4b1c4e6c175f2c445eeacdd73e580c37.ppt
- Количество слайдов: 36

Class objectives: l Highlight some important areas in environmental chemistry l present some of the common techniques that environmental chemists use to quantify process that occur in the environment • It is assumed that everyone has courses in calculus and general chemistry.

Class objectives: • We will cover general topics: Global warming, Strat. O 3, aerosols, photochemical smog, acid rain, etc. • Develop relationships will be used to help quantify equilibrium and kinetic processes

Important Environmental Issues l Global warming and stratospheric ozone depletion l Concentration of environmental pollutants at the poles; pesticides in foods, etc. l Buildup of environmental chemicals in the oceans; contamination of soil and ground water l Particle exposure, photochemical oxidant exposure, acid deposition l Energy shortages
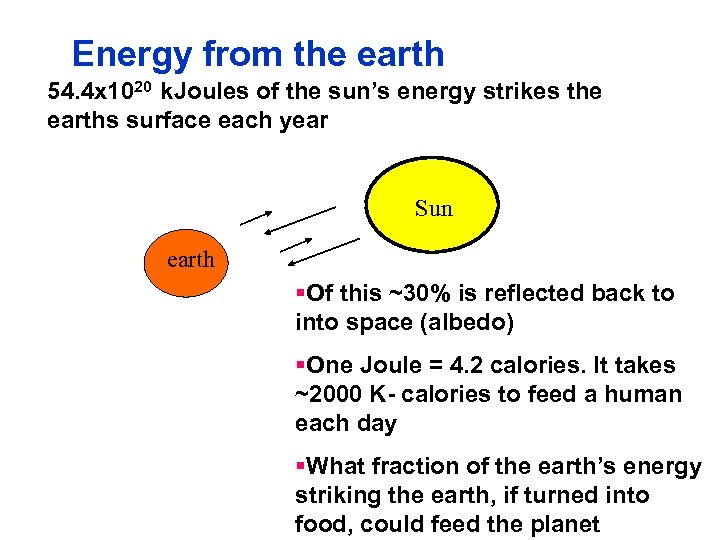
Energy from the earth 54. 4 x 1020 k. Joules of the sun’s energy strikes the earths surface each year Sun earth §Of this ~30% is reflected back to into space (albedo) §One Joule = 4. 2 calories. It takes ~2000 K calories to feed a human each day §What fraction of the earth’s energy striking the earth, if turned into food, could feed the planet
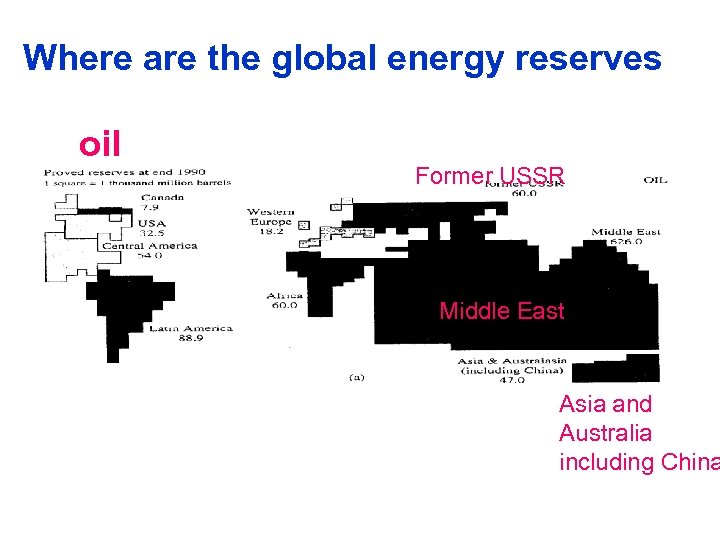
Where are the global energy reserves oil Figure 1. 5 Spiro Former USSR page 10 Middle East Asia and Australia including China
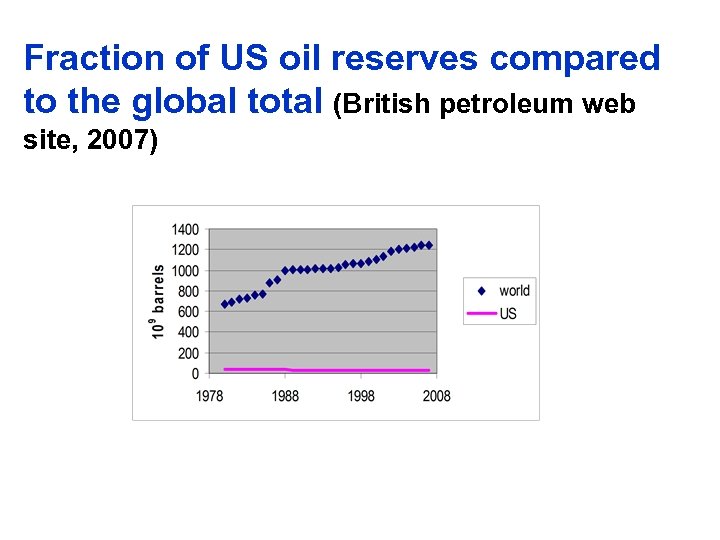
Fraction of US oil reserves compared to the global total (British petroleum web site, 2007)

The atmospheric compartment l. How much does it weigh? l. Temperature and pressure l. Circulation and mixing l. Where did Oxygen come from l. Particle emissions l. Emissions of other pollutants

How thin is the air at the top of Mt. Everest? u. Mt. Everest is 8882 meters high or 8. 88 km high ulog P = 0. 06 x 8. 88 u. P = 10 0. 06 x 8. 88 = 0. 293 bars u. Assume there are 1. 01 bars/atm. u. This means there is < 1/3 of the air

The quantity d is called the dry adiabatic lapse rate u d = - d. T/dz = 9. 8 o. K/kilometer u. If the air is saturated with water the lapse rate is often called s u Near the surface sis ~ 4 o. K/km and at 6 km and – 5 o. K/km it is ~ 6 K/km at 7 km high
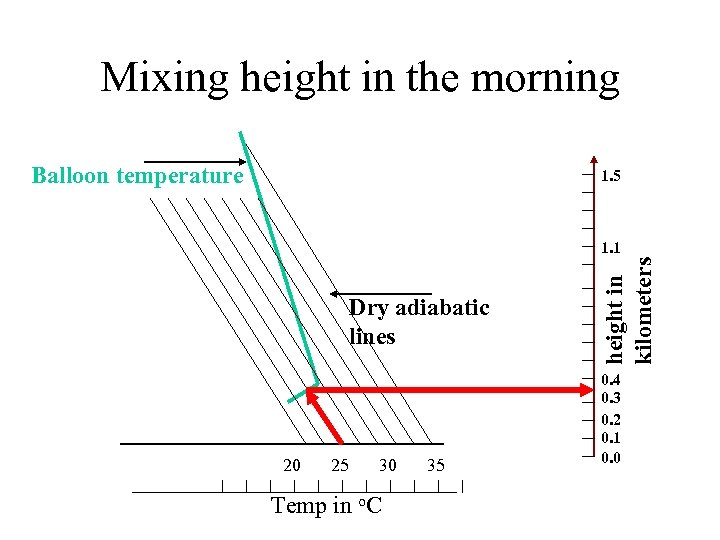
Mixing height in the morning Balloon temperature 1. 5 Dry adiabatic lines 20 25 30 Temp in o. C 35 height in kilometers 1. 1 0. 4 0. 3 0. 2 0. 1 0. 0

What is Global Warming and how can it Change the Climate?

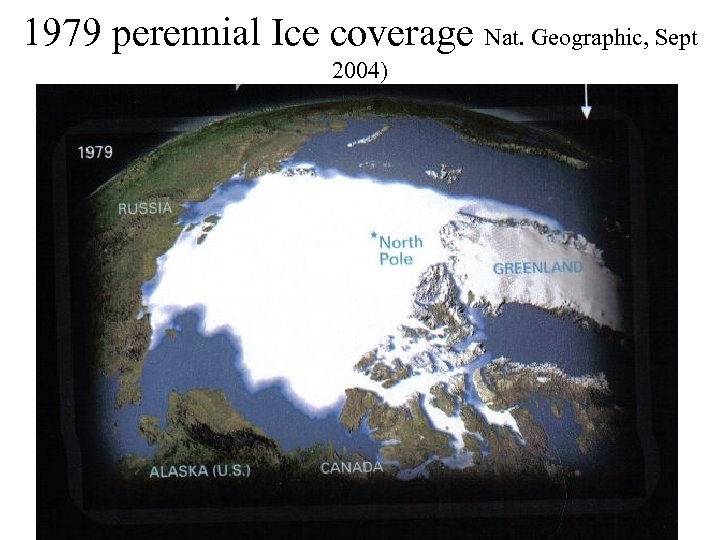
1979 perennial Ice coverage Nat. Geographic, Sept 2004)

2003 perennial Ice coverage
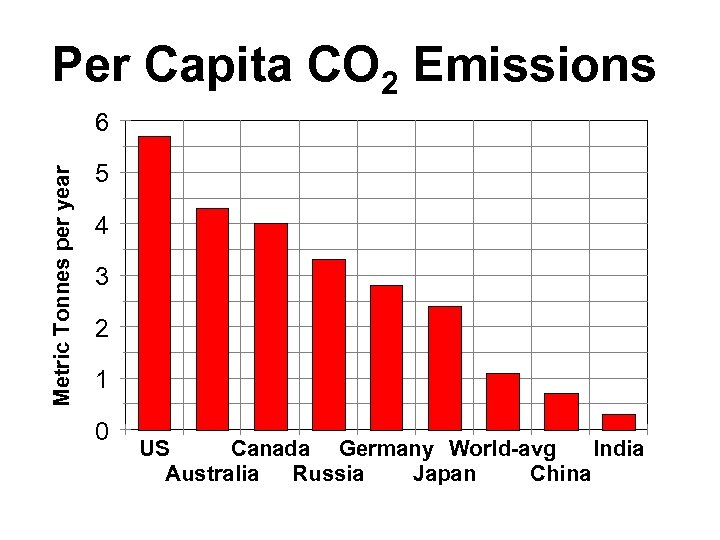
Per Capita CO 2 Emissions Metric Tonnes per year 6 5 4 3 2 1 0 US Canada Germany World avg India Australia Russia Japan China
![Kinetics: 1 st order reactions A > B d [A] /dt = krate [A] Kinetics: 1 st order reactions A > B d [A] /dt = krate [A]](https://present5.com/presentation/4b1c4e6c175f2c445eeacdd73e580c37/image-16.jpg)
Kinetics: 1 st order reactions A > B d [A] /dt = krate [A] d [A]/[A] = kratedt [A]t= [A]0 e kt
![Some time vs conc. data Hr Conc [A] Ln[A] 0 2. 718 1 0. Some time vs conc. data Hr Conc [A] Ln[A] 0 2. 718 1 0.](https://present5.com/presentation/4b1c4e6c175f2c445eeacdd73e580c37/image-17.jpg)
Some time vs conc. data Hr Conc [A] Ln[A] 0 2. 718 1 0. 3 2. 117 0. 75 0. 6 1. 649 0. 50 0. 9 1. 284 0. 25 1. 2 1. 000 0. 00 1. 5 0. 779 0. 25
![A plot of the ln[conc] vs. time for a 1 st order reaction gives A plot of the ln[conc] vs. time for a 1 st order reaction gives](https://present5.com/presentation/4b1c4e6c175f2c445eeacdd73e580c37/image-18.jpg)
A plot of the ln[conc] vs. time for a 1 st order reaction gives a straight line with a slope of the 1 st order rate constant.
![ln [A]/[A]o= k t 1/2 ; ln 2 /k =t 1/2 2 nd order ln [A]/[A]o= k t 1/2 ; ln 2 /k =t 1/2 2 nd order](https://present5.com/presentation/4b1c4e6c175f2c445eeacdd73e580c37/image-19.jpg)
ln [A]/[A]o= k t 1/2 ; ln 2 /k =t 1/2 2 nd order reactions A + B products d. A/dt = k 2 nd [A][B] If B is constant kpseudo 1 st = k 2 nd [B]
![kpseudo 1 st = k 2 nd [B] ln 2 /k =t 1/2 1. kpseudo 1 st = k 2 nd [B] ln 2 /k =t 1/2 1.](https://present5.com/presentation/4b1c4e6c175f2c445eeacdd73e580c37/image-20.jpg)
kpseudo 1 st = k 2 nd [B] ln 2 /k =t 1/2 1. constant OH radicals in the atmosphere kpseudo 1 st = k 2 nd [OH. ] 2. constant p. H kpseudo 1 st = k 2 nd [OH ]
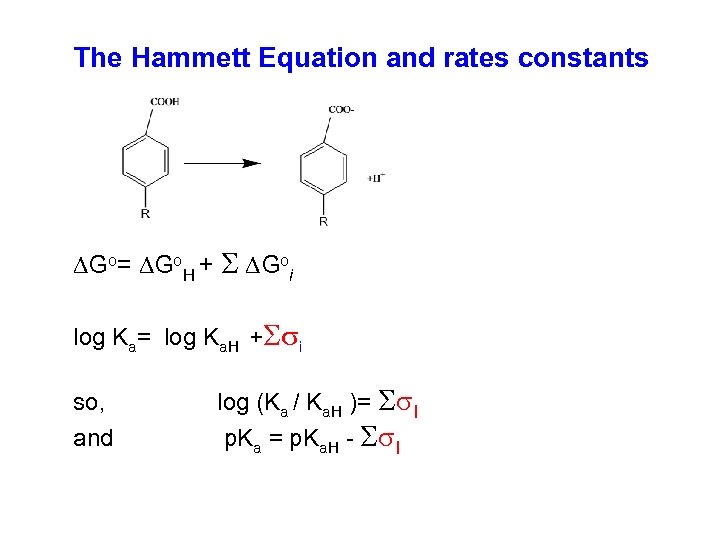
The Hammett Equation and rates constants DGo= DGo. H + S DGoi log Ka= log Ka. H +Ssi so, log (Ka / Ka. H )= Ss. I and p. Ka = p. Ka. H Ss. I



It is also possible to show that: log(krate) = log krate. H + r sm, p or log(krate/krate. H) = r sm, p
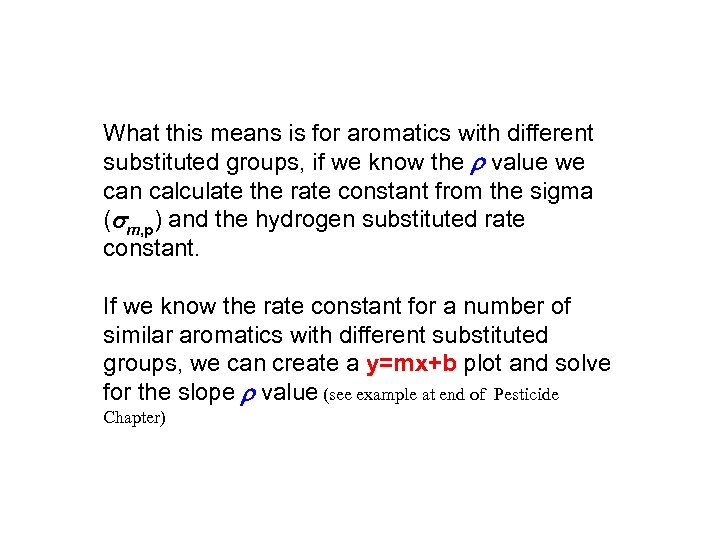
What this means is for aromatics with different substituted groups, if we know the r value we can calculate the rate constant from the sigma (sm, p) and the hydrogen substituted rate constant. If we know the rate constant for a number of similar aromatics with different substituted groups, we can create a y=mx+b plot and solve for the slope r value (see example at end of Pesticide Chapter)
![ln [A]/[A]o= k t 1/2 ; ln 2 /k =t 1/2 2 nd order ln [A]/[A]o= k t 1/2 ; ln 2 /k =t 1/2 2 nd order](https://present5.com/presentation/4b1c4e6c175f2c445eeacdd73e580c37/image-26.jpg)
ln [A]/[A]o= k t 1/2 ; ln 2 /k =t 1/2 2 nd order reactions A + B products d. A/dt = k 2 nd [A][B] If B is constant kpseudo 1 st = k 2 nd [B]
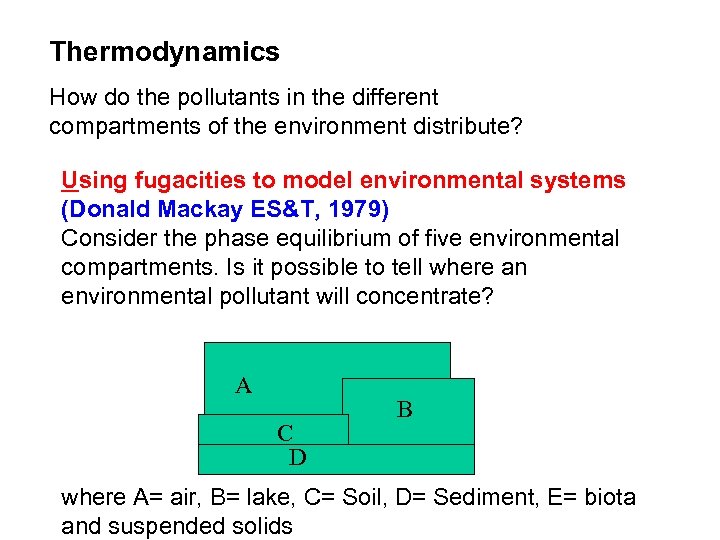
Thermodynamics How do the pollutants in the different compartments of the environment distribute? Using fugacities to model environmental systems (Donald Mackay ES&T, 1979) Consider the phase equilibrium of five environmental compartments. Is it possible to tell where an environmental pollutant will concentrate? A C D B C where A= air, B= lake, C= Soil, D= Sediment, E= biota and suspended solids
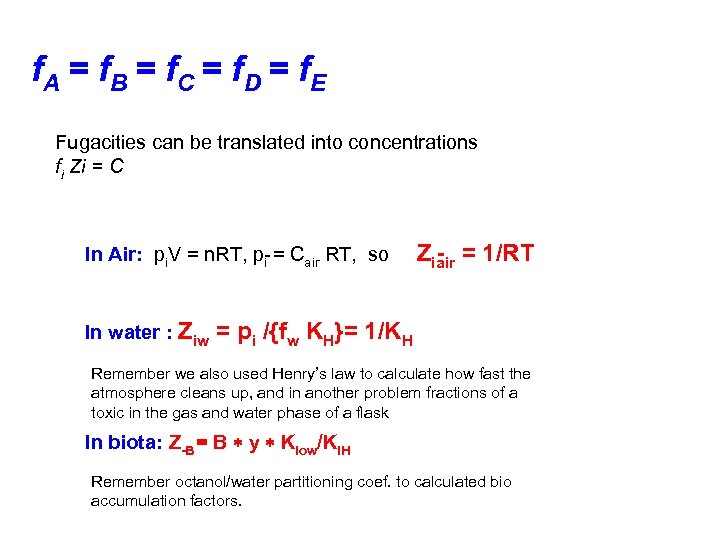
f. A = f B = f C = f D = f E Fugacities can be translated into concentrations fi Zi = C In Air: pi. V = n. RT, p = Cair RT, so Zi = 1/RT i air In water : Ziw = pi /{fw KH}= 1/KH Remember we also used Henry’s law to calculate how fast the atmosphere cleans up, and in another problem fractions of a toxic in the gas and water phase of a flask In biota: Z B = B y Kiow/Ki. H Remember octanol/water partitioning coef. to calculated bio accumulation factors.
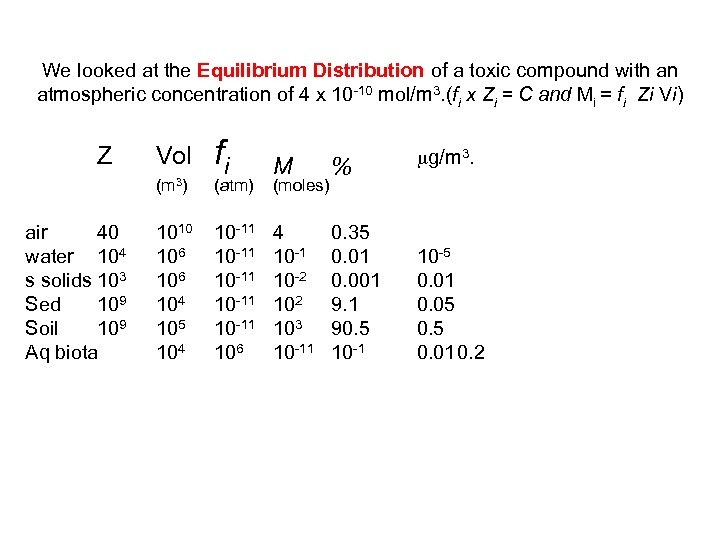
We looked at the Equilibrium Distribution of a toxic compound with an atmospheric concentration of 4 x 10 10 mol/m 3. (fi x Zi = C and Mi = fi Zi Vi) Z Vol (m 3) air 40 water 104 s solids 103 Sed 109 Soil 109 Aq biota 1010 106 104 105 104 fi M % 10 11 10 11 106 4 10 1 10 2 103 10 11 0. 35 0. 01 0. 001 9. 1 90. 5 10 1 (atm) (moles) mg/m 3. 10 5 0. 01 0. 05 0. 010. 2
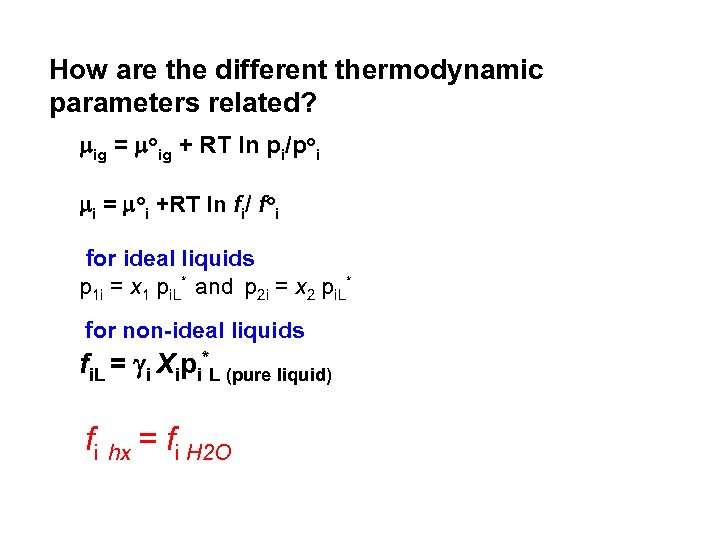
How are the different thermodynamic parameters related? mig = moig + RT ln pi/poi mi = moi +RT ln fi/ foi for ideal liquids p 1 i = x 1 pi. L* and p 2 i = x 2 pi. L* for non ideal liquids fi. L = i Xipi*L (pure liquid) fi hx = fi H 2 O
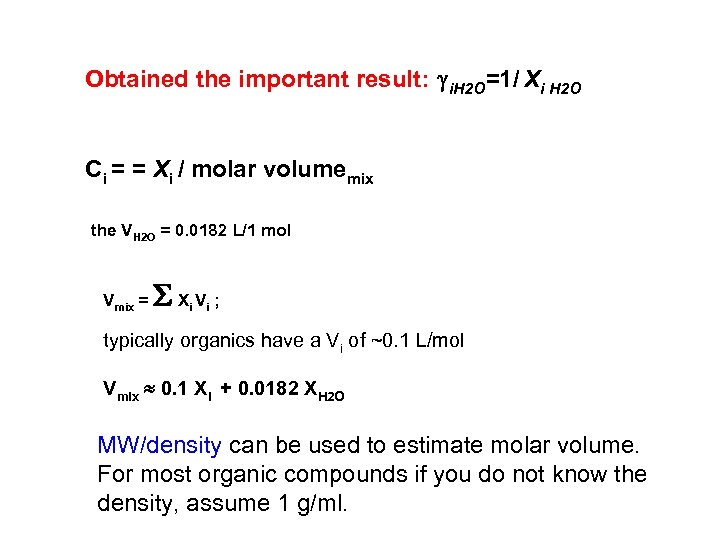
Obtained the important result: i. H 2 O=1/ Xi H 2 O Ci = = Xi / molar volumemix the VH 2 O = 0. 0182 L/1 mol Vmix = S X V ; i i typically organics have a Vi of ~0. 1 L/mol Vmix 0. 1 Xi + 0. 0182 XH 2 O MW/density can be used to estimate molar volume. For most organic compounds if you do not know the density, assume 1 g/ml.

From the saturated concentration of an organic in water (Ciwsat) can you calculate the mole fraction and activity coefficient? Remember the toluene homework where you were given a maximum saturation concentration in water of 515 mg/liter H 2 O. Convert this to moles per liter which is a Ciwsat = mole fraction/molar vol.
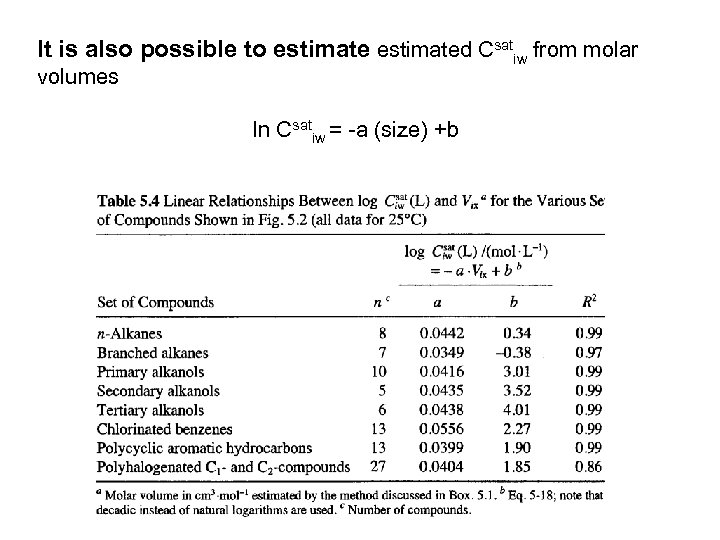
It is also possible to estimated Csatiw from molar volumes ln Csatiw = a (size) +b
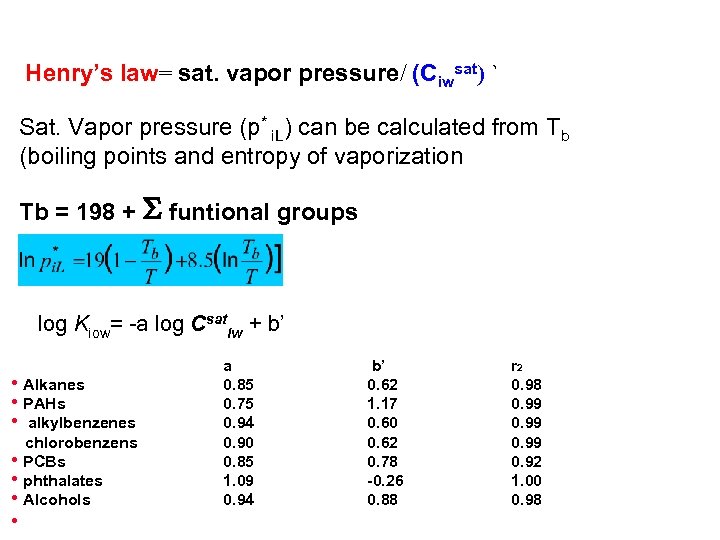
Henry’s law= sat. vapor pressure/ (Ciwsat) ` Sat. Vapor pressure (p* i. L) can be calculated from Tb (boiling points and entropy of vaporization Tb = 198 + S funtional groups log Kiow= a log Csatiw + b’ h. Alkanes h. PAHs h alkylbenzenes chlorobenzens h. PCBs hphthalates h. Alcohols h a 0. 85 0. 75 0. 94 0. 90 0. 85 1. 09 0. 94 b’ 0. 62 1. 17 0. 60 0. 62 0. 78 0. 26 0. 88 r 2 0. 98 0. 99 0. 92 1. 00 0. 98
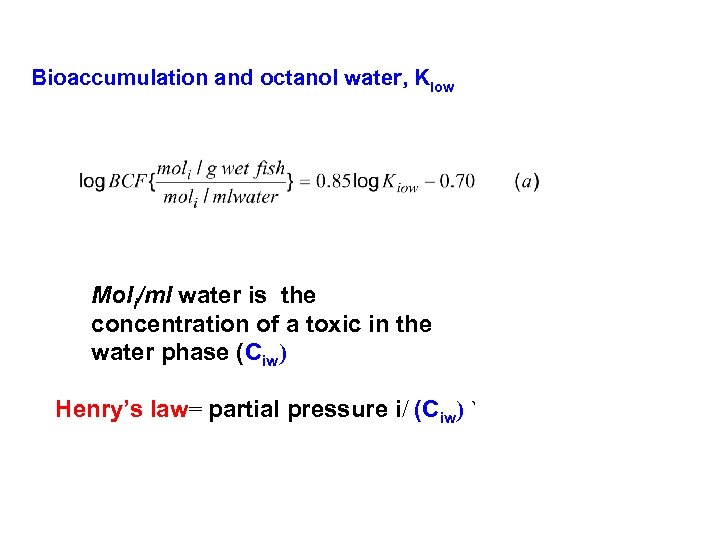
Bioaccumulation and octanol water, Kiow Moli/ml water is the concentration of a toxic in the water phase (Ciw) Henry’s law= partial pressure i/ (Ciw) `

Go over problems I did at the board, problems that were covered from the notes during class, and homework problems from the short and long homework sets. Look at the natural waters homework/with answer link The exam will cover thermo, vapor pressure, henry’s law, water octanol, surface and water purification, pesticides and heavy toxic metals. It will have problems and some short questions. Good luck to all
4b1c4e6c175f2c445eeacdd73e580c37.ppt