60e8926de5b3eb5683d79c185659e967.ppt
- Количество слайдов: 14
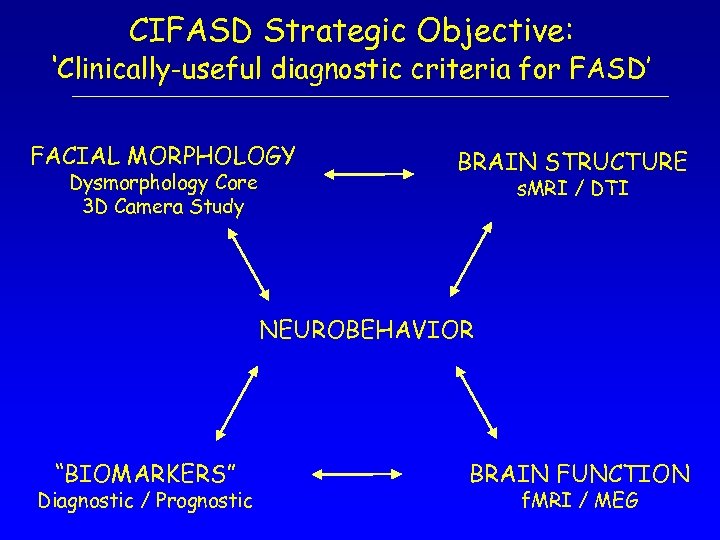
CIFASD Strategic Objective: ‘Clinically-useful diagnostic criteria for FASD’ FACIAL MORPHOLOGY Dysmorphology Core 3 D Camera Study BRAIN STRUCTURE s. MRI / DTI NEUROBEHAVIOR “BIOMARKERS” Diagnostic / Prognostic BRAIN FUNCTION f. MRI / MEG
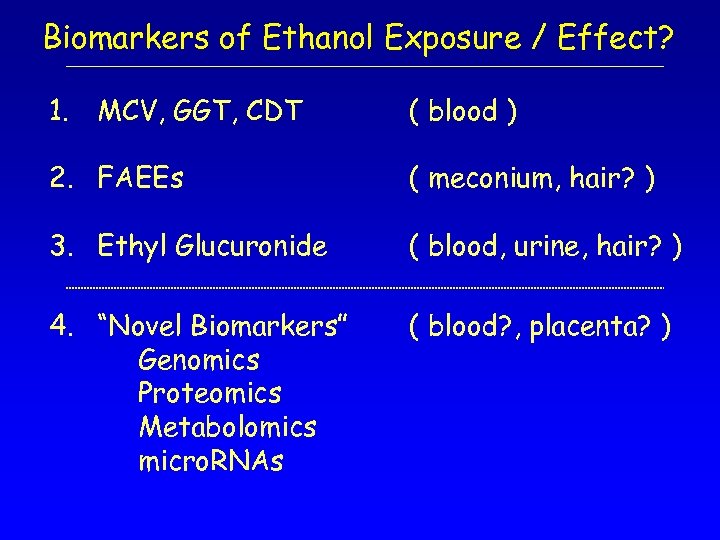
Biomarkers of Ethanol Exposure / Effect? 1. MCV, GGT, CDT ( blood ) 2. FAEEs ( meconium, hair? ) 3. Ethyl Glucuronide ( blood, urine, hair? ) 4. “Novel Biomarkers” Genomics Proteomics Metabolomics micro. RNAs ( blood? , placenta? )

Prenatal Ethanol Exposure Paradigm VOLUNTARY DRINKING - 5% Ethanol in 0. 066% saccharin water - Two-bottle choice paradigm - Intermittent consumption (4 hr/day) - Peak maternal serum Et. OH: Long-Evans rats: 84 mg / d. L Black 6/J mice: 125 mg / d. L - Saccharin water, volume-matched control

Altered Gene Expression Cannabinoid Receptor 1 S 100 Calcium Binding Protein A 4 Galanin Receptor 2 Integrin Alpha 7 Gremlin 1 S 100 Calcium Binding Protein G Hemoglobin Epsilon 1 Secreted Frizzle-related Protein 4 Matrix Metalloproteinase 2 S 100 Calcium Binding Protein B Deiodinase Iodothyronine 2 Lipopolysaccharide Binding Protein Galanin Hemoglobin Delta Toll-like Receptor 4 Placental Growth Factor Transforming Growth Factor Alpha Heart & Neural Crest Derivative 2 11 b-Hydroxysteroid Dehydrogenase 2 Transient Receptor Potential Cation Channel 4 Serine Peptidase Inhibitor, Kazal Type 5 EGF-containing Fibulin-like Extracellular Matrix Protein 1
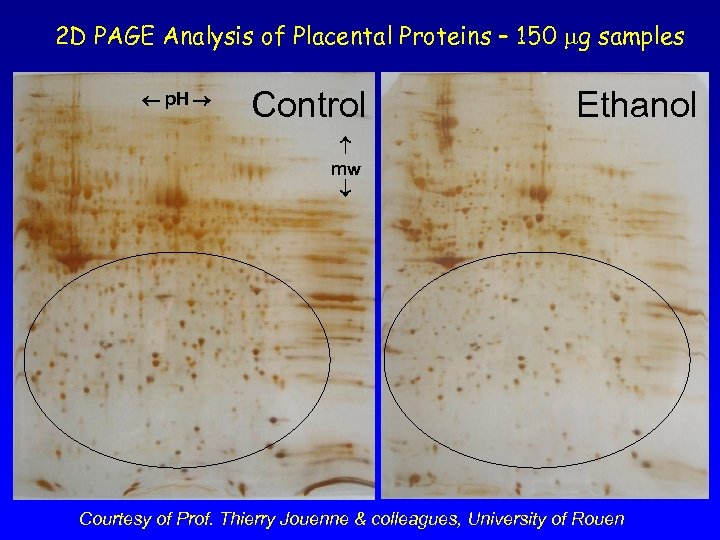
2 D PAGE Analysis of Placental Proteins – 150 mg samples p. H Control Ethanol mw Courtesy of Prof. Thierry Jouenne & colleagues, University of Rouen
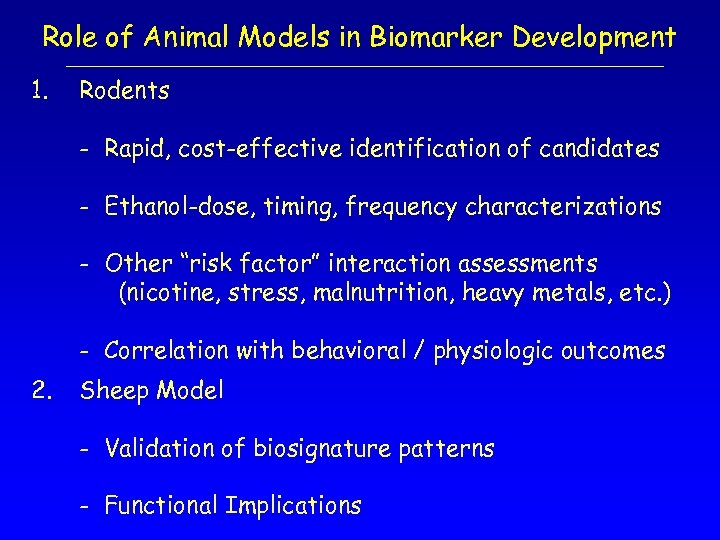
Role of Animal Models in Biomarker Development 1. Rodents - Rapid, cost-effective identification of candidates - Ethanol-dose, timing, frequency characterizations - Other “risk factor” interaction assessments (nicotine, stress, malnutrition, heavy metals, etc. ) - Correlation with behavioral / physiologic outcomes 2. Sheep Model - Validation of biosignature patterns - Functional Implications
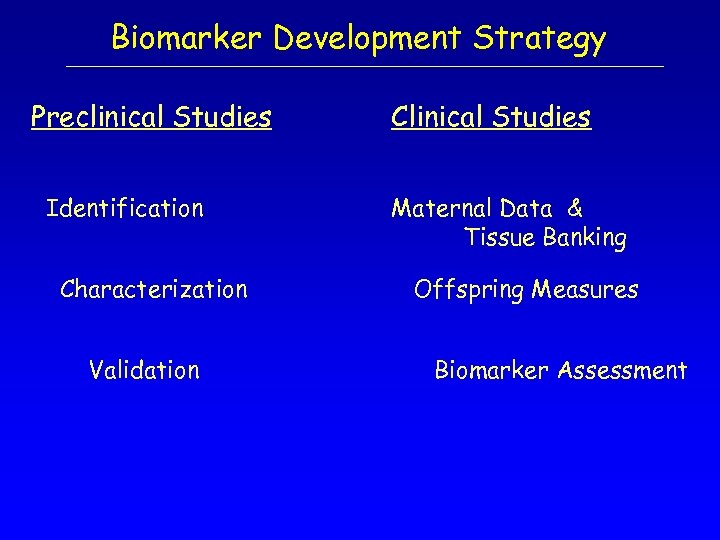
Biomarker Development Strategy Preclinical Studies Identification Characterization Validation Clinical Studies Maternal Data & Tissue Banking Offspring Measures Biomarker Assessment
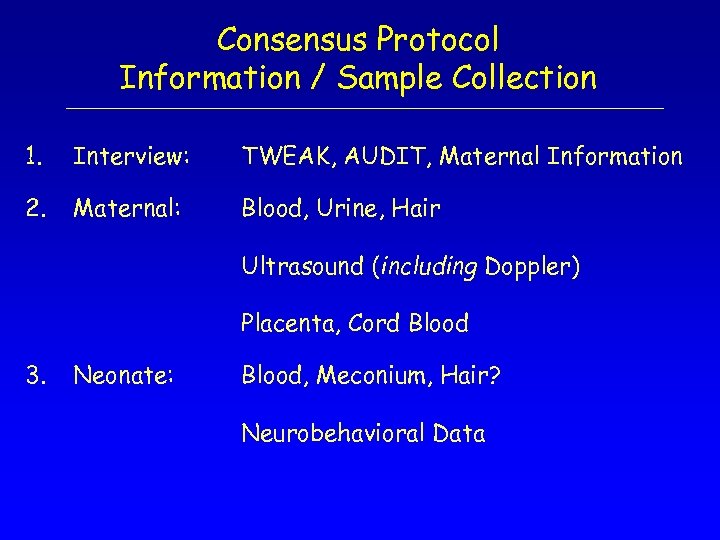
Consensus Protocol Information / Sample Collection 1. Interview: TWEAK, AUDIT, Maternal Information 2. Maternal: Blood, Urine, Hair Ultrasound (including Doppler) Placenta, Cord Blood 3. Neonate: Blood, Meconium, Hair? Neurobehavioral Data

Consensus Protocol Information / Sample Collection Times 1. First Prenatal Visit: (1 st trimester) 2. Follow-up Visit: (3 rd trimester) 3. Delivery: 4. Follow-up: (6 to 12 months of age)
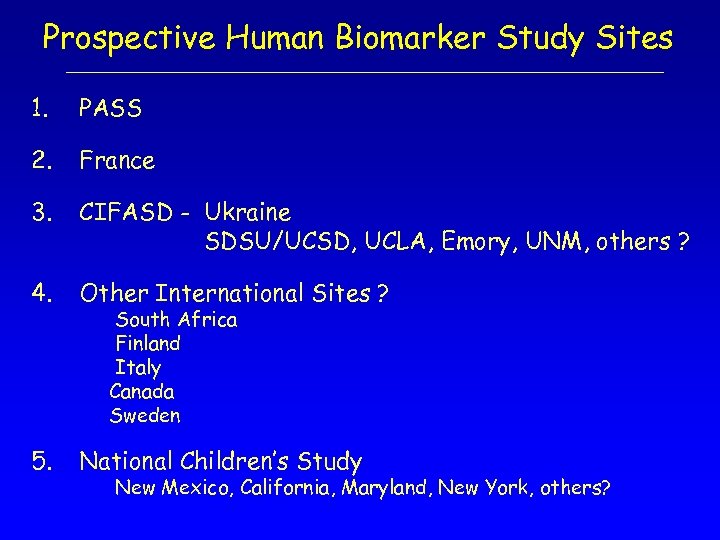
Prospective Human Biomarker Study Sites 1. PASS 2. France 3. CIFASD - Ukraine SDSU/UCSD, UCLA, Emory, UNM, others ? 4. Other International Sites ? 5. National Children’s Study South Africa Finland Italy Canada Sweden New Mexico, California, Maryland, New York, others?


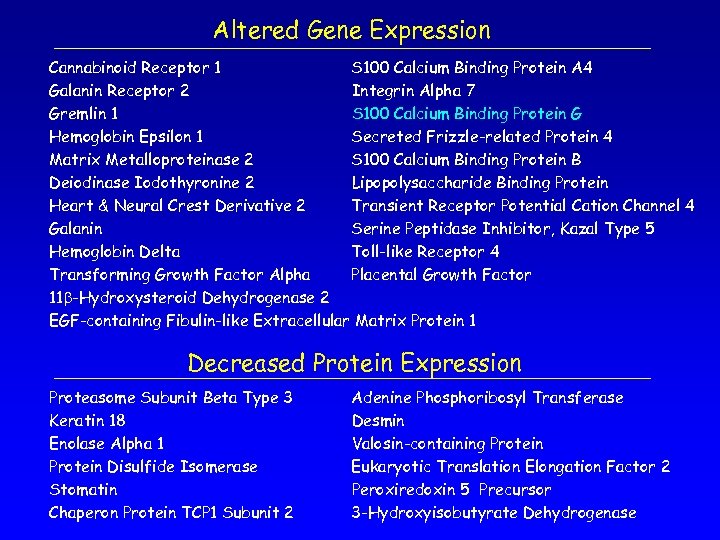
Altered Gene Expression Cannabinoid Receptor 1 S 100 Calcium Binding Protein A 4 Galanin Receptor 2 Integrin Alpha 7 Gremlin 1 S 100 Calcium Binding Protein G Hemoglobin Epsilon 1 Secreted Frizzle-related Protein 4 Matrix Metalloproteinase 2 S 100 Calcium Binding Protein B Deiodinase Iodothyronine 2 Lipopolysaccharide Binding Protein Heart & Neural Crest Derivative 2 Transient Receptor Potential Cation Channel 4 Galanin Serine Peptidase Inhibitor, Kazal Type 5 Hemoglobin Delta Toll-like Receptor 4 Transforming Growth Factor Alpha Placental Growth Factor 11 b-Hydroxysteroid Dehydrogenase 2 EGF-containing Fibulin-like Extracellular Matrix Protein 1 Decreased Protein Expression Proteasome Subunit Beta Type 3 Keratin 18 Enolase Alpha 1 Protein Disulfide Isomerase Stomatin Chaperon Protein TCP 1 Subunit 2 Adenine Phosphoribosyl Transferase Desmin Valosin-containing Protein Eukaryotic Translation Elongation Factor 2 Peroxiredoxin 5 Precursor 3 -Hydroxyisobutyrate Dehydrogenase
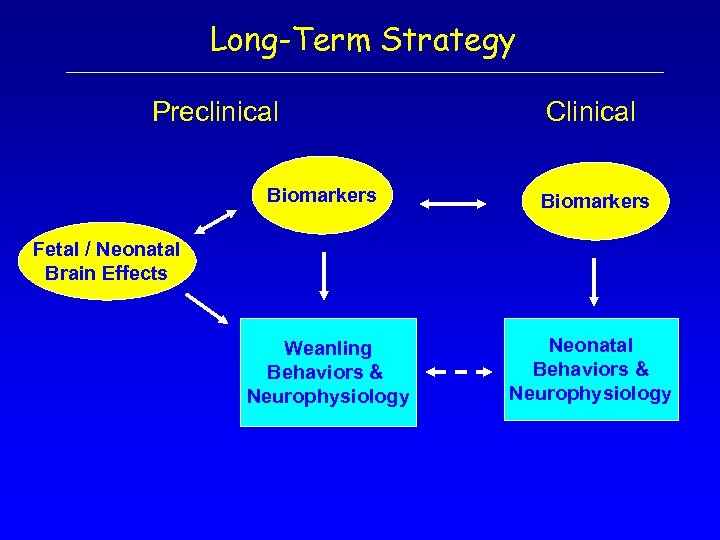
Long-Term Strategy Preclinical Clinical Biomarkers Weanling Behaviors & Neurophysiology Neonatal Behaviors & Neurophysiology Fetal / Neonatal Brain Effects
60e8926de5b3eb5683d79c185659e967.ppt