like the platonic solid with 12 regular pentagon faces, these faces are not regular. The possible forms are {h 0 l} or {0 kl} and each of thefaces that make up the form have 5 sides.
Closed isometric Forms 8 - Pyritohedron or pentagondodecahedron 102 The pyritohedron is a 12 -faced form (special form) that occurs in the crystal class 2/m. Note that there are no 4 -fold axes in this class. Faces are each perpendicular to a mirror plane, reducing the number of faces to 12 pentagonal faces. Although this superficially looks like the platonic solid with 12 regular pentagon faces, these faces are not regular. The possible forms are {h 0 l} or {0 kl} and each of thefaces that make up the form have 5 sides.
 Closed isometric Forms 9 - Diploid 321 The diploid is the general form {hkl} for the diploidal class (2/m). 24 congruent irregular quadrilateral faces. The name comes from a Latin root for half, because half of the 48 faces for full isometric symmetry are present. [Again there are no 4 -fold axes]
Closed isometric Forms 9 - Diploid 321 The diploid is the general form {hkl} for the diploidal class (2/m). 24 congruent irregular quadrilateral faces. The name comes from a Latin root for half, because half of the 48 faces for full isometric symmetry are present. [Again there are no 4 -fold axes]
 Closed isometric Forms 10 - Tetartoids are general forms in the tetartoidal class (233) which only has 3 -fold axes and 2 fold axes with no mirror planes. 12 congruent irregular pentagonal faces. The name comes from a Greek root for onefourth because only a quarter of the 48 faces for full isometric symmetry are present.
Closed isometric Forms 10 - Tetartoids are general forms in the tetartoidal class (233) which only has 3 -fold axes and 2 fold axes with no mirror planes. 12 congruent irregular pentagonal faces. The name comes from a Greek root for onefourth because only a quarter of the 48 faces for full isometric symmetry are present.
 Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
 Combining forms • Restrictions on types of forms within a crystal – All forms must be in the same crystal system – All forms must have symmetry of one crystal class • Tetragonal prism has a single 4 -fold rotation, only found in tetragonal crystal class with single 4 -fold rotation axis • Pedions never occur in mineral with center of symmetry Zones, Forms and habit. ppp
Combining forms • Restrictions on types of forms within a crystal – All forms must be in the same crystal system – All forms must have symmetry of one crystal class • Tetragonal prism has a single 4 -fold rotation, only found in tetragonal crystal class with single 4 -fold rotation axis • Pedions never occur in mineral with center of symmetry Zones, Forms and habit. ppp
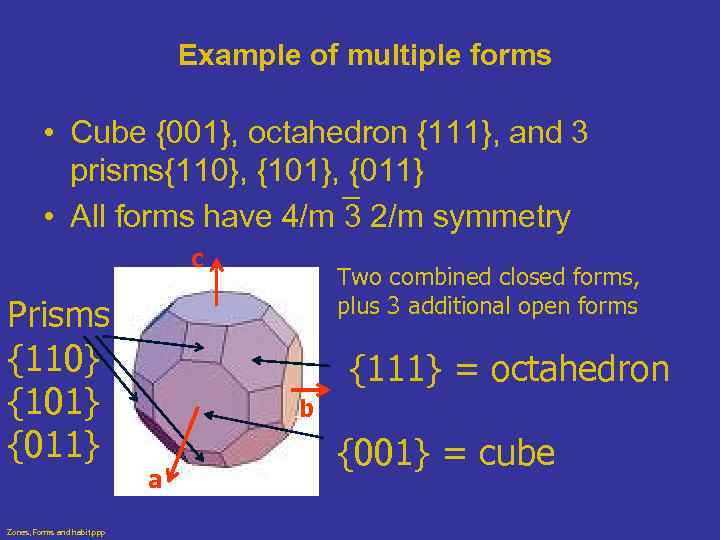 Example of multiple forms • Cube {001}, octahedron {111}, and 3 prisms{110}, {101}, {011} • All forms have 4/m 3 2/m symmetry c Prisms {110} {101} {011} Zones, Forms and habit. ppp Two combined closed forms, plus 3 additional open forms {111} = octahedron b a {001} = cube
Example of multiple forms • Cube {001}, octahedron {111}, and 3 prisms{110}, {101}, {011} • All forms have 4/m 3 2/m symmetry c Prisms {110} {101} {011} Zones, Forms and habit. ppp Two combined closed forms, plus 3 additional open forms {111} = octahedron b a {001} = cube
 Multi-face forms • !!!! Multi-faced forms are not composed of several simpler forms –A cube is not 6 pedions – or 3 pinacoids Zones, Forms and habit. ppp
Multi-face forms • !!!! Multi-faced forms are not composed of several simpler forms –A cube is not 6 pedions – or 3 pinacoids Zones, Forms and habit. ppp
 Relationship between forms • Enantiomorphous forms – Two forms related to each other by mirror planes – Mirror planes missing within the form itself • Positive and negative forms – Two forms related to each other by rotation axis – Rotation axis missing within the forms Zones, Forms and habit. ppp
Relationship between forms • Enantiomorphous forms – Two forms related to each other by mirror planes – Mirror planes missing within the form itself • Positive and negative forms – Two forms related to each other by rotation axis – Rotation axis missing within the forms Zones, Forms and habit. ppp
 Enantiomorphous Forms • Lack center of symmetry and mirrors • Since they are mirror images, they are right and left handed forms • Individual crystal of enantiomorphic mineral may be right or left handed, but not both • Originate from a type of symmetry called screw axis (may spiral right or left) • Quartz is common example Zones, Forms and habit. ppp
Enantiomorphous Forms • Lack center of symmetry and mirrors • Since they are mirror images, they are right and left handed forms • Individual crystal of enantiomorphic mineral may be right or left handed, but not both • Originate from a type of symmetry called screw axis (may spiral right or left) • Quartz is common example Zones, Forms and habit. ppp
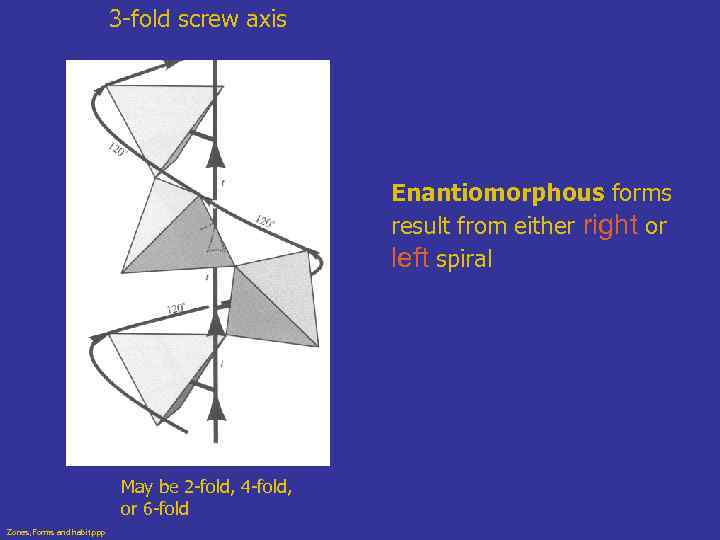 3 -fold screw axis Enantiomorphous forms result from either right or left spiral May be 2 -fold, 4 -fold, or 6 -fold Zones, Forms and habit. ppp
3 -fold screw axis Enantiomorphous forms result from either right or left spiral May be 2 -fold, 4 -fold, or 6 -fold Zones, Forms and habit. ppp
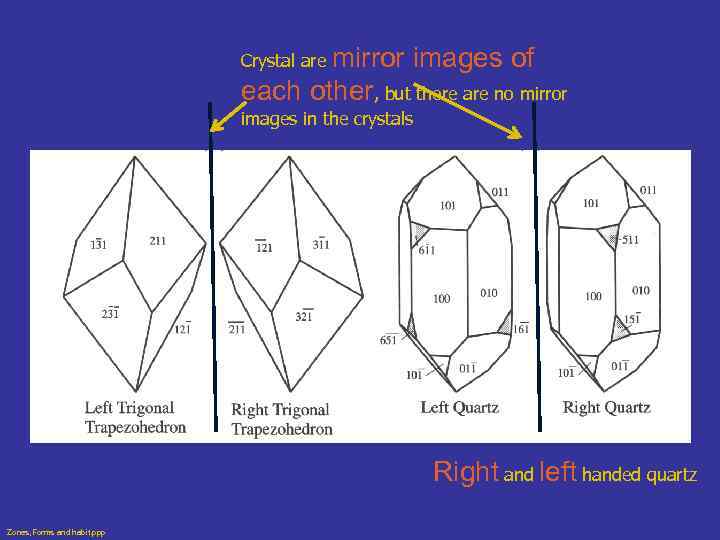 mirror images of each other, but there are no mirror Crystal are images in the crystals Right and left handed quartz Zones, Forms and habit. ppp
mirror images of each other, but there are no mirror Crystal are images in the crystals Right and left handed quartz Zones, Forms and habit. ppp
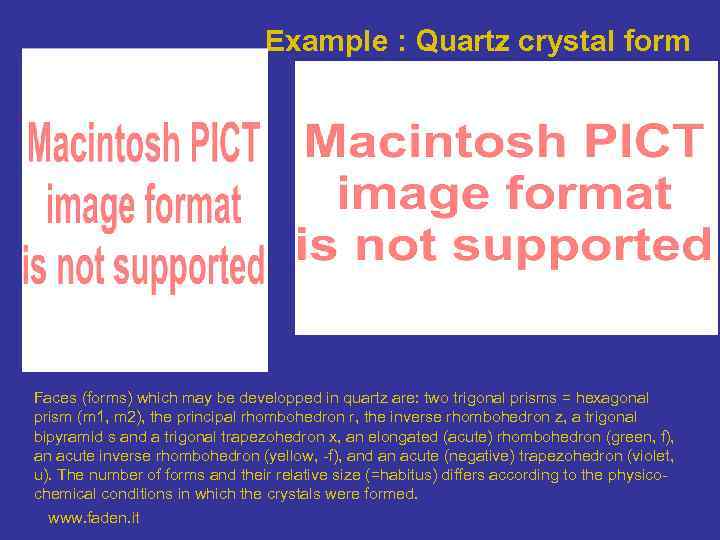 Example : Quartz crystal form Faces (forms) which may be developped in quartz are: two trigonal prisms = hexagonal prism (m 1, m 2), the principal rhombohedron r, the inverse rhombohedron z, a trigonal bipyramid s and a trigonal trapezohedron x, an elongated (acute) rhombohedron (green, f), an acute inverse rhombohedron (yellow, -f), and an acute (negative) trapezohedron (violet, u). The number of forms and their relative size (=habitus) differs according to the physicochemical conditions in which the crystals were formed. www. faden. it
Example : Quartz crystal form Faces (forms) which may be developped in quartz are: two trigonal prisms = hexagonal prism (m 1, m 2), the principal rhombohedron r, the inverse rhombohedron z, a trigonal bipyramid s and a trigonal trapezohedron x, an elongated (acute) rhombohedron (green, f), an acute inverse rhombohedron (yellow, -f), and an acute (negative) trapezohedron (violet, u). The number of forms and their relative size (=habitus) differs according to the physicochemical conditions in which the crystals were formed. www. faden. it
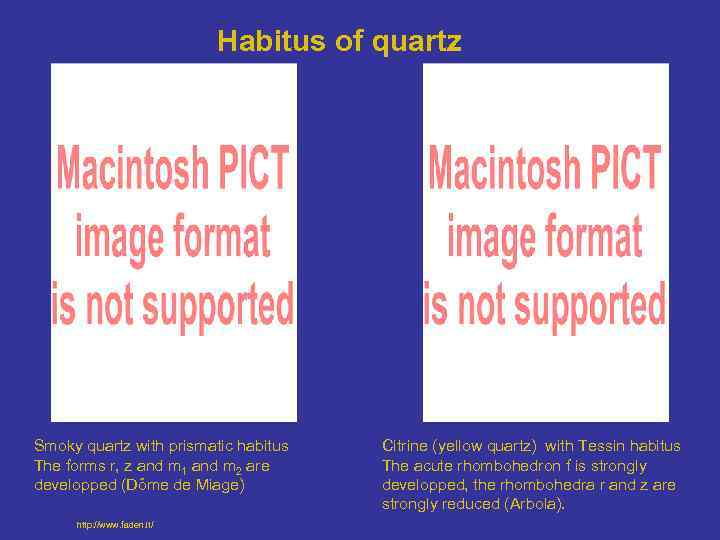 Habitus of quartz Smoky quartz with prismatic habitus The forms r, z and m 1 and m 2 are developped (Dôme de Miage) http: //www. faden. it/ Citrine (yellow quartz) with Tessin habitus The acute rhombohedron f is strongly developped, the rhombohedra r and z are strongly reduced (Arbola).
Habitus of quartz Smoky quartz with prismatic habitus The forms r, z and m 1 and m 2 are developped (Dôme de Miage) http: //www. faden. it/ Citrine (yellow quartz) with Tessin habitus The acute rhombohedron f is strongly developped, the rhombohedra r and z are strongly reduced (Arbola).
 Positive and Negative Form • Two forms related by rotation • Two possible rotations: – 60º on 3 -fold rotation axis – 90º on 4 - or 2 -fold rotation axis Zones, Forms and habit. ppp
Positive and Negative Form • Two forms related by rotation • Two possible rotations: – 60º on 3 -fold rotation axis – 90º on 4 - or 2 -fold rotation axis Zones, Forms and habit. ppp
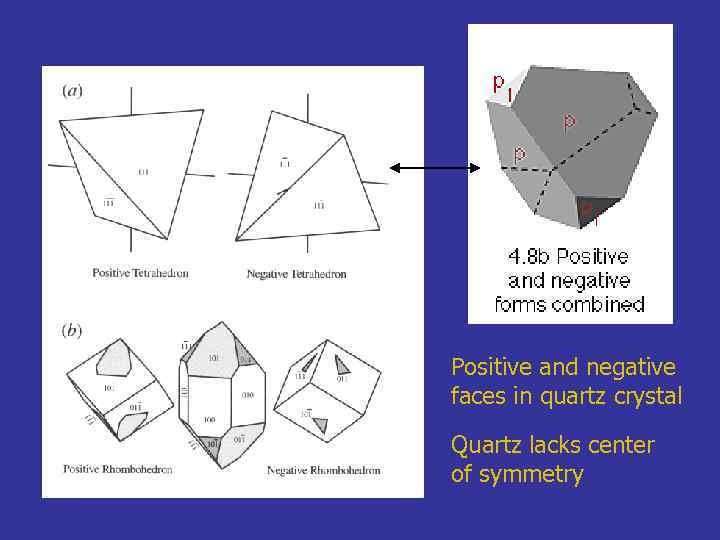 Positive and negative faces in quartz crystal Quartz lacks center of symmetry
Positive and negative faces in quartz crystal Quartz lacks center of symmetry
 General forms and special forms A general form is a form in a particular crystal class that contains faces that intersect all crystallographic axes at different lengths. It has the form symbol {hkl}. All other forms that may be present are called special forms. In the monoclinic, triclinic, and orthorhombic crystal systems, the form {111} is a general form because in these systems faces of this form will intersect the a, b, and c axes at different lengths because the unit lengths are different on each axis. In crystals of higher symmetry, where two or more of the axes have equal length, a general form must intersect the equal length axes at different multiples of the unit length. Thus in the tetragonal system the form {121} is a general form. In the isometric system a general form would have to be something like {123}. Zones, Forms and habit. ppp
General forms and special forms A general form is a form in a particular crystal class that contains faces that intersect all crystallographic axes at different lengths. It has the form symbol {hkl}. All other forms that may be present are called special forms. In the monoclinic, triclinic, and orthorhombic crystal systems, the form {111} is a general form because in these systems faces of this form will intersect the a, b, and c axes at different lengths because the unit lengths are different on each axis. In crystals of higher symmetry, where two or more of the axes have equal length, a general form must intersect the equal length axes at different multiples of the unit length. Thus in the tetragonal system the form {121} is a general form. In the isometric system a general form would have to be something like {123}. Zones, Forms and habit. ppp
 Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
 Forms in the Six Crystal System • Forms control orientation of crystallographic axes of the 6 crystal system • Systematic relationship between form, symmetry present, and Hermann-Mauguin symbols • Following slides show these relationships Zones, Forms and habit. ppp
Forms in the Six Crystal System • Forms control orientation of crystallographic axes of the 6 crystal system • Systematic relationship between form, symmetry present, and Hermann-Mauguin symbols • Following slides show these relationships Zones, Forms and habit. ppp
 Triclinic system n. Common symmetry: 1 -fold rotation nc-axis parallels prominent zone axis nb and a axes parallel crystal edges na and b typically > 90º n. Single Hermann-Mauguin symbol n. Common Zones, Forms and habit. ppp minerals: plagioclase and microcline
Triclinic system n. Common symmetry: 1 -fold rotation nc-axis parallels prominent zone axis nb and a axes parallel crystal edges na and b typically > 90º n. Single Hermann-Mauguin symbol n. Common Zones, Forms and habit. ppp minerals: plagioclase and microcline
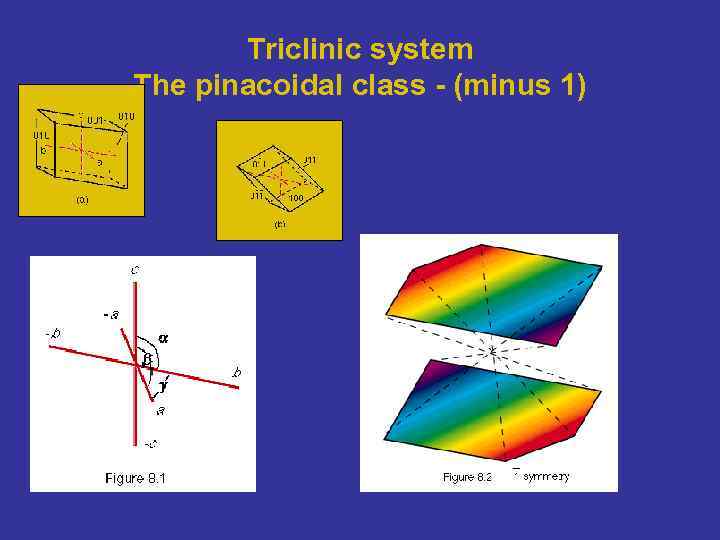 Triclinic system The pinacoidal class - (minus 1)
Triclinic system The pinacoidal class - (minus 1)
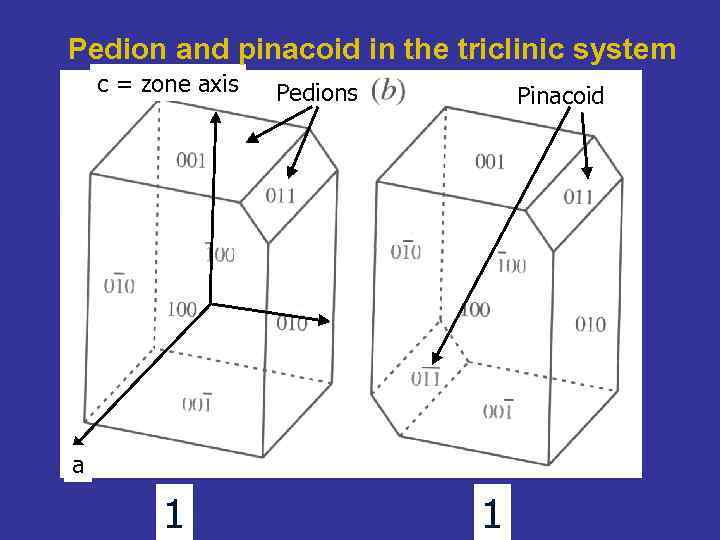 Pedion and pinacoid in the triclinic system c = zone axis Pedions Pinacoid a 1 1
Pedion and pinacoid in the triclinic system c = zone axis Pedions Pinacoid a 1 1
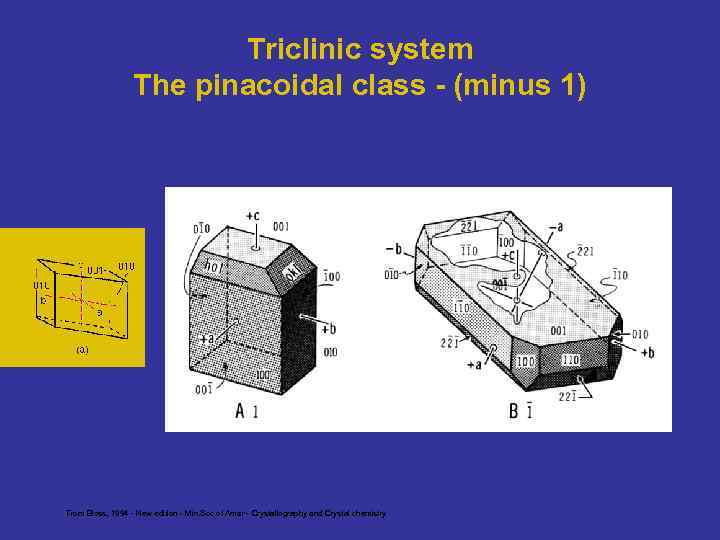 Triclinic system The pinacoidal class - (minus 1) From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Triclinic system The pinacoidal class - (minus 1) From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
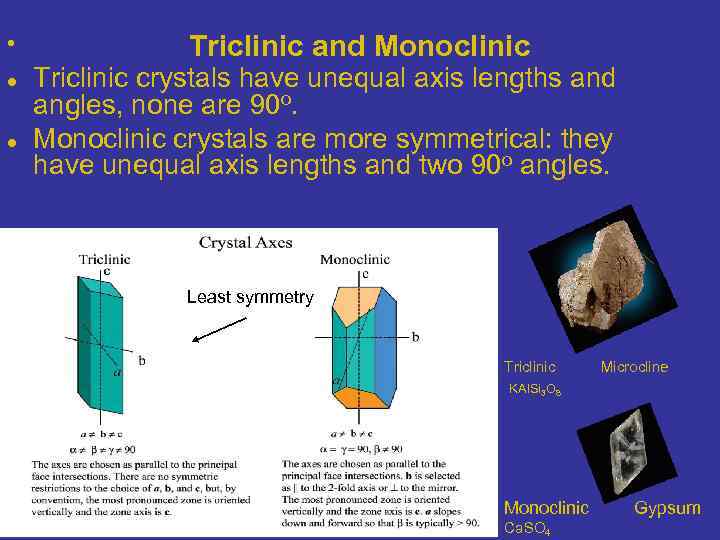 • Triclinic and Monoclinic Triclinic crystals have unequal axis lengths and angles, none are 90 o. Monoclinic crystals are more symmetrical: they have unequal axis lengths and two 90 o angles. Least symmetry Triclinic Microcline KAl. Si 3 O 8 Monoclinic Gypsum Ca. SO 4
• Triclinic and Monoclinic Triclinic crystals have unequal axis lengths and angles, none are 90 o. Monoclinic crystals are more symmetrical: they have unequal axis lengths and two 90 o angles. Least symmetry Triclinic Microcline KAl. Si 3 O 8 Monoclinic Gypsum Ca. SO 4
 Monoclinic system n. Common symmetry: 2 -fold rotation and/or single mirror plane nb axis commonly parallel the 2 -fold rotation and/or perpendicular to mirror plane nc axis parallel to prominent zone na axis down and to front so b > 90 n. Single H-M symbol (2, m, or 2/m) n. Common Zones, Forms and habit. ppp minerals: amphiboles, pyroxenes, micas
Monoclinic system n. Common symmetry: 2 -fold rotation and/or single mirror plane nb axis commonly parallel the 2 -fold rotation and/or perpendicular to mirror plane nc axis parallel to prominent zone na axis down and to front so b > 90 n. Single H-M symbol (2, m, or 2/m) n. Common Zones, Forms and habit. ppp minerals: amphiboles, pyroxenes, micas
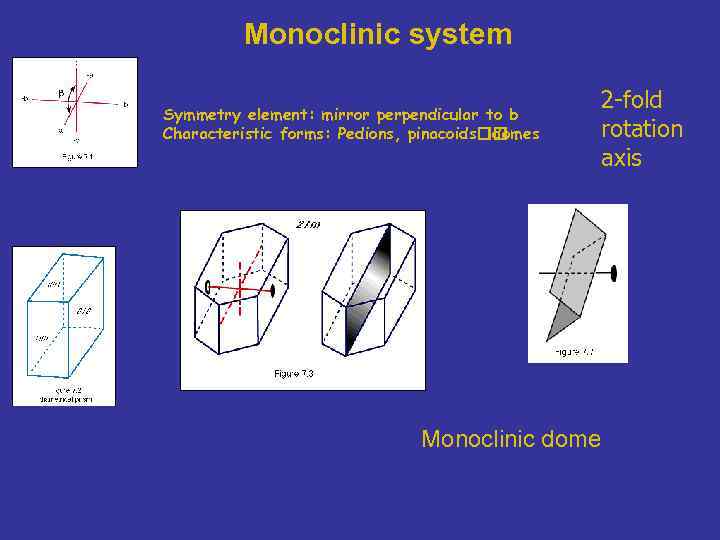 Monoclinic system Symmetry element: mirror perpendicular to b Characteristic forms: Pedions, pinacoids domes 2 -fold rotation axis Monoclinic dome
Monoclinic system Symmetry element: mirror perpendicular to b Characteristic forms: Pedions, pinacoids domes 2 -fold rotation axis Monoclinic dome
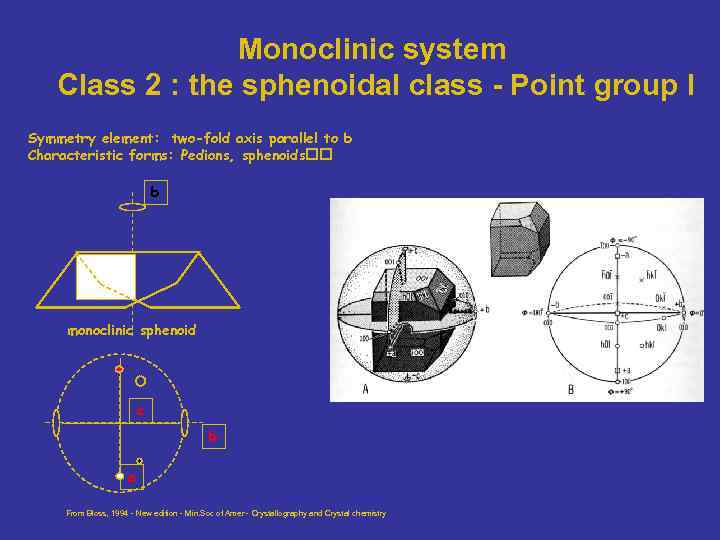 Monoclinic system Class 2 : the sphenoidal class - Point group I Symmetry element: two-fold axis parallel to b Characteristic forms: Pedions, sphenoids b monoclinic sphenoid c b a From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Monoclinic system Class 2 : the sphenoidal class - Point group I Symmetry element: two-fold axis parallel to b Characteristic forms: Pedions, sphenoids b monoclinic sphenoid c b a From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
 Monoclinic system Class m : the domatic class - point group II b Point group: m Symmetry element: mirror perpendicular to b Characteristic forms: Pedions, pinacoids domes c b a From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Monoclinic system Class m : the domatic class - point group II b Point group: m Symmetry element: mirror perpendicular to b Characteristic forms: Pedions, pinacoids domes c b a From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
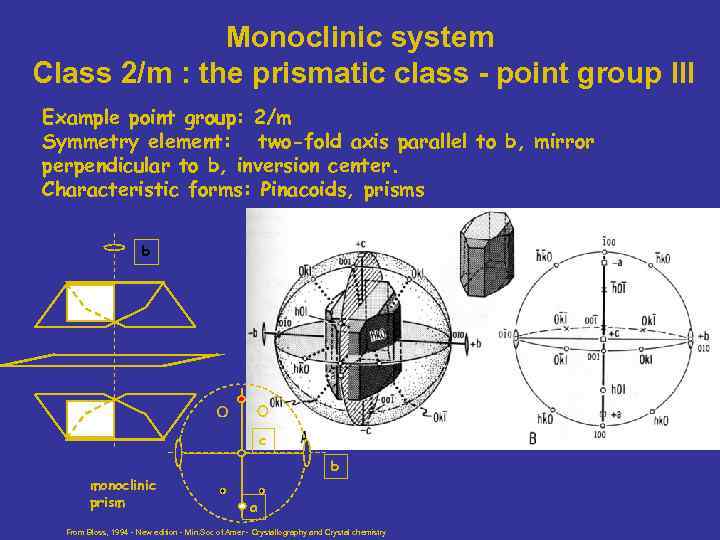 Monoclinic system Class 2/m : the prismatic class - point group III Example point group: 2/m Symmetry element: two-fold axis parallel to b, mirror perpendicular to b, inversion center. Characteristic forms: Pinacoids, prisms b c monoclinic prism b a From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Monoclinic system Class 2/m : the prismatic class - point group III Example point group: 2/m Symmetry element: two-fold axis parallel to b, mirror perpendicular to b, inversion center. Characteristic forms: Pinacoids, prisms b c monoclinic prism b a From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
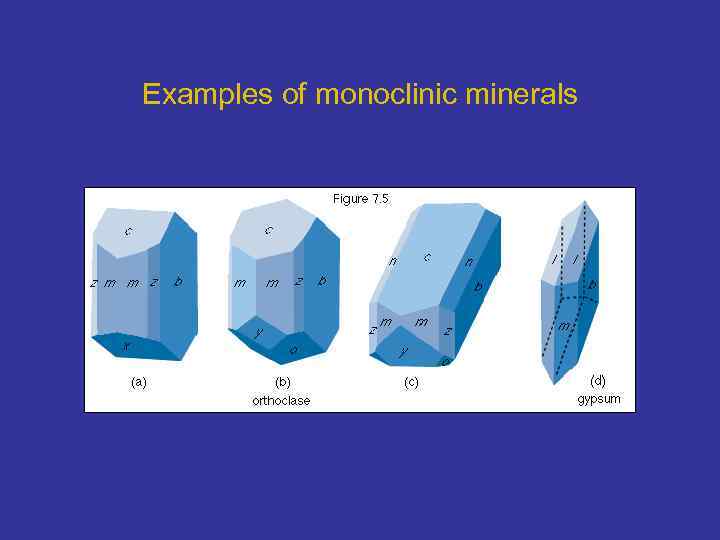 Examples of monoclinic minerals
Examples of monoclinic minerals
 Monoclinic minerals Several twinned (two crystals “glued” together in an oriented fashion) green crystals of titanite (sphene) on matrix. Capelinha, Minas Gerais, Brazil. 5. 5 x 5 x 3. 5 cm. http: //webmineral. com Transparent, bluish crystal aggregate of vivianite on matrix. Santa Eulalia, Chihuahua, Mexico. Faces of the monoclinic prism
Monoclinic minerals Several twinned (two crystals “glued” together in an oriented fashion) green crystals of titanite (sphene) on matrix. Capelinha, Minas Gerais, Brazil. 5. 5 x 5 x 3. 5 cm. http: //webmineral. com Transparent, bluish crystal aggregate of vivianite on matrix. Santa Eulalia, Chihuahua, Mexico. Faces of the monoclinic prism
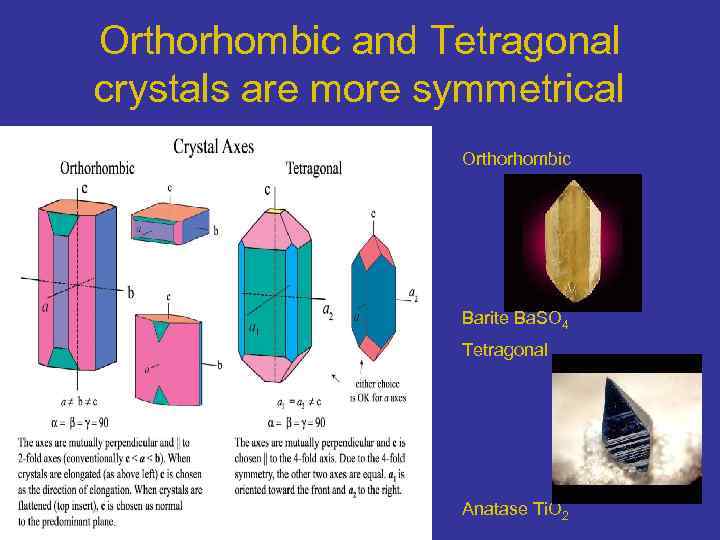 Orthorhombic and Tetragonal crystals are more symmetrical Orthorhombic Barite Ba. SO 4 Tetragonal Anatase Ti. O 2
Orthorhombic and Tetragonal crystals are more symmetrical Orthorhombic Barite Ba. SO 4 Tetragonal Anatase Ti. O 2
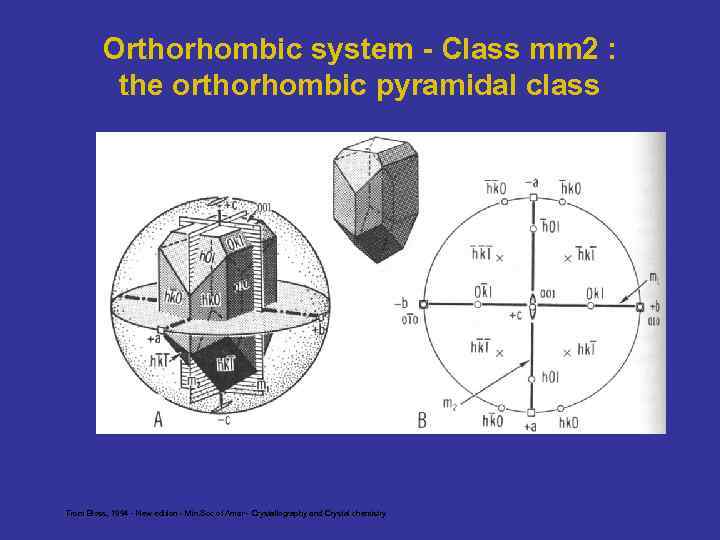 Orthorhombic system - Class mm 2 : the orthorhombic pyramidal class From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Orthorhombic system - Class mm 2 : the orthorhombic pyramidal class From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
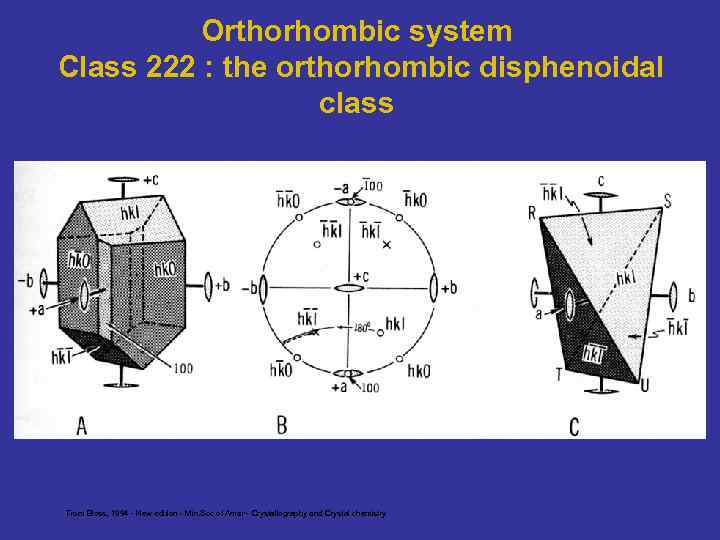 Orthorhombic system Class 222 : the orthorhombic disphenoidal class From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Orthorhombic system Class 222 : the orthorhombic disphenoidal class From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
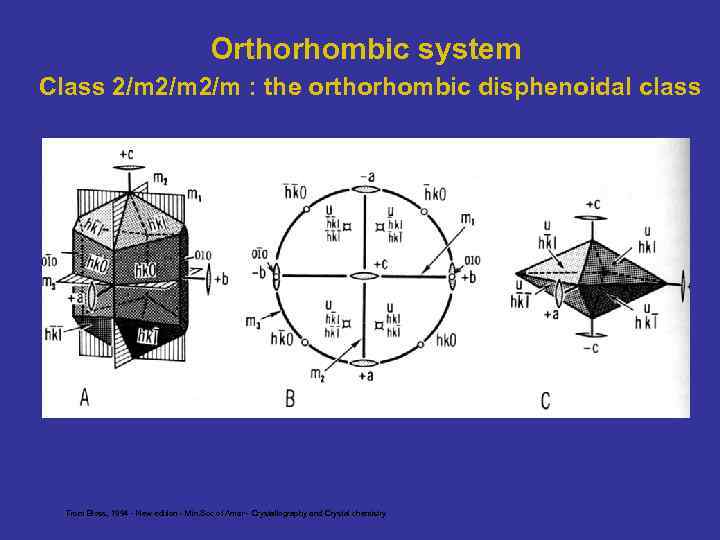 Orthorhombic system Class 2/m 2/m : the orthorhombic disphenoidal class From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Orthorhombic system Class 2/m 2/m : the orthorhombic disphenoidal class From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
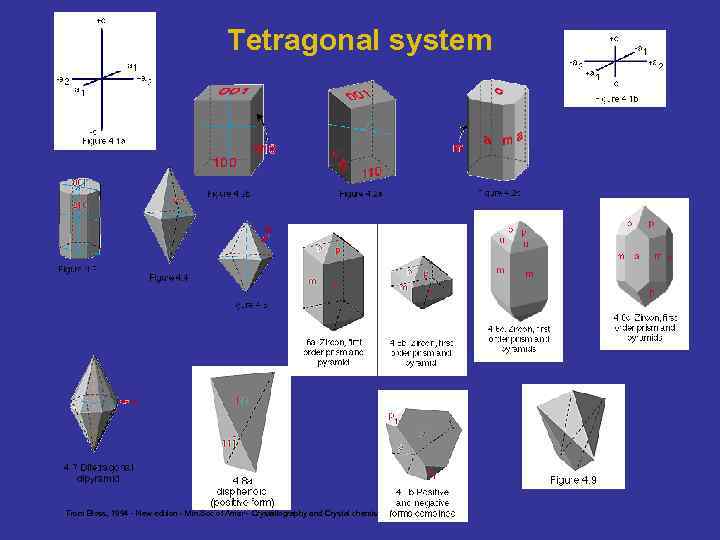 Tetragonal system From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Tetragonal system From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
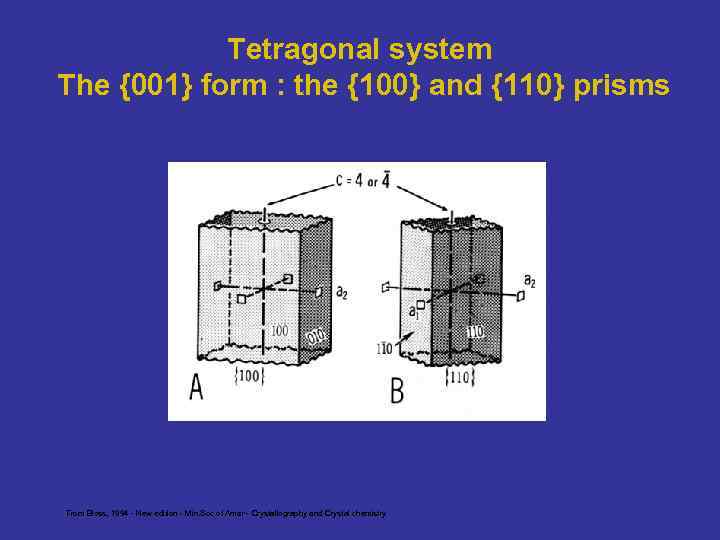 Tetragonal system The {001} form : the {100} and {110} prisms From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Tetragonal system The {001} form : the {100} and {110} prisms From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
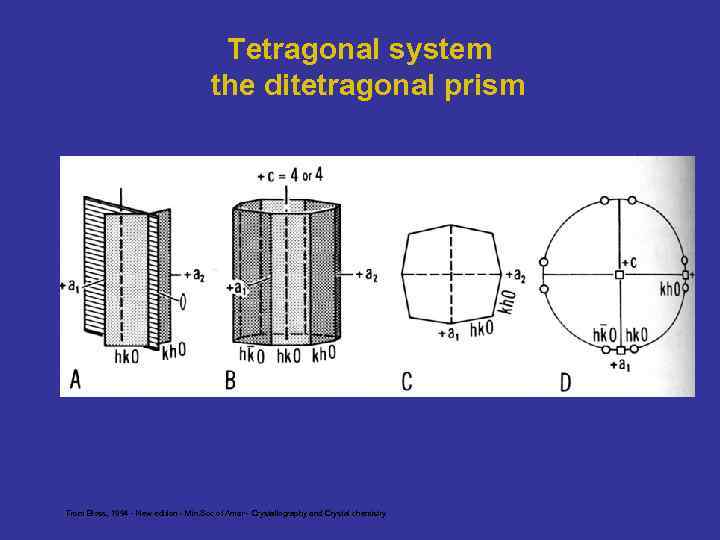 Tetragonal system the ditetragonal prism From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Tetragonal system the ditetragonal prism From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
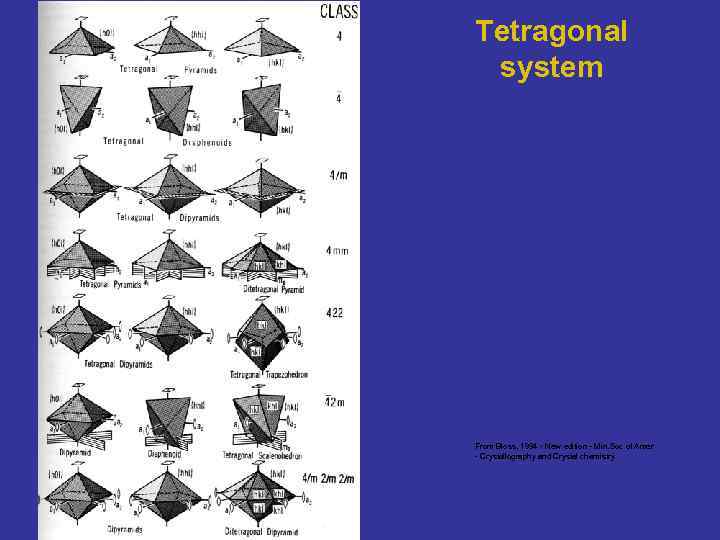 Tetragonal system From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Tetragonal system From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
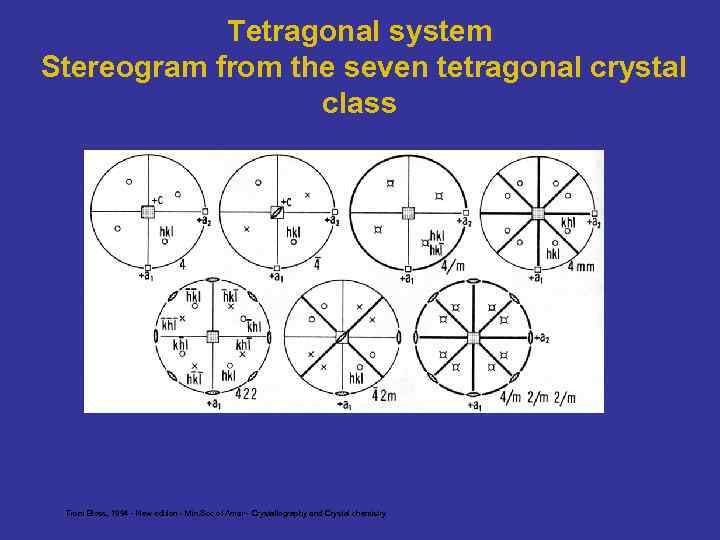 Tetragonal system Stereogram from the seven tetragonal crystal class From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Tetragonal system Stereogram from the seven tetragonal crystal class From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
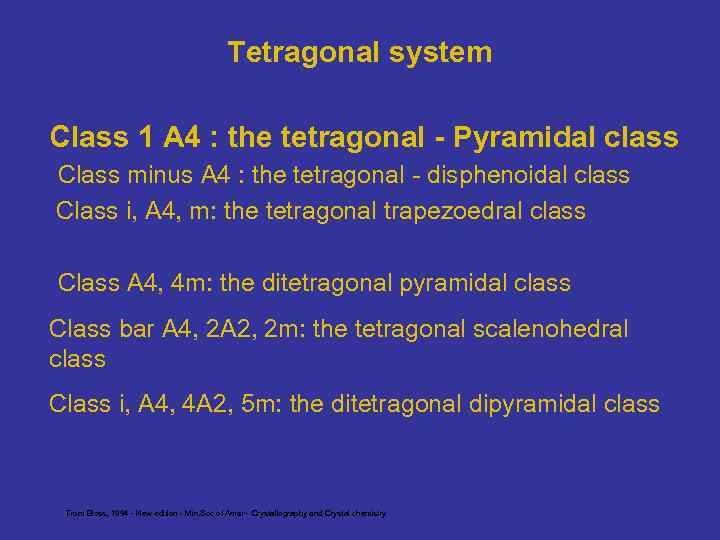 Tetragonal system Class 1 A 4 : the tetragonal - Pyramidal class Class minus A 4 : the tetragonal - disphenoidal class Class i, A 4, m: the tetragonal trapezoedral class Class A 4, 4 m: the ditetragonal pyramidal class Class bar A 4, 2 A 2, 2 m: the tetragonal scalenohedral class Class i, A 4, 4 A 2, 5 m: the ditetragonal dipyramidal class From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Tetragonal system Class 1 A 4 : the tetragonal - Pyramidal class Class minus A 4 : the tetragonal - disphenoidal class Class i, A 4, m: the tetragonal trapezoedral class Class A 4, 4 m: the ditetragonal pyramidal class Class bar A 4, 2 A 2, 2 m: the tetragonal scalenohedral class Class i, A 4, 4 A 2, 5 m: the ditetragonal dipyramidal class From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
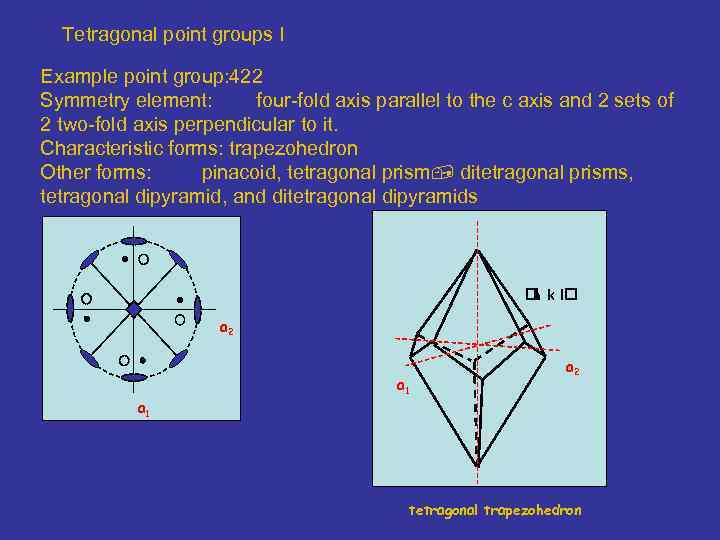 Tetragonal point groups I Example point group: 422 Symmetry element: four-fold axis parallel to the c axis and 2 sets of 2 two-fold axis perpendicular to it. Characteristic forms: trapezohedron Other forms: pinacoid, tetragonal prism ditetragonal prisms, tetragonal dipyramid, and ditetragonal dipyramids k l h a 2 a 1 tetragonal trapezohedron
Tetragonal point groups I Example point group: 422 Symmetry element: four-fold axis parallel to the c axis and 2 sets of 2 two-fold axis perpendicular to it. Characteristic forms: trapezohedron Other forms: pinacoid, tetragonal prism ditetragonal prisms, tetragonal dipyramid, and ditetragonal dipyramids k l h a 2 a 1 tetragonal trapezohedron
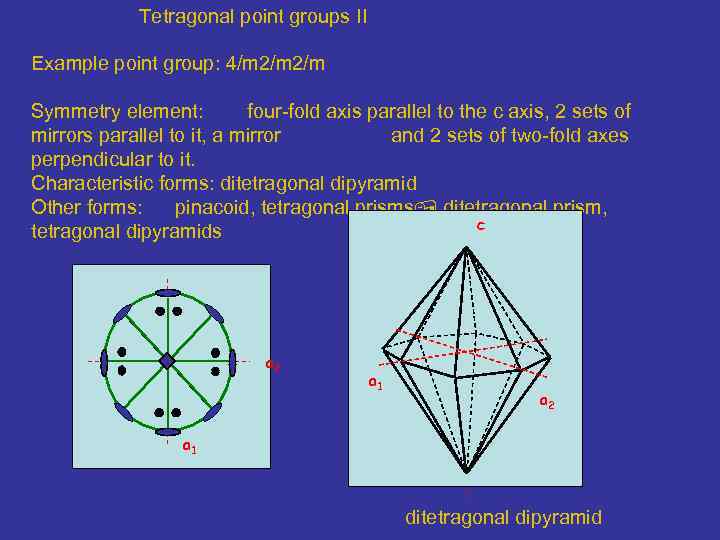 Tetragonal point groups II Example point group: 4/m 2/m Symmetry element: four-fold axis parallel to the c axis, 2 sets of mirrors parallel to it, a mirror and 2 sets of two-fold axes perpendicular to it. Characteristic forms: ditetragonal dipyramid Other forms: pinacoid, tetragonal prisms ditetragonal prism, c tetragonal dipyramids a 2 a 1 ditetragonal dipyramid
Tetragonal point groups II Example point group: 4/m 2/m Symmetry element: four-fold axis parallel to the c axis, 2 sets of mirrors parallel to it, a mirror and 2 sets of two-fold axes perpendicular to it. Characteristic forms: ditetragonal dipyramid Other forms: pinacoid, tetragonal prisms ditetragonal prism, c tetragonal dipyramids a 2 a 1 ditetragonal dipyramid
 Tetragonal crystals Narsarsukite Na 2(Ti, Fe)Si 2 (O, F)11 Mont Saint-Hilaire, Quebec, Canada. Point group: 4/m 2/m http: //webmineral. com Mackayite Fe. Te 2 O 5(OH) Mc. Guinnity shaft, Goldfield, Esmeralda County, Nevada, USA. Point group: 4/m 2/m Schematic projection of the narsarsukite crystal
Tetragonal crystals Narsarsukite Na 2(Ti, Fe)Si 2 (O, F)11 Mont Saint-Hilaire, Quebec, Canada. Point group: 4/m 2/m http: //webmineral. com Mackayite Fe. Te 2 O 5(OH) Mc. Guinnity shaft, Goldfield, Esmeralda County, Nevada, USA. Point group: 4/m 2/m Schematic projection of the narsarsukite crystal
 Tetragonal crystals Zircon - Zr. Si. O 4 Finnmark, Norway. Crystal diameter 6 cm. Point group 4/m 2/m. The crystal shape is composed of a dipyramid and a prism. http: //webmineral. brgm. fr: 8003 Scheelite Ca. WO 4 Traversella, Piemont, Italy. Crystal diameter 4. 5 cm. Point group 4/m. The crystal shape is composed of a dipyramid and if only the macroscopic shape is taken the resulting point group would be the same as for zircon. The lowering of the point group is due to the microscopic symmetry.
Tetragonal crystals Zircon - Zr. Si. O 4 Finnmark, Norway. Crystal diameter 6 cm. Point group 4/m 2/m. The crystal shape is composed of a dipyramid and a prism. http: //webmineral. brgm. fr: 8003 Scheelite Ca. WO 4 Traversella, Piemont, Italy. Crystal diameter 4. 5 cm. Point group 4/m. The crystal shape is composed of a dipyramid and if only the macroscopic shape is taken the resulting point group would be the same as for zircon. The lowering of the point group is due to the microscopic symmetry.
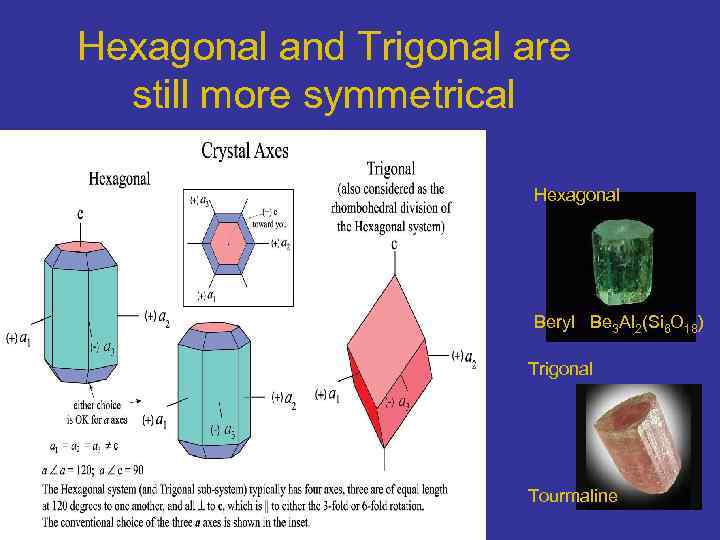 Hexagonal and Trigonal are still more symmetrical Hexagonal Beryl Be 3 Al 2(Si 6 O 18) Trigonal Tourmaline
Hexagonal and Trigonal are still more symmetrical Hexagonal Beryl Be 3 Al 2(Si 6 O 18) Trigonal Tourmaline
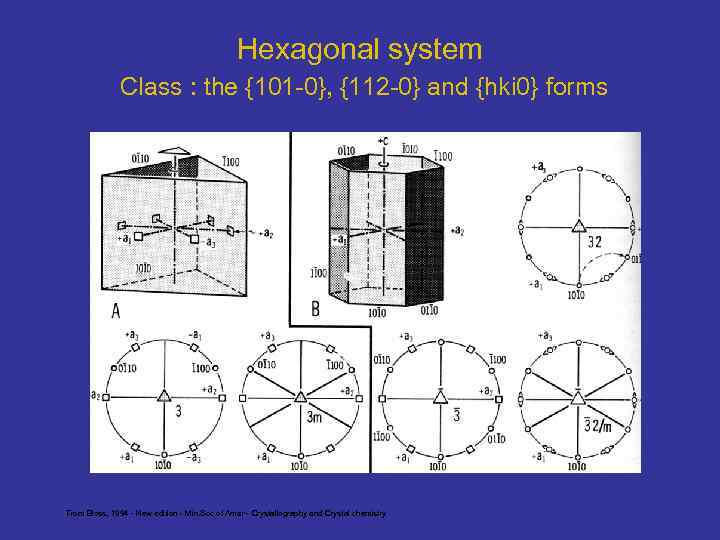 Hexagonal system Class : the {101 -0}, {112 -0} and {hki 0} forms From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Hexagonal system Class : the {101 -0}, {112 -0} and {hki 0} forms From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
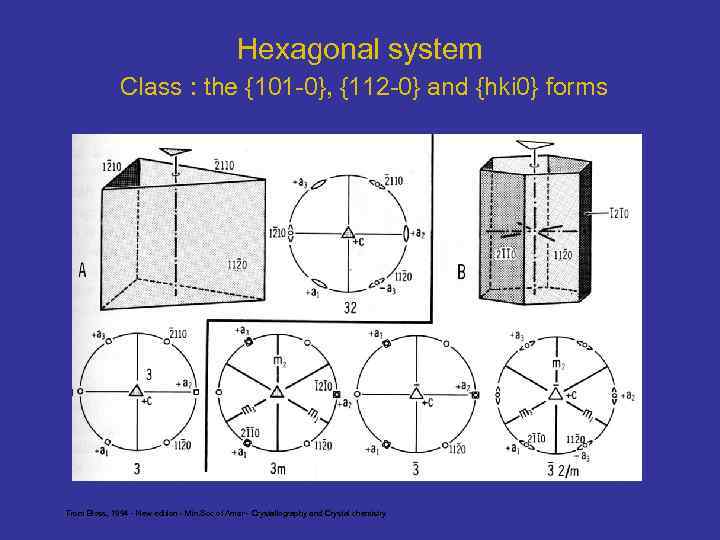 Hexagonal system Class : the {101 -0}, {112 -0} and {hki 0} forms From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Hexagonal system Class : the {101 -0}, {112 -0} and {hki 0} forms From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
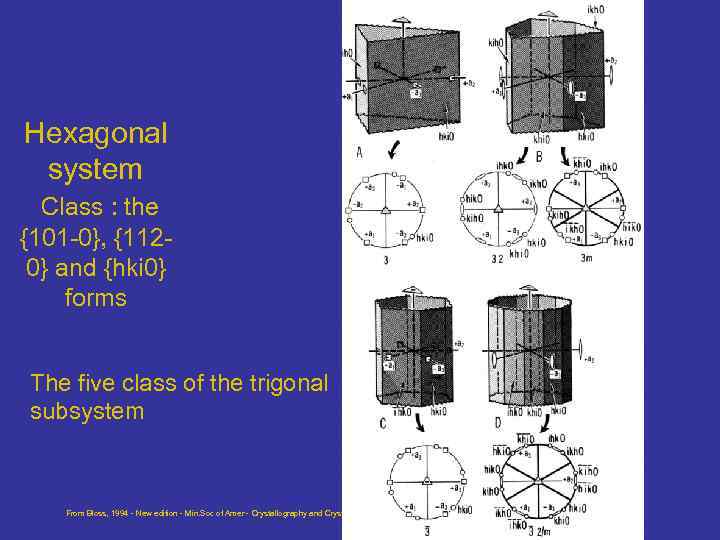 Hexagonal system Class : the {101 -0}, {1120} and {hki 0} forms The five class of the trigonal subsystem From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Hexagonal system Class : the {101 -0}, {1120} and {hki 0} forms The five class of the trigonal subsystem From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
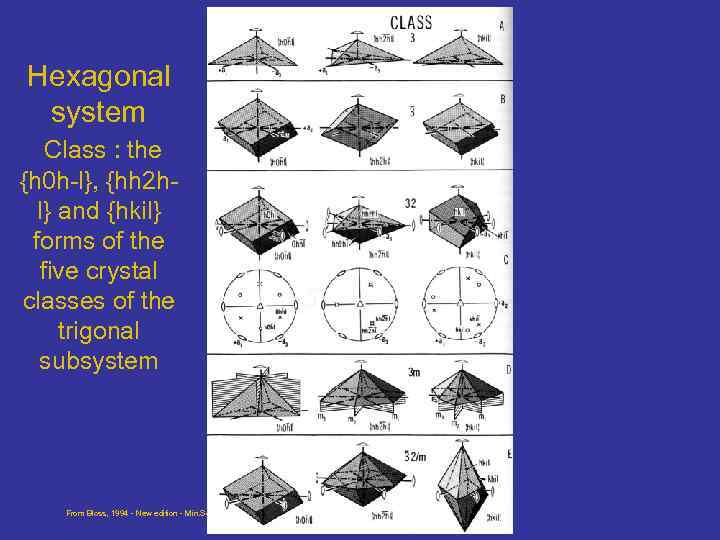 Hexagonal system Class : the {h 0 h-l}, {hh 2 hl} and {hkil} forms of the five crystal classes of the trigonal subsystem From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Hexagonal system Class : the {h 0 h-l}, {hh 2 hl} and {hkil} forms of the five crystal classes of the trigonal subsystem From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
 Hexagonal subsystem Class : the {101 -0}, {112 -0} and {hki 0} forms From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Hexagonal subsystem Class : the {101 -0}, {112 -0} and {hki 0} forms From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
 Hexagonal subsystem Class : the {h 0 h-l}, {hh 2 h-l} and {hkil} forms From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
Hexagonal subsystem Class : the {h 0 h-l}, {hh 2 h-l} and {hkil} forms From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
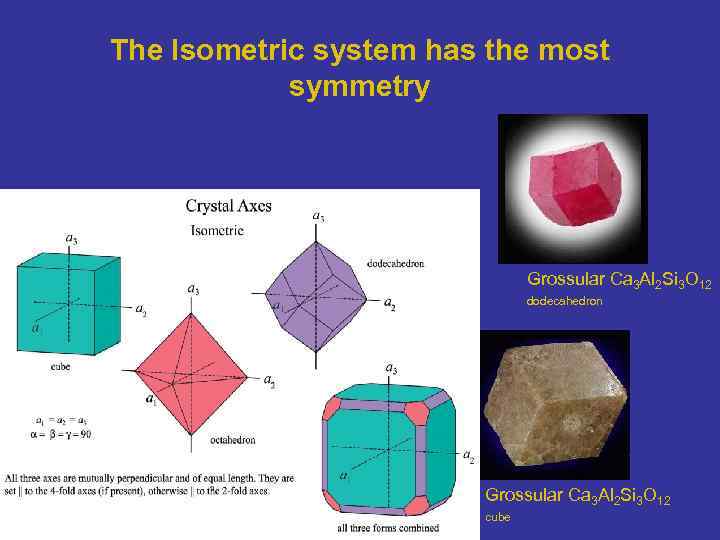 The Isometric system has the most symmetry Grossular Ca 3 Al 2 Si 3 O 12 dodecahedron Grossular Ca 3 Al 2 Si 3 O 12 cube
The Isometric system has the most symmetry Grossular Ca 3 Al 2 Si 3 O 12 dodecahedron Grossular Ca 3 Al 2 Si 3 O 12 cube
 Cubic forms
Cubic forms
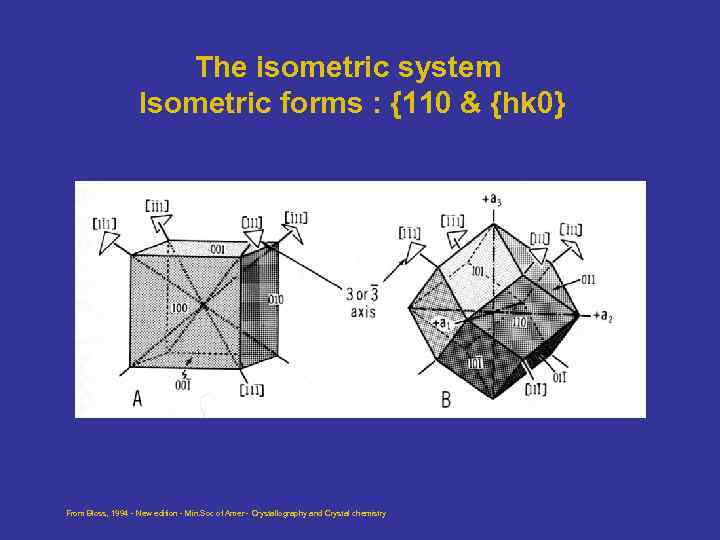 The isometric system Isometric forms : {110 & {hk 0} From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
The isometric system Isometric forms : {110 & {hk 0} From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
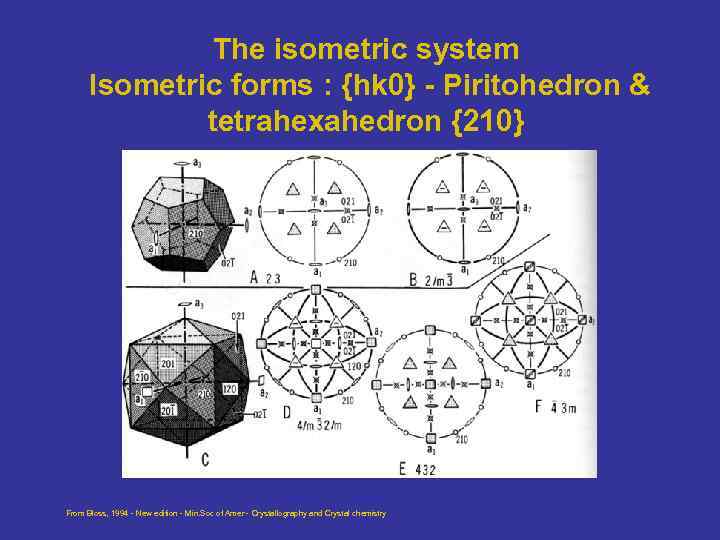 The isometric system Isometric forms : {hk 0} - Piritohedron & tetrahexahedron {210} From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
The isometric system Isometric forms : {hk 0} - Piritohedron & tetrahexahedron {210} From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
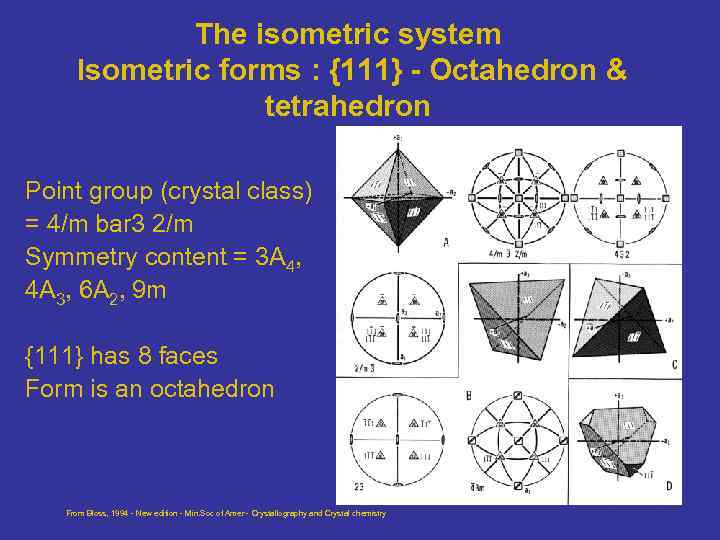 The isometric system Isometric forms : {111} - Octahedron & tetrahedron Point group (crystal class) = 4/m bar 3 2/m Symmetry content = 3 A 4, 4 A 3, 6 A 2, 9 m {111} has 8 faces Form is an octahedron From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
The isometric system Isometric forms : {111} - Octahedron & tetrahedron Point group (crystal class) = 4/m bar 3 2/m Symmetry content = 3 A 4, 4 A 3, 6 A 2, 9 m {111} has 8 faces Form is an octahedron From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
 The isometric system Isometric forms : {hhl} From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
The isometric system Isometric forms : {hhl} From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
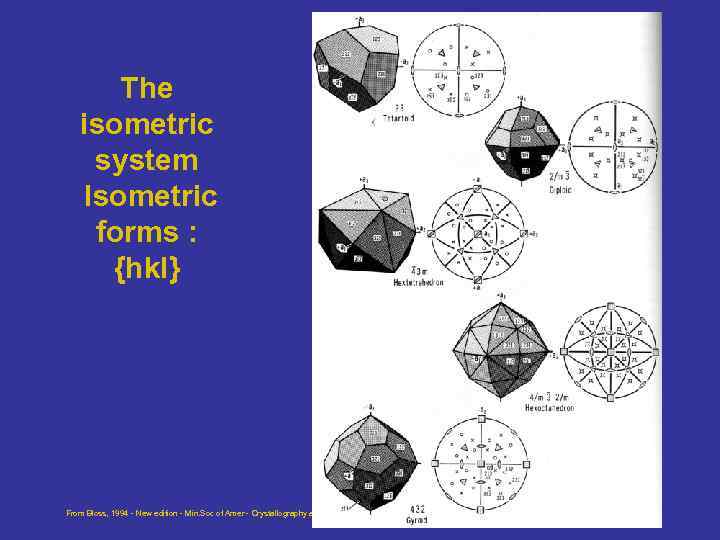 The isometric system Isometric forms : {hkl} From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
The isometric system Isometric forms : {hkl} From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
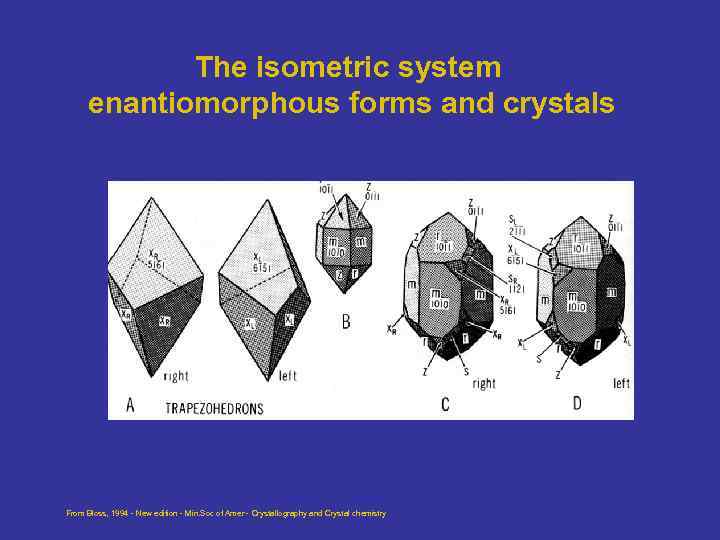 The isometric system enantiomorphous forms and crystals From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
The isometric system enantiomorphous forms and crystals From Bloss, 1994 - New edition - Min. Soc of Amer - Crystallography and Crystal chemistry
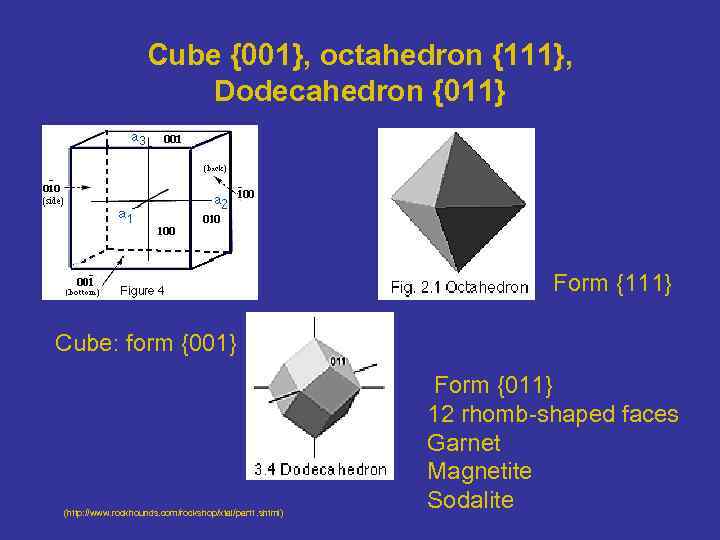 Cube {001}, octahedron {111}, Dodecahedron {011} Form {111} Cube: form {001} (http: //www. rockhounds. com/rockshop/xtal/part 1. shtml) Form {011} 12 rhomb-shaped faces Garnet Magnetite Sodalite
Cube {001}, octahedron {111}, Dodecahedron {011} Form {111} Cube: form {001} (http: //www. rockhounds. com/rockshop/xtal/part 1. shtml) Form {011} 12 rhomb-shaped faces Garnet Magnetite Sodalite
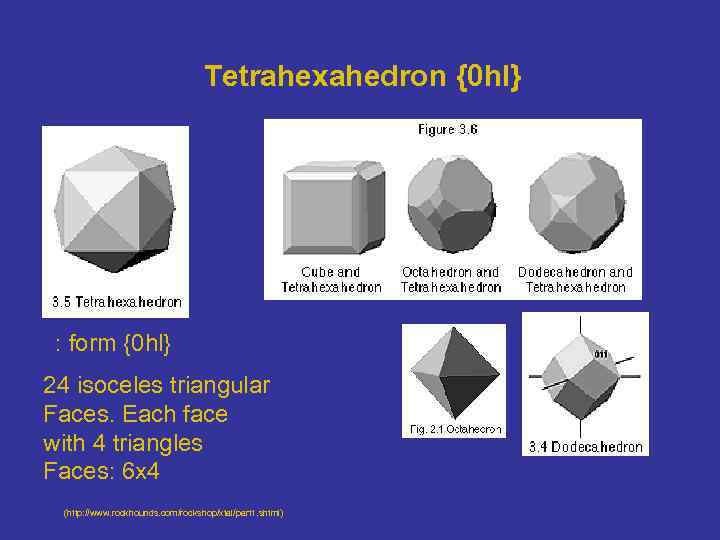 Tetrahexahedron {0 hl} : form {0 hl} 24 isoceles triangular Faces. Each face with 4 triangles Faces: 6 x 4 (http: //www. rockhounds. com/rockshop/xtal/part 1. shtml)
Tetrahexahedron {0 hl} : form {0 hl} 24 isoceles triangular Faces. Each face with 4 triangles Faces: 6 x 4 (http: //www. rockhounds. com/rockshop/xtal/part 1. shtml)
 Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
Crystal form 1 - Introduction - Definitions 2 - Form : Models in wood, porcelain, plastic, cardboard 3 - Open and closed forms 4 - Labelling forms 5 - Non-isometric open forms 6 - Non-isometric closed forms 7 - Isometric form - Closed form 8 - Combining forms - relationships between forms 9 - Forms in the 6 crsytal systems 10 - Form and habits of crystals
 Don’t mix form and habit HABIT is the correct term to indicate outward appearance. Habit, when applied to natural crystals and minerals, includes such descriptive terms as tabular, equidimensional, acicular, massive, reniform, drusy, and encrusting. A crystal form is a set of crystal faces that are related to each other by the elements of symmetry of a given crystal system. A set of crystal faces have the same arrangement of atoms.
Don’t mix form and habit HABIT is the correct term to indicate outward appearance. Habit, when applied to natural crystals and minerals, includes such descriptive terms as tabular, equidimensional, acicular, massive, reniform, drusy, and encrusting. A crystal form is a set of crystal faces that are related to each other by the elements of symmetry of a given crystal system. A set of crystal faces have the same arrangement of atoms.
 Zones, Forms, Habits • Quantitative description of orientation in minerals – use Miller indices: – Zone - Lines, or linear directions within minerals – Form - Shapes of three dimensional objects • Qualitative description of mineral shapes: – Habit Zones, Forms and habit. ppp
Zones, Forms, Habits • Quantitative description of orientation in minerals – use Miller indices: – Zone - Lines, or linear directions within minerals – Form - Shapes of three dimensional objects • Qualitative description of mineral shapes: – Habit Zones, Forms and habit. ppp
 Crystal Habit • Qualitative terminology to describe individual minerals and aggregates of minerals – Shape of individual minerals – Intergrowths of several mineral grains – Shape of masses of grains Zones, Forms and habit. ppp
Crystal Habit • Qualitative terminology to describe individual minerals and aggregates of minerals – Shape of individual minerals – Intergrowths of several mineral grains – Shape of masses of grains Zones, Forms and habit. ppp
 Some common crystal Habit Cubic - cube shapes Octahedral - shaped like octahedrons Tabular - rectangular shapes. Equant - a term used to describe minerals that have all of their boundaries of approximately equal length. Fibrous - elongated clusters of fibers. Acicular - long, slender crystals. Prismatic - abundance of prism faces. Bladed - like a wedge or knife blade Dendritic - tree-like growths Botryoidal - smooth bulbous shapes
Some common crystal Habit Cubic - cube shapes Octahedral - shaped like octahedrons Tabular - rectangular shapes. Equant - a term used to describe minerals that have all of their boundaries of approximately equal length. Fibrous - elongated clusters of fibers. Acicular - long, slender crystals. Prismatic - abundance of prism faces. Bladed - like a wedge or knife blade Dendritic - tree-like growths Botryoidal - smooth bulbous shapes
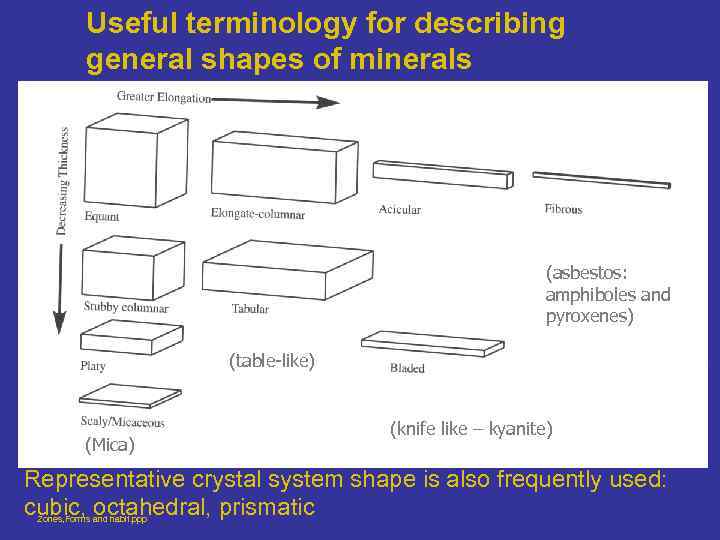 Useful terminology for describing general shapes of minerals (asbestos: amphiboles and pyroxenes) (table-like) (Mica) (knife like – kyanite) Representative crystal system shape is also frequently used: cubic, octahedral, prismatic Zones, Forms and habit. ppp
Useful terminology for describing general shapes of minerals (asbestos: amphiboles and pyroxenes) (table-like) (Mica) (knife like – kyanite) Representative crystal system shape is also frequently used: cubic, octahedral, prismatic Zones, Forms and habit. ppp
 Fibrous tremolite: amphibole Bladed kyanite: silicate Zones, Forms and habit. ppp Fibrous tremolite
Fibrous tremolite: amphibole Bladed kyanite: silicate Zones, Forms and habit. ppp Fibrous tremolite
 Texture of mineral assemblage (shape of mass of grains) Colloform - finely crystalline, concentric mineral layer Globular (spherulitic) - radiating, concentrically arranged acicular minerals Botryoidal - smooth bulbous shapes, like a bunch of grapes Reniform - kidney shaped Mammillary - similar, but larger than botryoidal, breast-like or portions of spheres Drusy - Surface covered with layer of small crystals Dendritic - tree-like growths Zones, Forms and habit. ppp
Texture of mineral assemblage (shape of mass of grains) Colloform - finely crystalline, concentric mineral layer Globular (spherulitic) - radiating, concentrically arranged acicular minerals Botryoidal - smooth bulbous shapes, like a bunch of grapes Reniform - kidney shaped Mammillary - similar, but larger than botryoidal, breast-like or portions of spheres Drusy - Surface covered with layer of small crystals Dendritic - tree-like growths Zones, Forms and habit. ppp
 Drusy quartz Globular hematite Zones, Forms and habit. ppp
Drusy quartz Globular hematite Zones, Forms and habit. ppp


